Effects of Nutritional Interventions in the Control of Musculoskeletal Pain: An Integrative Review
Abstract
1. Introduction
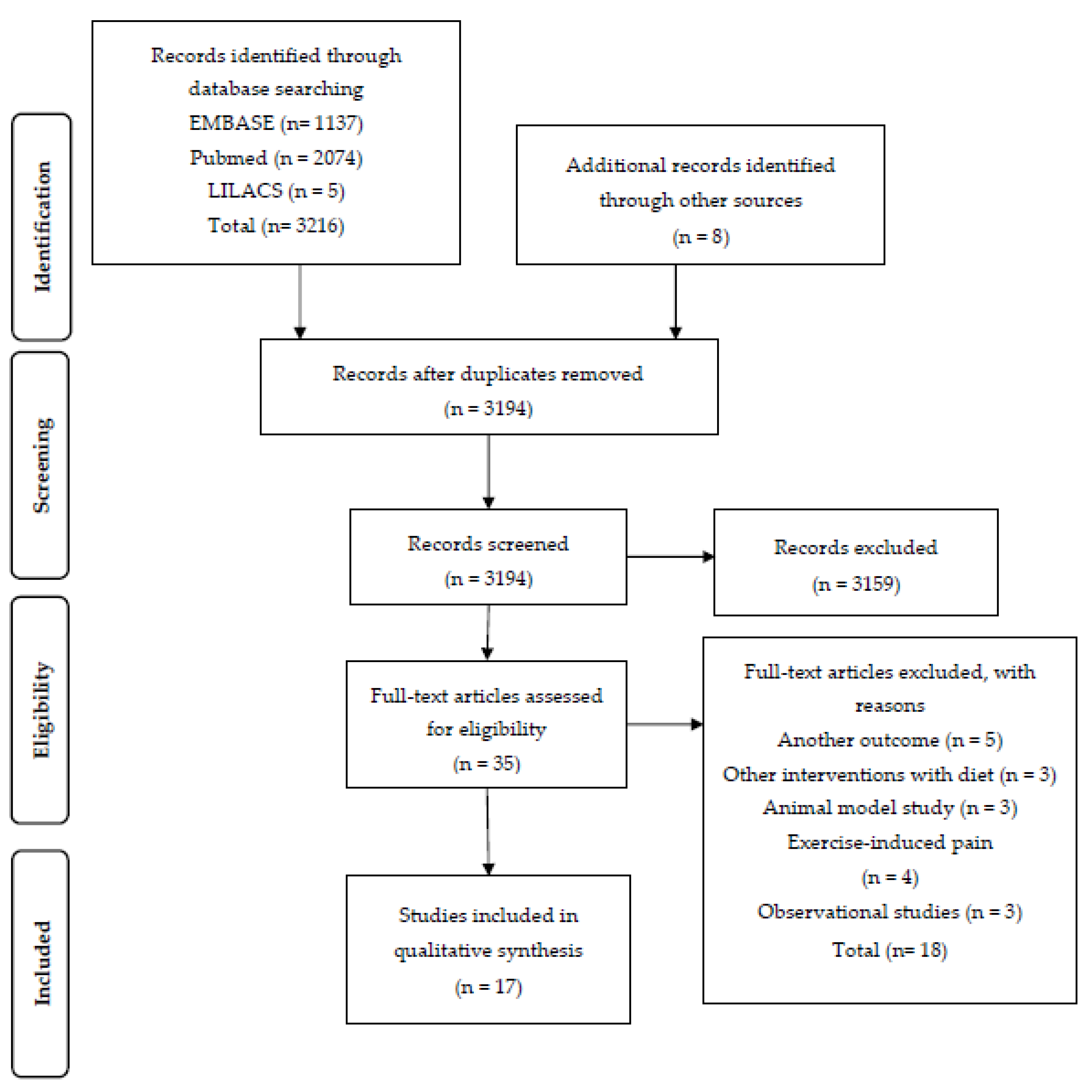
2. Materials and Methods
3. Results
3.1. Results of Nutritional Interventions
3.1.1. Specific Diets
3.1.2. Nutritional Intervention with Fruits
3.1.3. Nutritional Effects of the Use of Olive Oil, Omega-3, and Other Oils
3.1.4. Vitamin D and Other Food Supplements
3.1.5. Pain Assessment Instruments
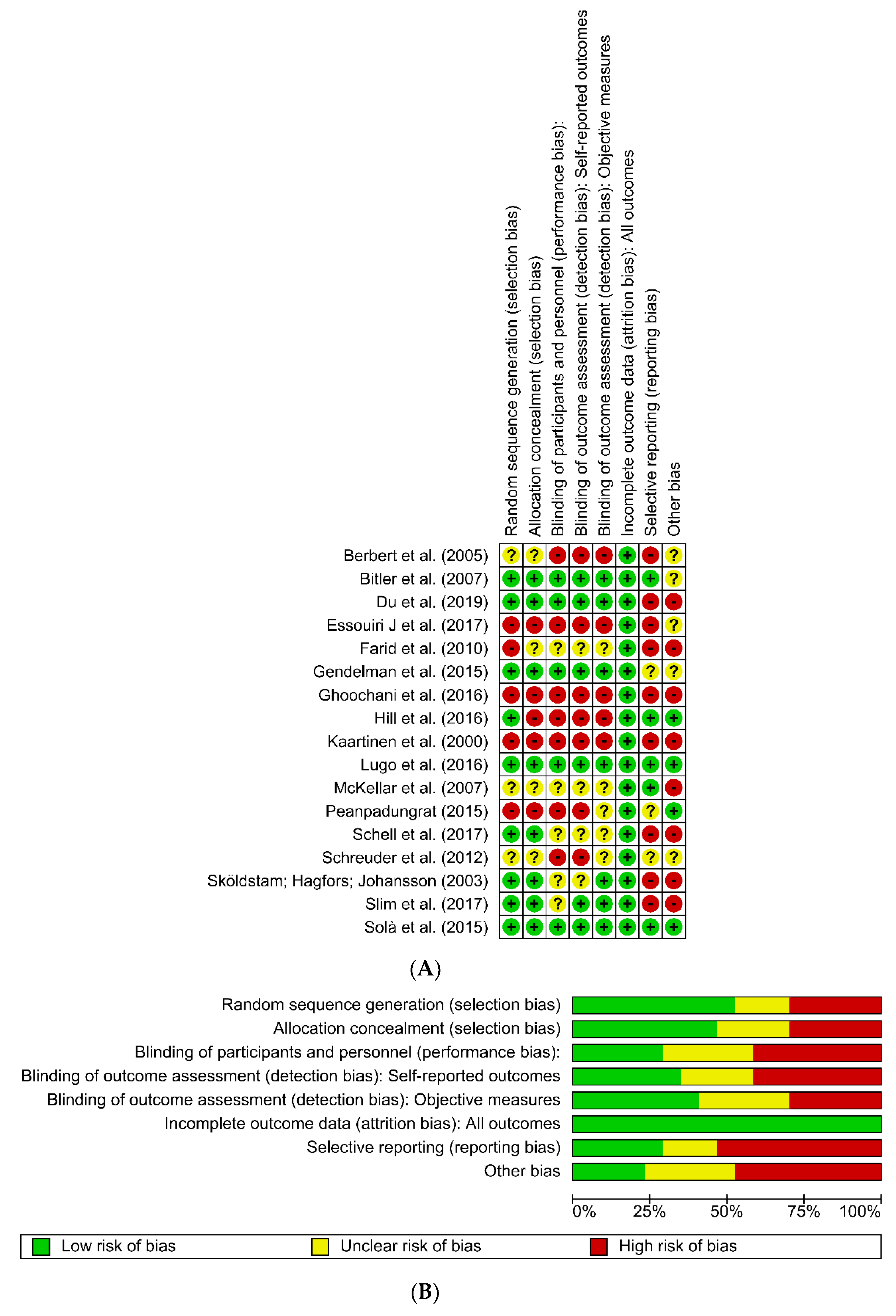
4. Discussion
4.1. Specific Diets
4.2. Nutritional Interventions with Fruits
4.3. Effect of the Use of Olive Oil, Omega-3, and Other Oils
4.4. Vitamins and Other Food Supplements
4.5. Strong Points
4.6. Limitations
4.7. Future Recommendations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clark, S.; Horton, R. Low back pain: A major global challenge. Lancet 2018, 391, 2302. [Google Scholar] [CrossRef]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef]
- Buchbinder, R.; Underwood, M.; Hartvigsen, J.; Maher, C.G. The Lancet Series call to action to reduce low value care for low back pain: An update. Pain 2020, 161, S57–S64. [Google Scholar] [CrossRef]
- Anandacoomarasamy, A.; Caterson, I.; Sambrook, P.; Fransen, M.; March, L. The impact of obesity on the musculoskeletal system. Int. J. Obes. 2008, 32, 211–222. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Fernández-de-Las-Peñas, C.; Graven-Nielsen, T. Basic aspects of musculoskeletal pain: From acute to chronic pain. J. Man. Manip. Ther. 2011, 19, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Skelly, A.C.; Chou, R.; Dettori, J.R.; Turner, J.A.; Friedly, J.L.; Rundell, S.D.; Fu, R.; Brodt, E.D.; Wasson, N.; Winter, C.; et al. AHRQ Comparative Effectiveness Reviews. In Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2018. [Google Scholar]
- Foster, N.E.; Anema, J.R.; Cherkin, D.; Chou, R.; Cohen, S.P.; Gross, D.P.; Ferreira, P.H.; Fritz, J.M.; Koes, B.W.; Peul, W.; et al. Prevention and treatment of low back pain: Evidence, challenges, and promising directions. Lancet 2018, 391, 2368–2383. [Google Scholar] [CrossRef]
- Du, C.; Smith, A.; Avalos, M.; South, S.; Crabtree, K.; Wang, W.; Kwon, Y.H.; Vijayagopal, P.; Juma, S. Blueberries Improve Pain, Gait Performance, and Inflammation in Individuals with Symptomatic Knee Osteoarthritis. Nutrients 2019, 11, 290. [Google Scholar] [CrossRef]
