Beverages in Rheumatoid Arthritis: What to Prefer or to Avoid
Abstract
1. Introduction
1.1. The Mosaic of Autoimmunity in RA and the Role of Nutrition
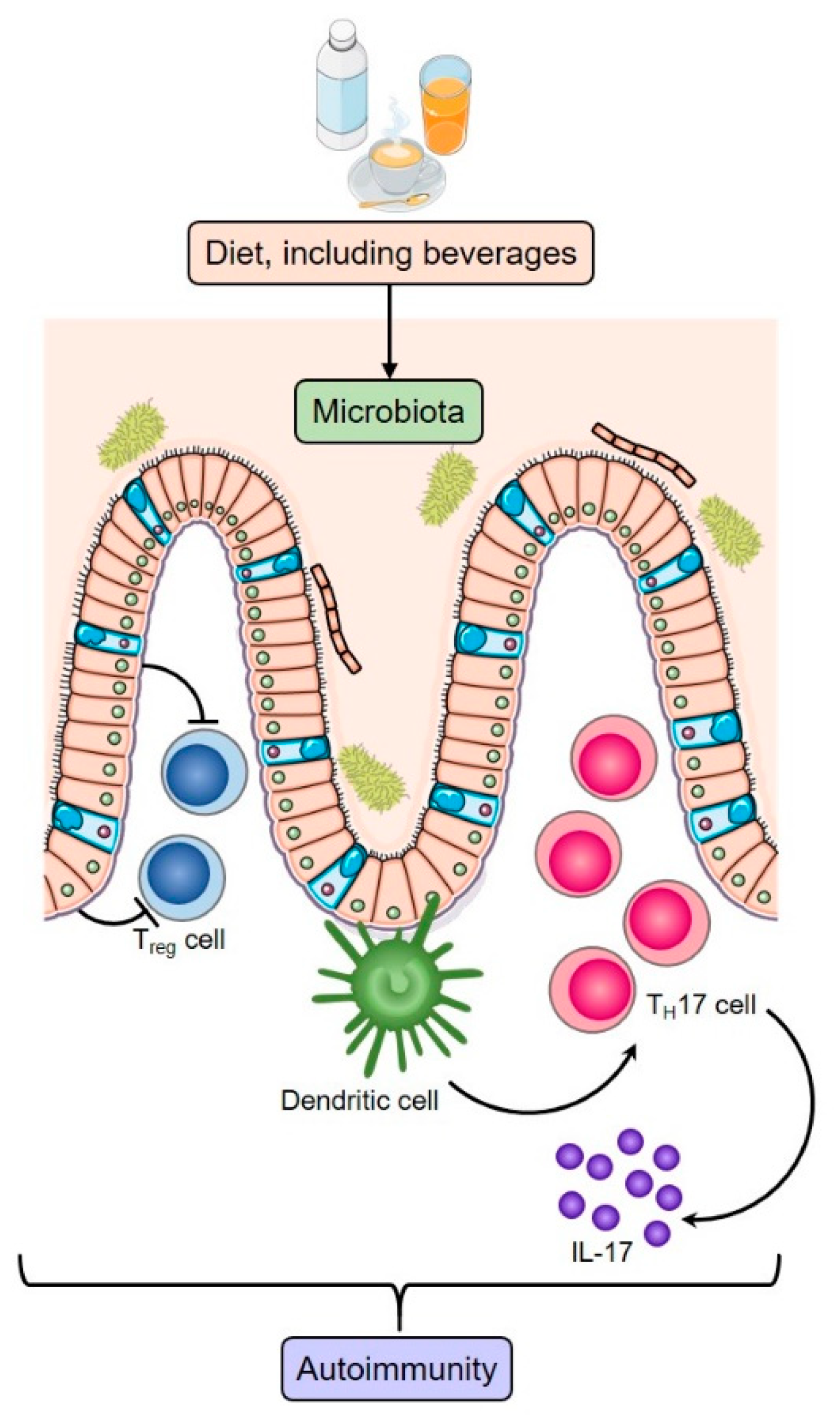
1.2. Diet and the Microbiome
2. Beverages in the Context of Wider Dietary Patterns
2.1. Western Diet
2.2. Mediterranean Diet
2.3. Vegetarian and Vegan Diets
2.4. The Impact of Fasting on RA Disease Outcomes
3. An Evolving Multidisciplinary Team: The Importance of the Dietician
4. The Role of Water and Fresh Fruit Juices
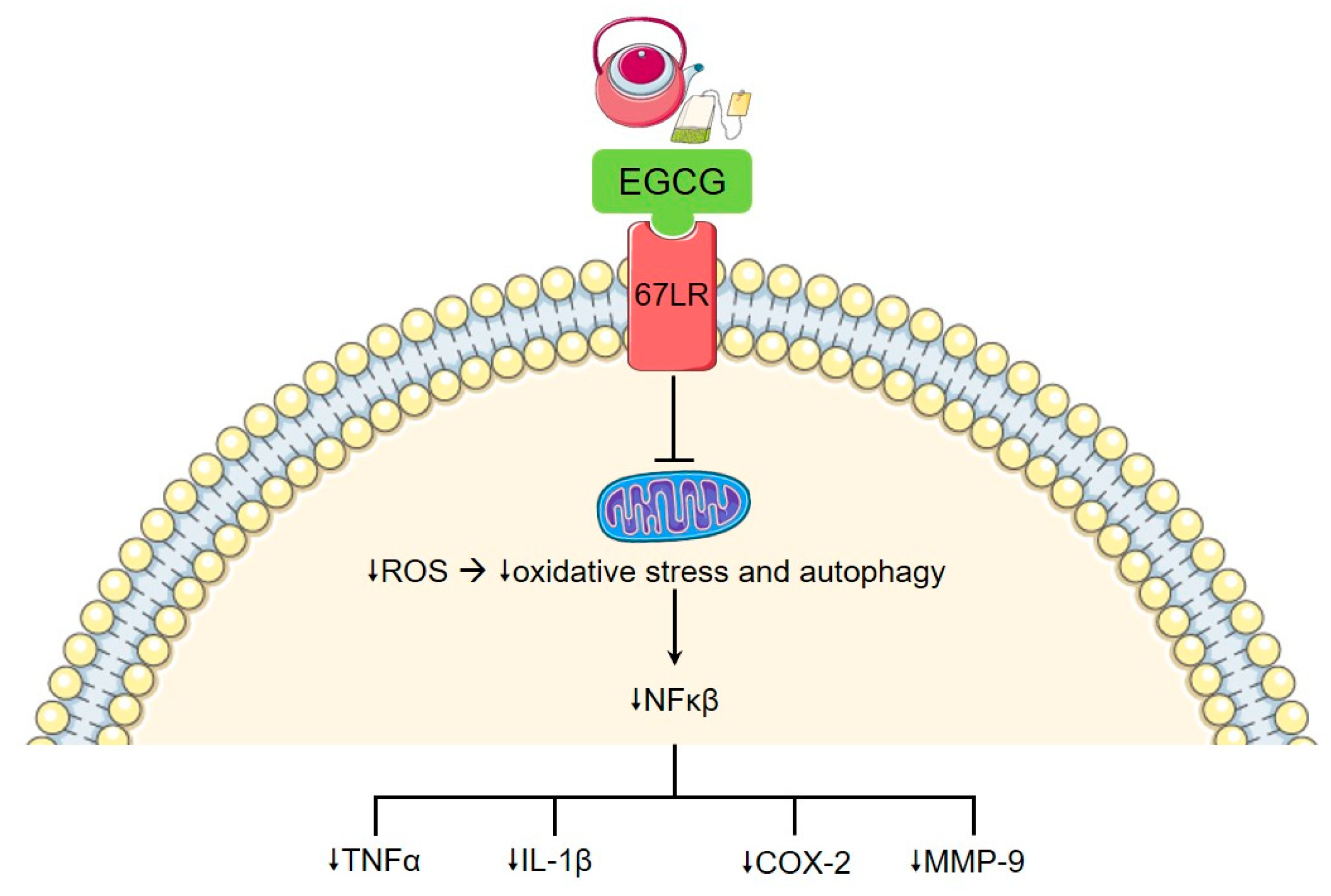
5. Tea and Coffee
6. Cocoa-Based Beverages and Milk
7. Sugar-Sweetened Soft Drinks
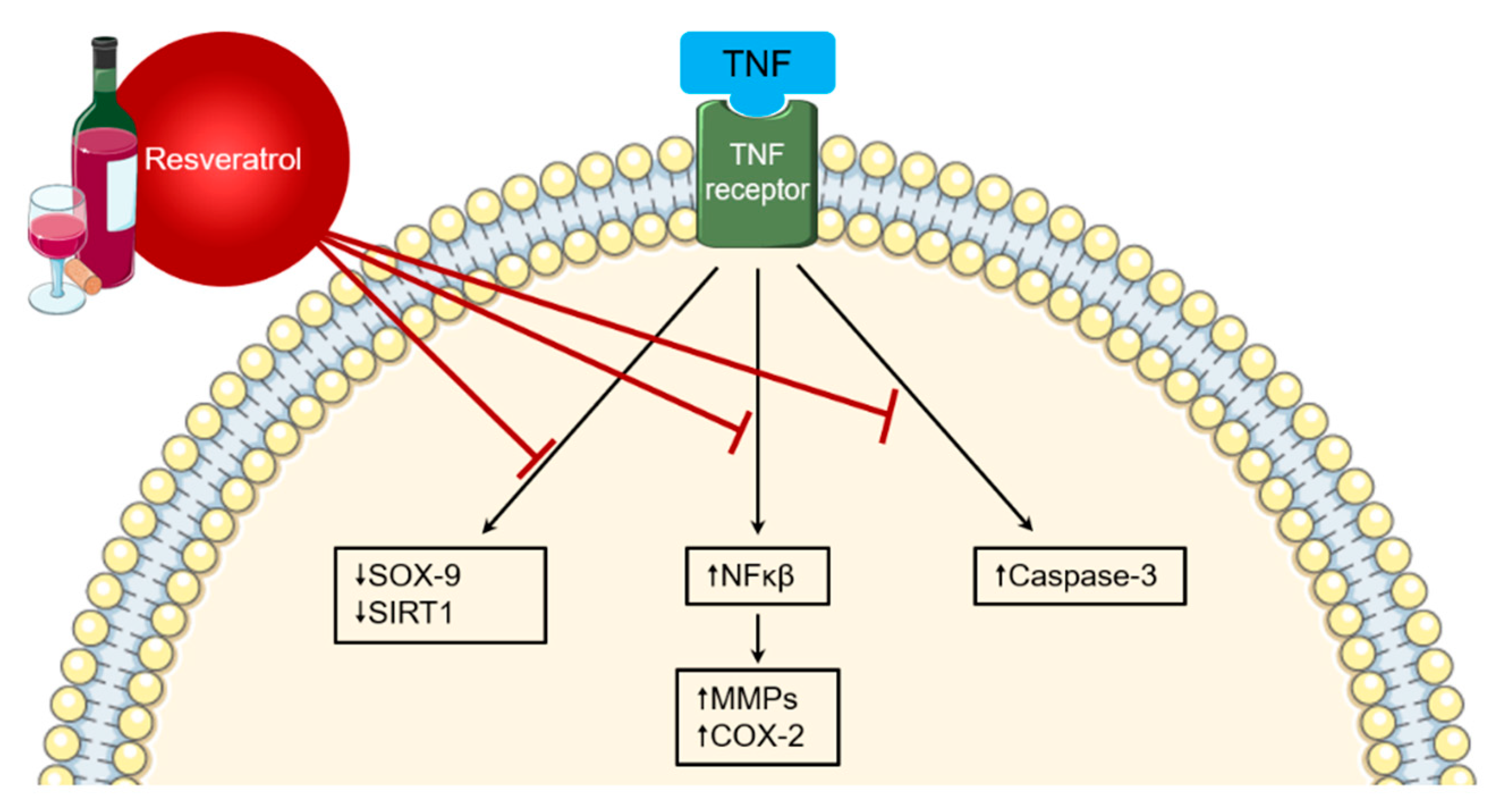
8. Alcohol Intake
9. The Role of Beverages during Treatment of RA
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jawaheer, D.; Seldin, M.F.; Amos, C.I.; Chen, W.; Shigeta, R.; Etzel, C.; Damle, A.; Xiao, X.; Chen, N.; Lum, R.F.; et al. Screening the genome for rheumatoid arthritis susceptibility genes: A replication study and combined analysis of 512 multicase families. Arthritis Rheum. 2003, 48, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Nikiphorou, E. Don’t neglect nutrition in rheumatoid arthritis! RMD Open 2018, 4, e000591. [Google Scholar] [CrossRef] [PubMed]
- Silman, A.J.; Pearson, J.E. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002, 4, S265–S272. [Google Scholar] [CrossRef]
- Catrina, A.I.; Deane, K.D.; Scher, J.U. Gene, environment, microbiome and mucosal immune tolerance in rheumatoid arthritis. Rheumatology 2014, 55, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tedeschi, S.K.; Barbhaiya, M.; Leatherwood, C.L.; Speyer, C.B.; Lu, B.; Costenbader, K.H.; Karlson, E.W.; Sparks, J.A. Impact and Timing of Smoking Cessation on Reducing Risk of Rheumatoid Arthritis Among Women in the Nurses’ Health Studies. Arthritis Rheum. 2019, 71, 914–924. [Google Scholar] [CrossRef]
- Walker, J.G.; Littlejohn, G.O.; McMurray, N.E.; Cutolo, M. Stress system response and rheumatoid arthritis: A multilevel approach. Rheumatology 1999, 38, 1050–1057. [Google Scholar] [CrossRef][Green Version]
- Alunno, A.; Nikiphorou, E.; Philippou, E.; Daien, C.; Wiek, D.; Kouloumas, M.; Cutolo, M. Nutrition in RMDs: Is it really food for thought? Focus on rheumatoid arthritis. BMC Rheumatol. 2020, 4, 1–5. [Google Scholar] [CrossRef]
- Philippou, E.; Petersson, S.D.; Rodomar, C.; Nikiphorou, E. Rheumatoid arthritis and dietary interventions: Systematic review of clinical trials. Nutr. Rev. 2020. [Google Scholar] [CrossRef]
- Dahan, S.; Segal, Y.; Shoenfeld, Y. Dietary factors in rheumatic autoimmune diseases: A recipe for therapy? Nat. Rev. Rheumatol. 2017, 13, 348–358. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Isenberg, D.A. The mosaic of autoimmunity. Immunol. Today 1989, 10, 123–126. [Google Scholar] [CrossRef]
- Cooper, G.S.; Miller, F.W.; Pandey, J.P. The role of genetic factors in autoimmune disease: Implications for environmental research. Environ. Health Perspect. 1999, 107, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kutateladze, T.G. Diet and the epigenome. Nat. Commun. 2018, 9, 3375. [Google Scholar] [CrossRef] [PubMed]
- Ek, W.E.; Tobi, E.W.; Ahsan, M.; Lampa, E.; Ponzi, E.; Kyrtopoulos, S.A.; Georgiadis, P.; Lumey, L.H.; Heijmans, B.T.; Botsivali, M.; et al. Tea and coffee consumption in relation to DNA methylation in four European cohorts. Hum. Mol. Genet. 2017, 26, 3221–3231. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wei, J.; He, X.; An, P.; Wang, H.; Jiang, L.; Shao, D.; Liang, H.; Li, Y.; Wang, F.; et al. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur. J. Cancer 2015, 51, 2820–2832. [Google Scholar] [CrossRef]
- Ozemek, C.; Laddu, D.R.; Arena, R.; Lavie, C.J. The role of diet for prevention and management of hypertension. Curr. Opin. Cardiol. 2018, 33, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Jayalath, V.H.; De Souza, R.J.; Ha, V.; Mirrahimi, A.; Blanco-Mejia, S.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; Wolever, T.M.S.; Beyene, J.; et al. Sugar-sweetened beverage consumption and incident hypertension: A systematic review and meta-analysis of prospective cohorts. Am. J. Clin. Nutr. 2015, 102, 914–921. [Google Scholar] [CrossRef]
- Pase, M.P.; Himali, J.J.; Beiser, A.S.; Aparicio, H.J.; Satizabal, C.L.; Vasan, R.S.; Seshadri, S.; Jacques, P.F. Sugar- and Artificially Sweetened Beverages and the Risks of Incident Stroke and Dementia: A Prospective Cohort Study. Stroke 2017, 48, 1139–1146. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Young, B.A.; Katz, R.; Tucker, K.L.; Carithers, T.C.; Norwood, A.F.; Correa, A. Patterns of beverages consumed and risk of incident kidney disease. Clin. J. Am. Soc. Nephrol. 2018, 14, 49–56. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2018, 195, 74–85. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nat. Cell Biol. 2013, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Noble, E.E.; Hsu, T.M.; Jones, R.B.; Fodor, A.A.; Goran, M.I.; Kanoski, S.E. Early-life sugar consumption affects the rat microbiome independently of obesity. J. Nutr. 2016, 147, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Alderete, T.L.; Kim, J.S.; Millstein, J.; Gilliland, F.D.; Goran, M.I. High intake of dietary fructose in overweight/obese teenagers associated with depletion of Eubacterium and Streptococcus in gut microbiome. Gut Microbes 2019, 10, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Cowan, T.E.; Palmnäs, M.S.A.; Yang, J.; Bomhof, M.R.; Ardell, K.L.; Reimer, R.A.; Vogel, H.J.; Shearer, J. Chronic coffee consumption in the diet-induced obese rat: Impact on gut microbiota and serum metabolomics. J. Nutr. Biochem. 2014, 25, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Nishitsuji, K.; Watanabe, S.; Xiao, J.; Nagatomo, R.; Ogawa, H.; Tsunematsu, T.; Umemoto, H.; Morimoto, Y.; Akatsu, H.; Inoue, K.; et al. Effect of coffee or coffee components on gut microbiome and short-chain fatty acids in a mouse model of metabolic syndrome. Sci. Rep. 2018, 8, 16173. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Bergot, A.S.; Giri, R.; Thomas, R. The microbiome and rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101497. [Google Scholar] [CrossRef]
- Brusca, S.B.; Abramson, S.B.; Scher, J.U. Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr. Opin. Rheumatol. 2014, 26, 101–107. [Google Scholar] [CrossRef]
- Alpizar-Rodriguez, D.; Lesker, T.R.; Gronow, A.; Gilbert, B.; Raemy, E.; Lamacchia, C.; Gabay, C.; Finckh, A.; Strowig, T. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Jena, P.K.; Sheng, L.; Di Lucente, J.; Jin, L.W.; Maezawa, I.; Wan, Y.J.Y. Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet-induced systemic inflammation, microglial activation, and reduced neuroplasticity. FASEB J. 2018, 32, 2866–2877. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Tang, R.; Zhang, G.; Zeng, H.; Wood, R.J.; Liu, Z. High Fat Diet Alters Gut Microbiota and the Expression of Paneth Cell-Antimicrobial Peptides Preceding Changes of Circulating Inflammatory Cytokines. Mediat. Inflamm. 2017, 2017, 9474896. [Google Scholar] [CrossRef]
- Philippou, E.; Nikiphorou, E. Are we really what we eat? Nutrition and its role in the onset of rheumatoid arthritis. Autoimmun. Rev. 2018, 17, 1074–1077. [Google Scholar] [CrossRef]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61 (Suppl. 6), 1402S–1406S. [Google Scholar] [CrossRef]
- Nordmann, A.J.; Suter-Zimmermann, K.; Bucher, H.C.; Shai, I.; Tuttle, K.R.; Estruch, R.; Briel, M. Meta-analysis comparing mediterranean to low-fat diets for modification of cardiovascular risk factors. Am. J. Med. 2011, 124, 841–851.e2. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A journey into a Mediterranean diet and type 2 diabetes: A systematic review with meta-analyses. BMJ Open 2015, 5, e008222. [Google Scholar] [CrossRef]
- Berti, V.; Walters, M.; Sterling, J.; Quinn, C.G.; Logue, M.; Andrews, R.; Matthews, D.C.; Osorio, R.S.; Pupi, A.; Vallabhajosula, S.; et al. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology 2018, 90, e1789–e1798. [Google Scholar] [CrossRef] [PubMed]
- Pocovi-Gerardino, G.; Correa-Rodríguez, M.; Callejas-Rubio, J.-L.; Ríos-Fernández, R.; Martín-Amada, M.; Cruz-Caparros, M.-G.; Rueda-Medina, B.; Ortego-Centeno, N. Beneficial effect of Mediterranean diet on disease activity and cardiovascular risk in systemic lupus erythematosus patients: A cross-sectional study. Rheumatology 2020. [Google Scholar] [CrossRef]