- Essouiri, J.; Harzy, T.; Benaicha, N.; Errasfa, M.; Abourazzak, F.E. Effectiveness of Argan Oil Consumption on Knee Osteoarthritis Symptoms: A Randomized Controlled Clinical Trial. Curr. Rheumatol. Rev. 2017, 13, 231–235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Slim, M.; Calandre, E.P.; Garcia-Leiva, J.M.; Rico-Villademoros, F.; Molina-Barea, R.; Rodriguez-Lopez, C.M.; Morillas-Arques, P. The Effects of a Gluten-free Diet Versus a Hypocaloric Diet Among Patients With Fibromyalgia Experiencing Gluten Sensitivity-like Symptoms: A Pilot, Open-Label Randomized Clinical Trial. J. Clin. Gastroenterol. 2017, 51, 500–507. [Google Scholar] [CrossRef]
- Elma, Ö.; Yilmaz, S.T.; Deliens, T.; Clarys, P.; Nijs, J.; Coppieters, I.; Polli, A.; Malfliet, A. Chronic Musculoskeletal Pain and Nutrition: Where Are We and Where Are We Heading? PM R 2020. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Miccono, A.; Naso, M.; Nichetti, M.; Riva, A.; Guerriero, F.; De Gregori, M.; Peroni, G.; Perna, S. Food pyramid for subjects with chronic pain: Foods and dietary constituents as anti-inflammatory and antioxidant agents. Nutr. Res. Rev. 2018, 31, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Berbert, A.A.; Kondo, C.R.; Almendra, C.L.; Matsuo, T.; Dichi, I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition 2005, 21, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Unger, J.M.; Crew, K.D.; Awad, D.; Dakhil, S.R.; Gralow, J.; Greenlee, H.; Lew, D.L.; Minasian, L.M.; Till, C.; et al. Randomized Multicenter Placebo-Controlled Trial of Omega-3 Fatty Acids for the Control of Aromatase Inhibitor-Induced Musculoskeletal Pain: SWOG S0927. J. Clin. Oncol. 2015, 33, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L.; March, L.M.; Aitken, D.; Lester, S.E.; Battersby, R.; Hynes, K.; Fedorova, T.; Proudman, S.M.; James, M.; Cleland, L.G.; et al. Fish oil in knee osteoarthritis: A randomised clinical trial of low dose versus high dose. Ann. Rheum. Dis. 2016, 75, 23–29. [Google Scholar] [CrossRef]
- Peanpadungrat, P. Efficacy and Safety of Fish Oil in Treatment of Knee Osteoarthritis. J. Med. Assoc. Thai. 2015, 98 (Suppl. 3), S110–S114. [Google Scholar]
- Bitler, C.M.; Matt, K.; Irving, M.; Hook, G.; Yusen, J.; Eagar, F.; Kirschner, K.; Walker, B.; Crea, R. Olive extract supplement decreases pain and improves daily activities in adults with osteoarthritis and decreases plasma homocysteine in those with rheumatoid arthritis. Nutr. Res. 2007, 27, 470–477. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Alcaraz, M.J.; Sanchez-Hidalgo, M.; Fernandez-Bolanos, J.G.; Alarcon-de-la-Lastra, C.; Ferrandiz, M.L. Anti-inflammatory and joint protective effects of extra-virgin olive-oil polyphenol extract in experimental arthritis. J. Nutr. Biochem. 2014, 25, 1275–1281. [Google Scholar] [CrossRef]