- Johansson, K.; Askling, J.; Alfredsson, L.; Di Giuseppe, D. Mediterranean diet and risk of rheumatoid arthritis: A population-based case-control study. Arthritis Res. Ther. 2018, 20, 175. [Google Scholar] [CrossRef]
- Hu, Y.; Costenbader, K.H.; Gao, X.; Hu, F.B.; Karlson, E.W.; Lu, B. Mediterranean diet and incidence of rheumatoid arthritis in women. Arthritis Rheum. 2015, 67, 597–606. [Google Scholar] [CrossRef] [PubMed]
- García-Morales, J.M.; Lozada-Mellado, M.; Hinojosa-Azaola, A.; Llorente, L.; Ogata-Medel, M.; Pineda-Juárez, J.A.; Alcocer-Varela, J.; Cervantes-Gaytán, R.; Castillo-Martínez, L. Effect of a Dynamic Exercise Program in Combination With Mediterranean Diet on Quality of Life in Women With Rheumatoid Arthritis. JCR J. Clin. Rheumatol. 2020, 26, S116–S122. [Google Scholar] [CrossRef]
- Gioxari, A.; Kaliora, A.C.; Marantidou, F.; Panagiotakos, D.P. Intake of ω-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition 2018, 45, 114–124.e4. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sugioka, Y.; Tada, M.; Okano, T.; Mamoto, K.; Inui, K.; Habu, D.; Koike, T. Monounsaturated fatty acids might be key factors in the Mediterranean diet that suppress rheumatoid arthritis disease activity: The TOMORROW study. Clin. Nutr. 2018, 37, 675–680. [Google Scholar] [CrossRef]
- Remans, P.H.J.; Sont, J.K.; Wagenaar, L.W.; Wouters-Wesseling, W.; Zuijderduin, W.M.; Jongma, A.; Breedveld, F.C.; van Laar, J.M. Nutrient supplementation with polyunsaturated fatty acids and micronutrients in rheumatoid arthritis: Clinical and biochemical effects. Eur. J. Clin. Nutr. 2004, 58, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Petersson, S.; Philippou, E.; Rodomar, C.; Nikiphorou, E. The Mediterranean diet, fish oil supplements and Rheumatoid arthritis outcomes: Evidence from clinical trials. Autoimmun. Rev. 2018, 17, 1105–1114. [Google Scholar] [CrossRef]
- Cleland, L.G.; French, J.K.; Betts, W.H.; Murphy, G.A.; Elliott, M.J. Clinical and biochemical effects of dietary fish oil supplements in rheumatoid arthritis. J. Rheumatol. 1988, 15, 1471–1475. [Google Scholar]
- Kremer, J.M.; Lawrence, D.A.; Jubiz, W.; Digiacomo, R.; Rynes, R.; Bartholomew, L.E.; Sherman, M. Dietary fish oil and olive oil supplementation in patients with Rheumatoid Arthritis clinical and immunologic effects. Arthritis Rheum. 1990, 33, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Alwarith, J.; Kahleova, H.; Rembert, E.; Yonas, W.; Dort, S.; Calcagno, M.; Burgess, N.; Crosby, L.; Barnard, N.D. Nutrition Interventions in Rheumatoid Arthritis: The Potential Use of Plant-Based Diets. A Review. Front. Nutr. 2019, 6, 141. [Google Scholar] [CrossRef]
- Kjeldsen-Kragh, J.; Borchgrevink, C.F.; Laerum, E.; Haugen, M.; Eek, M.; Forre, O.; Mowinkel, P.; Hovi, K. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902. [Google Scholar] [CrossRef]
- Elkan, A.C.; Sjöberg, B.; Kolsrud, B.; Ringertz, B.; Hafström, I.; Frostegård, J. Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: A randomized study. Arthritis Res. Ther. 2008, 10, R34. [Google Scholar] [CrossRef]
- Hafström, I.; Ringertz, B.; Spångberg, A.; Von Zweigbergk, L.; Brannemark, S.; Nylander, I.; Rönnelid, J.; Laasonen, L.; Klareskog, L. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: The effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology 2001, 40, 1175–1179. [Google Scholar] [CrossRef]
- Müller, H.; De Toledo, F.W.; Resch, K.L. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: A systematic review. Scand. J. Rheumatol. 2001, 30, 1–10. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Ben Nessib, D.; Maatallah, K.; Ferjani, H.; Kaffel, D.; Hamdi, W. Impact of Ramadan diurnal intermittent fasting on rheumatic diseases. Clin. Rheumatol. 2020, 39, 2433–2440. [Google Scholar] [CrossRef]
- Siddique, S.; Imran, Y.; Afzal, M.N.; Malik, U. Effect of ramadan fasting on disease activity in patients with rheumatoid arthritis presenting in tertiary care hospital. Pak. J. Med. Sci. 2020, 36, 1032–1035. [Google Scholar] [CrossRef]
- Fraser, D.A.; Thoen, J.; Djøseland, O.; Førre Kjeldsen-Kragh, J. Serum levels of interleukin-6 and dehydroepiandrosterone sulphate in response to either fasting or a ketogenic diet in rheumatoid arthritis patients. Clin. Exp. Rheumatol. 2000, 18, 357–362. [Google Scholar]
- Atzeni, F.; Nucera, V.; Masala, I.F.; Sarzi-Puttini, P.; Bonitta, G. Il-6 Involvement in pain, fatigue and mood disorders in rheumatoid arthritis and the effects of Il-6 inhibitor sarilumab. Pharmacol. Res. 2019, 149, 104402. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta Mol. Cell. Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Nakahara, H.; Song, J.; Sugimoto, M.; Hagihara, K.; Kishimoto, T.; Yoshizaki, K.; Nishimoto, N. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 1521–1529. [Google Scholar] [CrossRef]
- Dayer, J.M.; Choy, E. Therapeutic targets in rheumatoid arthritis: The interleukin-6 receptor. Rheumatology (Oxford) 2009, 49, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Lee, N.R.; Pi, R.H.; Lim, Y.S.; Lee, Y.M.; Lee, J.M.; Jeong, H.S.; Chung, S.H. IL-6 inhibitors for treatment of rheumatoid arthritis: Past, present, and future. Arch. Pharmacal Res. 2015, 38, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, S.; Remuzgo-Martínez, S.; López-Mejías, R.; Genre, F.; Calvo-Alén, J.; Llorente, I.; Aurrecoechea, E.; Ortiz, A.M.; Triguero, A.; Blanco, R.; et al. Rapid beneficial effect of the IL-6 receptor blockade on insulin resistance and insulin sensitivity in non-diabetic patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2018, 37, 465–473. [Google Scholar] [PubMed]
- Kurauti, M.A.; Costa, J.M.; Ferreira, S.M.; Santos, G.J.; Sponton, C.H.G.; Carneiro, E.M.; Telles, G.D.; Chacon-Mikahil, M.P.T.; Cavaglieri, C.R.; Rezende, L.F.; et al. Interleukin-6 increases the expression and activity of insulindegrading enzyme. Sci. Rep. 2017, 7, 46750. [Google Scholar] [CrossRef]
- Silvers, I.J.; Hovell, M.F.; Weisman, M.H.; Mueller, M.R. Assessing physician/patient perceptions in rheumatoid arthritis. A vital component in patient education. Arthritis Rheum. 1985, 28, 300–307. [Google Scholar] [CrossRef]
- Ndosi, M.; Ferguson, R.; Backhouse, M.R.; Bearne, L.; Ainsworth, P.; Roach, A.; Dennison, E.; Cherry, L. National variation in the composition of rheumatology multidisciplinary teams: A cross-sectional study. Rheumatol. Int. 2017, 37, 1453–1459. [Google Scholar] [CrossRef]
- Grotle, M.; Klokkerud, M.; Kjeken, I.; Bremander, A.; Hagel, S.; Strömbeck, B.; Hørslev-Petersen, K.; Meesters, J.; Vlieland, T.V.P.M.; Hagen, K.B.; et al. What’s in the black box of arthritis rehabilitation? A comparison of rehabilitation practice for patients with inflamatory arthritis in Northern Europe. J. Rehabil. Med. 2013, 45, 458–466. [Google Scholar] [CrossRef]
- Danesi, F.; Ferguson, L.R. Could pomegranate juice help in the control of inflammatory diseases? Nutrients 2017, 9, 958. [Google Scholar] [CrossRef]
- Ko, S.H.; Choi, S.W.; Ye, S.K.; Cho, B.L.; Kim, H.S.; Chung, M.H. Comparison of the antioxidant activities of nine different fruits in human plasma. J. Med. Food 2005, 8, 41–46. [Google Scholar] [CrossRef]
- Aviram, M.; Dornfeld, L.; Rosenblat, M.; Volkova, N.; Kaplan, M.; Coleman, R.; Hayek, T.; Presser, D.; Fuhrman, B. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E–deficient mice. Am. J. Clin. Nutr. 2000, 71, 1062–1076. [Google Scholar] [CrossRef]
- Kaplan, M.; Hayek, T.; Raz, A.; Coleman, R.; Dornfeld, L.; Vaya, J.; Aviram, M. Pomegranate Juice Supplementation to Atherosclerotic Mice Reduces Macrophage Lipid Peroxidation, Cellular Cholesterol Accumulation and Development of Atherosclerosis. J. Nutr. 2001, 131, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Zhang, W.; Li, J.; Du, L.; Lv, O.; Zhao, S.; Li, J. Beneficial Effects of Pomegranate on Lipid Metabolism in Metabolic Disorders. Mol. Nutr. Food Res. 2019, 63, e1800773. [Google Scholar] [CrossRef] [PubMed]