- Gaffey, A.; Slater, H.; Porritt, K.; Campbell, J.M. The effects of curcuminoids on musculoskeletal pain: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2017, 15, 486–516. [Google Scholar] [CrossRef]
- Shen, C.L.; Cao, J.J.; Dagda, R.Y.; Chanjaplammootil, S.; Lu, C.; Chyu, M.C.; Gao, W.; Wang, J.S.; Yeh, J.K. Green tea polyphenols benefits body composition and improves bone quality in long-term high-fat diet-induced obese rats. Nutr. Res. 2012, 32, 448–457. [Google Scholar] [CrossRef]
- Shen, C.L.; Smith, B.J.; Lo, D.F.; Chyu, M.C.; Dunn, D.M.; Chen, C.H.; Kwun, I.S. Dietary polyphenols and mechanisms of osteoarthritis. J. Nutr. Biochem. 2012, 23, 1367–1377. [Google Scholar] [CrossRef]
- Staurengo-Ferrari, L.; Ruiz-Miyazawa, K.W.; Pinho-Ribeiro, F.A.; Fattori, V.; Zaninelli, T.H.; Badaro-Garcia, S.; Borghi, S.M.; Carvalho, T.T.; Alves-Filho, J.C.; Cunha, T.M.; et al. Trans-Chalcone Attenuates Pain and Inflammation in Experimental Acute Gout Arthritis in Mice. Front. Pharmacol. 2018, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Lauche, R.; Graf, N.; Cramer, H.; Al-Abtah, J.; Dobos, G.; Saha, F.J. Efficacy of Cabbage Leaf Wraps in the Treatment of Symptomatic Osteoarthritis of the Knee: A Randomized Controlled Trial. Clin. J. Pain 2016, 32, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Selmi, C.; Mao, T.K.; Keen, C.L.; Schmitz, H.H.; Eric Gershwin, M. The anti-inflammatory properties of cocoa flavanols. J. Cardiovasc. Pharmacol. 2006, 47 (Suppl. 2), S163–S171. [Google Scholar] [CrossRef]
- Shen, W.; Xu, Y.; Lu, Y.H. Inhibitory effects of Citrus flavonoids on starch digestion and antihyperglycemic effects in HepG2 cells. J. Agric. Food Chem. 2012, 60, 9609–9619. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Winzenberg, T.; Nguo, K.; Lin, J.; Jones, G.; Ding, C. Association between serum levels of 25-hydroxyvitamin D and osteoarthritis: A systematic review. Rheumatology 2013, 52, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Hirani, V. Vitamin D status and pain: Analysis from the Health Survey for England among English adults aged 65 years and over. Br. J. Nutr. 2012, 107, 1080–1084. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, S.; Tu, L.; Cicuttini, F.; Han, W.; Zhu, Z.; Antony, B.; Wluka, A.; Winzenberg, T.; Meng, T.; Aitken, D.; et al. Effect of Vitamin D Supplementation on Depressive Symptoms in Patients With Knee Osteoarthritis. J. Am. Med. Dir. Assoc. 2019, 20, 1634–1640.e1. [Google Scholar] [CrossRef]