- Sohrab, G.; Roshan, H.; Ebrahimof, S.; Nikpayam, O.; Sotoudeh, G.; Siasi, F. Effects of pomegranate juice consumption on blood pressure and lipid profile in patients with type 2 diabetes: A single-blind randomized clinical trial. Clin. Nutr. ESPEN 2019, 29, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Banerjee, N.; Sirven, M.A.; Minamoto, Y.; Markel, M.E.; Suchodolski, J.S.; Talcott, S.T.; Mertens-Talcott, S.U. Pomegranate polyphenolics reduce inflammation and ulceration in intestinal colitis—Involvement of the miR-145/p70S6K1/HIF1α axis in vivo and in vitro. J. Nutr. Biochem. 2017, 43, 107–115. [Google Scholar] [CrossRef]
- Ghavipour, M.; Sotoudeh, G.; Tavakoli, E.; Mowla, K.; Hasanzadeh, J.; Mazloom, Z. Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in Rheumatoid Arthritis patients. Eur. J. Clin. Nutr. 2016, 71, 92–96. [Google Scholar] [CrossRef]
- Pattison, D.J.; Symmons, D.P.; Lunt, M.; Welch, A.; Bingham, S.A.; Day, N.E.; Silman, A.J. Dietary β-cryptoxanthin and inflammatory polyarthritis: Results from a population-based prospective study. Am. J. Clin. Nutr. 2005, 82, 451–455. [Google Scholar] [CrossRef]
- Assimiti, D. The Use of Beetroot as Natural Solutions for Reducing Inflammation—Case Studies from Thailand (P12-046-19). Curr. Dev. Nutr. 2019, 3, nzz035-P12. [Google Scholar] [CrossRef]
- Shepherd, A.I.; Costello, J.T.; Bailey, S.J.; Bishop, N.; Wadley, A.J.; Young-Min, S.; Gilchrist, M.; Mayes, H.; White, D.; Gorczynski, P.; et al. “beet” the cold: Beetroot juice supplementation improves peripheral blood flow, endothelial function, and anti-inflammatory status in individuals with Raynaud’s phenomenon. J. Appl. Physiol. 2019, 127, 1478–1490. [Google Scholar] [CrossRef]
- Pope, J.; Al-beshri, J. Raynaud’s phenomenon secondary to rheumatoid arthritis may be predictive of more erosive disease. Arthritis Res. Ther. 2004, 6, 75. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Manthou, E.; Nakopoulou, T.; Georgakouli, K.; Jamurtas, A. AB1215-HPR Effects of beetroot juice supplementation n endothelial function and markers of inflammation among patients with rheumatoid arthritis. Ann. Rheum. Dis. BMJ 2017, 76, 1536–1537. [Google Scholar] [CrossRef]
- Reyes-Izquierdo, T.; Pietrzkowski, Z.; Argumedo, R.; Shu, C.; Nemzer, B.; Wybraniec, S. Betalain-rich red beet concentrate improves reduced knee discomfort and joint function: A double blind, placebo-controlled pilot clinical study. Nutr. Diet. Suppl. 2014, 6, 9. [Google Scholar] [CrossRef]
- Wang, Y.; Gallegos, J.L.; Haskell-Ramsay, C.; Lodge, J.K. Effects of chronic consumption of specific fruit (berries, citrus and cherries) on CVD risk factors: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.M.U.P.; Caldas, A.P.S.; da Silva, B.P.; Hermsdorff, H.H.M.; Alfenas, R.d.C.G. Effects of blueberry and cranberry consumption on type 2 diabetes glycemic control: A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1816–1828. [Google Scholar] [CrossRef]
- Thimóteo, N.S.B.; Iryioda, T.M.V.; Alfieri, D.F.; Rego, B.E.F.; Scavuzzi, B.M.; Fatel, E.; Lozovoy, M.A.B.; Simão, A.N.C.; Dichi, I. Cranberry juice decreases disease activity in women with rheumatoid arthritis. Nutrition 2019, 60, 112–117. [Google Scholar] [CrossRef]
- Wang, P.; Yamabe, N.; Hong, C.-J.; Bai, H.-W.; Zhu, B.T. Caffeic acid phenethyl ester, a coffee polyphenol, inhibits DNA methylation in vitro and in vivo. Eur. J. Pharmacol. 2020, 887, 173464. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Shenouda, S.M.; Vita, J.A. Effects of flavonoid-containing beverages and EGCG on endothelial function. J. Am. Coll. Nutr. 2007, 26, 366S–372S. [Google Scholar] [CrossRef]
- Wu, D.; Wang, J.; Pae, M.; Meydani, S.N. Green tea EGCG, T cells, and T cell-mediated autoimmune diseases. Mol. Asp. Med. 2012, 33, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Marotte, H.; Ruth, J.H.; Campbell, P.L.; Koch, A.E.; Ahmed, S. Green tea extract inhibits chemokine production, but up-regulates chemokine receptor expression, in rheumatoid arthritis synovial fibroblasts and rat adjuvant-induced arthritis. Rheumatology (Oxford) 2009, 49, 467–479. [Google Scholar] [CrossRef]
- Fechtner, S.; Singh, A.; Chourasia, M.; Ahmed, S. Molecular insights into the differences in anti-inflammatory activities of green tea catechins on IL-1β signaling in rheumatoid arthritis synovial fibroblasts. Toxicol. Appl. Pharmacol. 2017, 329, 112–120. [Google Scholar] [CrossRef]
- Lamichhane, D.; Collins, C.; Constantinescu, F.; Walitt, B.; Pettinger, M.; Parks, C.; Howard, B.V. Coffee and Tea Consumption in Relation to Risk of Rheumatoid Arthritis in the Women’s Health Initiative Observational Cohort. JCR J. Clin. Rheumatol. 2019, 25, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Legrady, D.; Dyer, A.R.; Shekelle, R.B.; Stamler, J.; Liu, K.; Paul, O.; Lepper, M.; Shryock, A.M. Coffee consumption and mortality in the Chicago western electric company study. Am. J. Epidemiol. 1987, 126, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Tverdal, A.; Stensvold, I.; Solvoll, K.; Foss, O.P.; Lund-Larsen, P.; Bjartveit, K. Coffee consumption and death from coronary heart disease in middle aged Norwegian men and women. BMJ 1990, 300, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Je, Y.; Giovannucci, E. Coffee consumption and all-cause and cause-specific mortality: A meta-analysis by potential modifiers. Eur. J. Epidemiol. 2019, 34, 731–752. [Google Scholar] [CrossRef]
- Ritter, M.; Hohenberger, K.; Alter, P.; Herzum, M.; Tebbe, J.; Maisch, M. Caffeine inhibits cytokine expression in lymphocytes. Cytokine 2005, 30, 177–181. [Google Scholar] [CrossRef]
- Horrigan, L.A.; Kelly, J.P.; Connor, T.J. Immunomodulatory effects of caffeine: Friend or foe? Pharmacol. Ther. 2006, 111, 877–892. [Google Scholar] [CrossRef]
- Horrigan, L.A.; Kelly, J.P.; Connor, T.J. Caffeine suppresses TNF-α production via activation of the cyclic AMP/protein kinase A pathway. Int. Immunopharmacol. 2004, 4, 1409–1417. [Google Scholar] [CrossRef]
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Adawi, M.; Amital, H.; Shoenfeld, Y. Coffee and autoimmunity: More than a mere hot beverage! Autoimmun. Rev. 2017, 16, 712–721. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S.C.; Song, G.G. Coffee or tea consumption and the risk of rheumatoid arthritis: A meta-analysis. Clin. Rheumatol. 2014, 33, 1575–1583. [Google Scholar] [CrossRef]
- Heliovaara, M.; Aho, K.; Knekt, P.; Impivaara, O.; Reunanen, A.; Aromaa, A. Coffee consumption, rheumatoid factor, and the risk of rheumatoid arthritis. Ann. Rheum. Dis. 2000, 59, 631–635. [Google Scholar] [CrossRef]
- Montesinos, M.C.; Yap, J.S.; Desai, A.; Posadas, I.; McCrary, C.T.; Cronstein, B.N. Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine: Evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant. Arthritis Rheum. 2000, 43, 656–663. [Google Scholar] [CrossRef]
- Nesher, G.; Mates, M.; Zevin, S. Effect of caffeine consumption on efficacy of methotrexate in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 571–572. [Google Scholar] [CrossRef]
- Soukup, T.; Hloch, K.; Doseděl, M.; Tebbens, J.D.; Nekvindová, J.; Šembera, Š.; Veleta, T.; Pávek, P.; Barvík, I. The influence of coffee intake and genetics on adenosine pathway in rheumatoid arthritis. Pharmacogenomics 2020, 21, 735–749. [Google Scholar] [CrossRef]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The cocoa, cognition, and aging (CoCoA) study-A randomized controlled trial. Am. J. Clin. Nutr. 2014, 101, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Fakler, P.; Stocks, N.P. Effect of cocoa on blood pressure. Cochrane Database Syst. Rev. 2017, 4, CD008893. [Google Scholar] [CrossRef]
- Pereira, T.; Bergqvist, J.; Vieira, C.; Grüner Sveälv, B.; Castanheira, J.; Conde, J. Randomized study of the effects of cocoa-rich chocolate on the ventricle–arterial coupling and vascular function of young, healthy adults. Nutrition 2019, 64, 175–183. [Google Scholar] [CrossRef]
- Mao, T.K.; Powell, J.; Van De Water, J.; Keen, C.L.; Schmitz, H.H.; Hammerstone, J.F.; Gershwin, M.E. The effect of cocoa procyanidins on the transcription and secretion of interleukin 1β in peripheral blood mononuclear cells. Life Sci. 2000, 66, 1377–1386. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Pérez-Cano, F.J.; Pérez-Berezo, T.; Castellote, C.; Franch, A.; Castell, M. Effect of a cocoa flavonoid-enriched diet on experimental autoimmune arthritis. Br. J. Nutr. 2011, 107, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Welsh, C.J.R.; Hanglow, A.C.; Conn, P.; Barker, T.H.W.; Coombs, R.R.A. Early rheumatoid-like synovial lesions in rabbits drinking cow’s milk1: I. Joint pathology. Int. Arch. Allergy Immunol. 1985, 78, 145–151. [Google Scholar] [CrossRef]