- Misra, D.; Booth, S.L.; Tolstykh, I.; Felson, D.T.; Nevitt, M.C.; Lewis, C.E.; Torner, J.; Neogi, T. Vitamin K deficiency is associated with incident knee osteoarthritis. Am. J. Med. 2013, 126, 243–248. [Google Scholar] [CrossRef]
- Masuko, K.; Murata, M.; Suematsu, N.; Okamoto, K.; Yudoh, K.; Nakamura, H.; Kato, T. A metabolic aspect of osteoarthritis: Lipid as a possible contributor to the pathogenesis of cartilage degradation. Clin. Exp. Rheumatol. 2009, 27, 347–353. [Google Scholar]
- Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Marinho, M.C.S.; Hamann, E.M.; Lima, A.C.d.C.F. Práticas e mudanças no comportamento alimentar na população de Brasília, Distrito Federal, Brasil. Rev. Bras. Saude Mater. Infant. 2007, 7, 251–261. [Google Scholar] [CrossRef][Green Version]
- Chin, S.H.; Huang, W.L.; Akter, S.; Binks, M. Obesity and pain: A systematic review. Int. J. Obes. 2020, 44, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Dewell, A.; Weidner, G.; Sumner, M.D.; Chi, C.S.; Ornish, D. A very-low-fat vegan diet increases intake of protective dietary factors and decreases intake of pathogenic dietary factors. J. Am. Diet. Assoc. 2008, 108, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. JACC Cardiol. Oncol. 2006, 48, 677–685. [Google Scholar]
- Sabia, M.; Kalariya, J. Nutrition and its effects on inflammation and chronic pain. J. Public Health Nutr. 2018, 1, 2. [Google Scholar] [CrossRef]
- Lin, I.; Wiles, L.; Waller, R.; Goucke, R.; Nagree, Y.; Gibberd, M.; Straker, L.; Maher, C.G.; O’Sullivan, P.P.B. What does best practice care for musculoskeletal pain look like? Eleven consistent recommendations from high-quality clinical practice guidelines: Systematic review. Br. J. Sports Med. 2020, 54, 79–86. [Google Scholar] [CrossRef]
- Whittemore, R.; Knafl, K. The integrative review: Updated methodology. J. Adv. Nurs. 2005, 52, 546–553. [Google Scholar] [CrossRef]
- Soares, C.B.; Hoga, L.A.; Peduzzi, M.; Sangaleti, C.; Yonekura, T.; Silva, D.R. Integrative review: Concepts and methods used in nursing. Rev. Esc. Enferm. USP 2017, 48, 335–345. [Google Scholar] [CrossRef]
- Milner, K.A.; Cosme, S. The PICO Game: An Innovative Strategy for Teaching Step 1 in Evidence-Based Practice. Worldviews Evid. Based Nurs. 2017, 14, 514–516. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- McKellar, G.; Morrison, E.; McEntegart, A.; Hampson, R.; Tierney, A.; Mackle, G.; Scoular, J.; Scott, J.A.; Capell, H.A. A pilot study of a Mediterranean-type diet intervention in female patients with rheumatoid arthritis living in areas of social deprivation in Glasgow. Ann. Rheum. Dis. 2007, 66, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Skoldstam, L.; Hagfors, L.; Johansson, G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, K.; Lammi, K.; Hypen, M.; Nenonen, M.; Hanninen, O.; Rauma, A.L. Vegan diet alleviates fibromyalgia symptoms. Scand. J. Rheumatol. 2000, 29, 308–313. [Google Scholar] [CrossRef]
- Schell, J.; Scofield, R.H.; Barrett, J.R.; Kurien, B.T.; Betts, N.; Lyons, T.J.; Zhao, Y.D.; Basu, A. Strawberries Improve Pain and Inflammation in Obese Adults with Radiographic Evidence of Knee Osteoarthritis. Nutrients 2017, 9, 949. [Google Scholar] [CrossRef]
- Ghoochani, N.; Karandish, M.; Mowla, K.; Haghighizadeh, M.H.; Jalali, M.T. The effect of pomegranate juice on clinical signs, matrix metalloproteinases and antioxidant status in patients with knee osteoarthritis. J. Sci. Food Agric. 2016, 96, 4377–4381. [Google Scholar] [CrossRef]
- Farid, R.; Rezaieyazdi, Z.; Mirfeizi, Z.; Hatef, M.R.; Mirheidari, M.; Mansouri, H.; Esmaelli, H.; Bentley, G.; Lu, Y.; Foo, Y.; et al. Oral intake of purple passion fruit peel extract reduces pain and stiffness and improves physical function in adult patients with knee osteoarthritis. Nutr. Res. 2010, 30, 601–606. [Google Scholar] [CrossRef]
- Lugo, J.P.; Saiyed, Z.M.; Lane, N.E. Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: A multicenter randomized, double-blind, placebo-controlled study. Nutr. J. 2016, 15, 14. [Google Scholar] [CrossRef]
- Sola, R.; Valls, R.M.; Martorell, I.; Giralt, M.; Pedret, A.; Taltavull, N.; Romeu, M.; Rodriguez, A.; Morina, D.; Lopez de Frutos, V.; et al. A low-fat yoghurt supplemented with a rooster comb extract on muscle joint function in adults with mild knee pain: A randomized, double blind, parallel, placebo-controlled, clinical trial of efficacy. Food Funct. 2015, 6, 3531–3539. [Google Scholar] [CrossRef]
- Gendelman, O.; Itzhaki, D.; Makarov, S.; Bennun, M.; Amital, H. A randomized double-blind placebo-controlled study adding high dose vitamin D to analgesic regimens in patients with musculoskeletal pain. Lupus 2015, 24, 483–489. [Google Scholar] [CrossRef]
- Schreuder, F.; Bernsen, R.M.; van der Wouden, J.C. Vitamin D supplementation for nonspecific musculoskeletal pain in non-Western immigrants: A randomized controlled trial. Ann. Fam. Med. 2012, 10, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Noale, M.; Solmi, M.; Rizzoli, R.; Vaona, A.; Demurtas, J.; Crepaldi, G.; Maggi, S. Adherence to a Mediterranean diet is associated with lower incidence of frailty: A longitudinal cohort study. Clin. Nutr. 2018, 37, 1492–1497. [Google Scholar] [CrossRef]