- Arntz, O.J.; Pieters, B.C.H.; Oliveira, M.C.; Broeren, M.G.A.; Bennink, M.B.; De Vries, M.; van Lent, P.L.E.M.; Koenders, M.I.; van den Berg, W.B.; van der Kraan, P.M.; et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol. Nutr. Food Res. 2015, 59, 1701–1712. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Abdelghany, T.M.; Omar, H.A.; Arafa, E.S.A.; Alrobaian, M.M.; Maghrabi, I.A. Camel Milk Attenuates Rheumatoid Arthritis Via Inhibition of Mitogen Activated Protein Kinase Pathway. Cell. Physiol. Biochem. 2017, 43, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Khosraviani, S.; Noel, S.; Mohan, D.; Donner, T.; Hamad, A.R.A. Interleukin-10 paradox: A potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine 2015, 74, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Keystone, E.; Wherry, J.; Grint, P. IL-10 as a therapeutic strategy in the treatment of rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 1998, 24, 629–639. [Google Scholar] [CrossRef]
- Sundström, B.; Ljung, L.; Di Giuseppe, D. Consumption of meat and dairy products is not associated with the risk for rheumatoid arthritis among women: A population-based cohort study. Nutrients 2019, 11, 2825. [Google Scholar] [CrossRef]
- Rambod, M.; Nazarinia, M.; Raieskarimian, F. The impact of dietary habits on the pathogenesis of rheumatoid arthritis: A case-control study. Clin. Rheumatol. 2018, 37, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, H.; Chen, H.; Ji, Q.; Huang, S.; Yang, P.; Liu, Z.; Yang, B. The pathogenesis of rheumatoid arthritis is associated with milk or egg allergy. N. Am. J. Med. Sci. 2016, 8, 40–46. [Google Scholar] [CrossRef]
- Ramne, S.; Alves Dias, J.; González-Padilla, E.; Olsson, K.; Lindahl, B.; Engström, G.; Ericson, U.; Johansson, I.; Sonestedt, E. Association between added sugar intake and mortality is nonlinear and dependent on sugar source in 2 Swedish population–based prospective cohorts. Am. J. Clin. Nutr. 2018, 109, 411–423. [Google Scholar] [CrossRef]
- Greenwood, D.C.; Threapleton, D.E.; Evans, C.E.L.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: Systematic review and dose-response meta-analysis of prospective studies. Br. J. Nutr. 2014, 112, 725–734. [Google Scholar] [CrossRef]
- Ferreira-Pêgo, C.; Babio, N.; Bes-Rastrollo, M.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; Serra-Majem, L.; Arós, F.; Fiol, M.; et al. Frequent consumption of sugar- and artificially sweetened beverages and natural and bottled fruit juices is associated with an increased risk of metabolic syndrome in a mediterranean population at high cardiovascular disease risk. J. Nutr. 2016, 146, 1528–1536. [Google Scholar] [CrossRef]
- Hu, F.B. Resolved: There is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes. Rev. 2013, 14, 606–619. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, W.; Wu, R.; Li, J.; Park, S.A.; Tu, E.; Zanvit, P.; Xu, J.; Liu, O.; Cain, A.; et al. High Glucose Intake Exacerbates Autoimmunity through Reactive-Oxygen-Species-Mediated TGF-β Cytokine Activation. Immunity 2019, 51, 671–681.e5. [Google Scholar] [CrossRef]
- Moling, O.; Gandini, L. Sugar and the mosaic of autoimmunity. Am. J. Case Rep. 2019, 20, 1364–1368. [Google Scholar] [CrossRef]
- Hu, Y.; Costenbader, K.H.; Gao, X.; Al-Daabil, M.; Sparks, J.A.; Solomon, D.H.; Hu, F.B.; Karlson, E.W.; Lu, B. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am. J. Clin. Nutr. 2014, 100, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, S.K.; Frits, M.; Cui, J.; Zhang, Z.Z.; Mahmoud, T.; Iannaccone, C.; Lin, T.C.; Yoshida, K.; Weinblatt, M.E.; Shadick, N.A.; et al. Diet and Rheumatoid Arthritis Symptoms: Survey Results From a Rheumatoid Arthritis Registry. Arthritis Rheum. 2017, 69, 1920–1925. [Google Scholar] [CrossRef]
- De Christopher, L.R.; Uribarri, J.; Tucker, K.L. Intake of high-fructose corn syrup sweetened soft drinks, fruit drinks and apple juice is associated with prevalent arthritis in US adults, aged 20–30 years. Nutr. Diabetes 2016, 6, e199. [Google Scholar] [CrossRef]