- Brain, K.; Burrows, T.L.; Rollo, M.E.; Chai, L.K.; Clarke, E.D.; Hayes, C.; Hodson, F.J.; Collins, C.E. A systematic review and meta-analysis of nutrition interventions for chronic noncancer pain. J. Hum. Nutr. Diet. 2019, 32, 198–225. [Google Scholar] [CrossRef]
- Towery, P.; Guffey, J.S.; Doerflein, C.; Stroup, K.; Saucedo, S.; Taylor, J. Chronic musculoskeletal pain and function improve with a plant-based diet. Complement. Ther. Med. 2018, 40, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Marum, A.P.; Moreira, C.; Tomas-Carus, P.; Saraiva, F.; Guerreiro, C.S. A low fermentable oligo-di-mono-saccharides and polyols (FODMAP) diet is a balanced therapy for fibromyalgia with nutritional and symptomatic benefits. Nutr. Hosp. 2017, 34, 667–674. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2019, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Chang, C.B.; Lee, D.-C.; Lee, J.-Y. Relationship between total fruit and vegetable intake and self-reported knee pain in older adults. J. Nutr. Health Aging 2017, 21, 750–758. [Google Scholar] [CrossRef]
- Rayman, M.P. Diet, nutrition and osteoarthritis. BMC Musculoskelet. Disord. 2015, 16, S7. [Google Scholar] [CrossRef][Green Version]
- Gray, P.; Chappell, A.; Jenkinson, A.M.; Thies, F.; Gray, S.R. Fish oil supplementation reduces markers of oxidative stress but not muscle soreness after eccentric exercise. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 206–214. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. The effects of ingestion of omega-3 fatty acids on perceived pain and external symptoms of delayed onset muscle soreness in untrained men. Clin. J. Sport Med. 2009, 19, 115–119. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Yanagimoto, K.; Nakazato, K.; Hayamizu, K.; Ochi, E. Eicosapentaenoic and docosahexaenoic acids-rich fish oil supplementation attenuates strength loss and limited joint range of motion after eccentric contractions: A randomized, double-blind, placebo-controlled, parallel-group trial. Eur. J. Appl. Physiol. 2016, 116, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.J.; Katz, J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 2007, 129, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Bae, S.C.; Song, G.G. Omega-3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: A meta-analysis. Arch. Med. Res. 2012, 43, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.E.; Moghaddami, M.; Mas, E.; Phillips, M.; Cleland, L.G.; Mori, T.A. Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot. Essent. Fatty Acids 2016, 107, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, R.J.; Dilisio, M.F.; Agrawal, D.K. Vitamin D and Its Effects on Articular Cartilage and Osteoarthritis. Orthop. J. Sports Med. 2017, 5, 2325967117711376. [Google Scholar] [CrossRef] [PubMed]
- Gencoglu, H.; Orhan, C.; Sahin, E.; Sahin, K. Undenatured Type II Collagen (UC-II) in Joint Health and Disease: A Review on the Current Knowledge of Companion Animals. Animals 2020, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.; Crawford, C.; Berry, K.; Deuster, P. Conditional Recommendations for Specific Dietary Ingredients as an Approach to Chronic Musculoskeletal Pain: Evidence-Based Decision Aid for Health Care Providers, Participants, and Policy Makers. Pain Med. 2019, 20, 1430–1448. [Google Scholar] [CrossRef]
- Kongsted, A.; Hartvigsen, J.; Boyle, E.; Ris, I.; Kjaer, P.; Thomassen, L.; Vach, W. GLA:D® Back: Group-based patient education integrated with exercises to support self-management of persistent back pain—Feasibility of implementing standardised care by a course for clinicians. Pilot Feasibility Stud. 2019, 5, 65. [Google Scholar] [CrossRef]
- Palsson, T.S.; Boudreau, S.; Høgh, M.; Herrero, P.; Bellosta-Lopez, P.; Domenech-Garcia, V.; Langella, F.; Gagni, N.; Christensen, S.W.; Villumsen, M. Education as a strategy for managing occupational-related musculoskeletal pain: A scoping review. BMJ Open 2020, 10, e032668. [Google Scholar] [CrossRef]
- Kjaer, P.; Kongsted, A.; Ris, I.; Abbott, A.; Rasmussen, C.D.N.; Roos, E.M.; Skou, S.T.; Andersen, T.E.; Hartvigsen, J. GLA:D® Back group-based patient education integrated with exercises to support self-management of back pain-development, theories and scientific evidence. BMC Musculoskelet. Disord. 2018, 19, 418. [Google Scholar] [CrossRef]
- Qaseem, A.; Wilt, T.J.; McLean, R.M.; Forciea, M.A. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2017, 166, 514–530. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.; Mayo-Wilson, E.; Montgomery, P.; Macdonald, G.; Michie, S.; Hopewell, S.; Moher, D. CONSORT-SPI 2018 Explanation and Elaboration: Guidance for reporting social and psychological intervention trials. Trials 2018, 19, 406. [Google Scholar] [CrossRef] [PubMed]
| Authors/Year/ Country | Population | Type of Study | Follow-Up Period | Intervention | Outcomes Assessed | Pain Assessment Instrument Used | Main Results | Reduction of Pain |