- Jin, Z.; Xiang, C.; Cai, Q.; Wei, X.; He, J. Alcohol consumption as a preventive factor for developing rheumatoid arthritis: A dose-response meta-analysis of prospective studies. Ann. Rheum. Dis. 2013, 73, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Sageloli, F.; Quesada, J.L.; Fautrel, B.; Salliot, C.; Gaudin, P.; Baillet, A. Moderate alcohol consumption is associated with increased radiological progression in women, but not in men, with early rheumatoid arthritis: Results from the ESPOIR cohort (Étude et Suivi des Polyarthrites Indifférenciées Récentes). Scand. J. Rheumatol. 2018, 47, 440–446. [Google Scholar] [CrossRef]
- Martinez, J.; Moreno, J.J. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem. Pharmacol. 2000, 59, 865–870. [Google Scholar] [CrossRef]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol Suppresses TNF-Induced Activation of Nuclear Transcription Factors NF-κB, Activator Protein-1, and Apoptosis: Potential Role of Reactive Oxygen Intermediates and Lipid Peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef]
- Riveiro-Naveira, R.R.; Valcá Rcel-Ares, M.N.; Almonte-Becerril, M.; Vaamonde-García, C.; Loureiro, J.S.; Hermida-Carballo, L.; López-Peláez, E.; Blanco, F.J.; López-Armada, M.J. Resveratrol lowers synovial hyperplasia, inflammatory markers and oxidative damage in an acute antigen-induced arthritis model. Rheumatology (Oxford) 2016, 55, 1889–1900. [Google Scholar] [CrossRef]
- Yang, G.; Chang, C.C.; Yang, Y.; Yuan, L.; Xu, L.; Ho, C.T.; Li, S. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar] [CrossRef] [PubMed]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Elhakeim, E.H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin. Rheumatol. 2018, 37, 2035–2042. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.B.; White, A.G.; Scarpati, L.M.; Wan, G.; Nelson, W.W. Long-term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin. Ther. 2017, 39, 2216–2229. [Google Scholar] [CrossRef] [PubMed]
- Narum, S.; Westergren, T.; Klemp, M. Corticosteroids and risk of gastrointestinal bleeding: A systematic review and meta-analysis. BMJ Open 2014, 4, e004587. [Google Scholar] [CrossRef]
- Cutolo, M.; Nikiphorou, E. EULAR Online Course on Rheumatic Diseases: Nutrition in Rheumatic Diseases. 2020, Module 42d. Available online: https://www.eular.org/edu_online_course.cfm (accessed on 29 August 2020).
| Nutrient | Age Range (Years)/Physiological State | |||||
|---|---|---|---|---|---|---|
| ≥18 | Pregnancy | Lactation | ||||
| 1st Trimester <12 Weeks | 2nd Trimester 13 < 28 Weeks | 3rd Trimester ≥ 28 Weeks | 0–6 Months Post-Partum | >6 Months Post-Partum | ||
| Total carbohydrates a (E%) | 45–60 | |||||
| Dietary fibre b (g/day) | 25 | |||||
| Total fat a (E%) | 20–35 | 20–35 | 20–35 | |||
| SFA | ALAP | ALAP | ALAP | |||
| LA b (E%) | 4 | 4 | 4 | |||
| ALA b (E%) | 0.5 | 0.5 | 0.5 | |||
| EPA + DHA b (mg/day) | 250 | 250 | 250 | |||
| DHA b (mg/day) | +100–200 c | +100–200 c | ||||
| TFA | ALAP | ALAP | ALAP | |||
| Protein | ||||||
| AR d | 0.66 | +0.52 e g/day | +7.2 e g/day | +23 e g/day | +10 e g/day | +15 e g/day |
| PRI d (g/kg bw/day) | 0.83 | +1 f g/day | +9 f g/day | +28 f g/day | +19 f g/day | +23 f g/day |
| Water b,g (L/day) | ||||||
| Males | 2.5 | |||||
| Females | 2.0 | 2.3 | 2.7 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, M.; Cutolo, M.; Nikiphorou, E. Beverages in Rheumatoid Arthritis: What to Prefer or to Avoid. Nutrients 2020, 12, 3155. https://doi.org/10.3390/nu12103155
Dey M, Cutolo M, Nikiphorou E. Beverages in Rheumatoid Arthritis: What to Prefer or to Avoid. Nutrients. 2020; 12(10):3155. https://doi.org/10.3390/nu12103155
Chicago/Turabian StyleDey, Mrinalini, Maurizio Cutolo, and Elena Nikiphorou. 2020. "Beverages in Rheumatoid Arthritis: What to Prefer or to Avoid" Nutrients 12, no. 10: 3155. https://doi.org/10.3390/nu12103155
APA StyleDey, M., Cutolo, M., & Nikiphorou, E. (2020). Beverages in Rheumatoid Arthritis: What to Prefer or to Avoid. Nutrients, 12(10), 3155. https://doi.org/10.3390/nu12103155