|---|---|---|---|---|---|---|---|---|
| Slim et al. (2017) [10] Spain | n = 75 patients with fibromyalgia Age > 18 years | Randomized clinical trial | 24 weeks | Group 1: Gluten-free diet (n = 35) Group 2: Low-calorie diet (n = 40) | Pain | Brief Pain Inventory-Short Form (BPI-SF) | There was a slight reduction in pain that did not differ significantly between the two study groups (p = 0.982) | No |
| McKellar et al. (2007) [43] United Kingdom | n = 130 women with rheumatoid arthritis Age: 30–70 years | Pilot study of dietary intervention | 6 weeks | Group 1: Mediterranean diet (n = 75) Group 2: Healthy control diet (n = 55) | Pain Stiffness Inflammatory marker: CRP and IL-6 | Global Pain Scale | Pain score was lower in group 1 (p = 0.011 and 0.049) than that in the control group, and morning stiffness (p = 0.041) was more predominantly observed in group 1 compared to the control group | Yes |
| Sköldstam; Hagfors; Johansson (2003) [44] Sweden | n = 51 Age: 33–75 years | Randomized, parallel study | 13 weeks | Group1: Mediterranean diet (n = 26) Group 2: Control (regular diet) (n = 25) | Pain Swelling of the joints Stiffness | VAS | In group 1 there was an improvement in joint swelling (p = 0.001), and a reduction in pain (p = 0.006). | Yes |
| Kaartinen et al. (2000) [45] Finland | N = 28 with fibromyalgia Age: 34–62 years | Non-randomized clinical trial | 13 weeks | Group 1: Vegan diet “living food”: uncooked foods, fruits, vegetables, mushrooms, nuts, seeds, legumes, and cereals (n = 18) Group 2: Control with omnivorous diet (n = 15) | Pain Stiffness | VAS | There was a reduction in pain observed on the visual analogue pain scale (p = 0.005) and joint stiffness (p = 0.001) in group 1. | Yes |
| Authors/Year/ Country | Population | Type of Study | Follow-Up Period | Intervention | Outcomes Assessed | Pain Assessment Instrument Used | Main Results | Reduction of Pain |
|---|---|---|---|---|---|---|---|---|
| Du et al. (2019) [8] United States | n = 63 adults with self-reported symptomatic osteoarthritis Age: 45–79 years | Randomized, double-blind | 17 weeks | Group 1: 40-g freeze-dried blueberry powder, in 20-g packs for daily consumption. Consumed twice a day (n = 27) Placebo group 2: Consumption of 40 g of “control” placebo powder daily, divided in 20-g packs, consumed twice a day (n = 22) | Pain Stiffness Inflammatory markers: Interleukin (IL)-1β, IL-6, IL-10, IL-13, TNF-α, MMP-3, MMP-13, and MCP-1 | WOMAC questionnaire | There was a significant reduction of pain in the treatment group with blueberry (p < 0.05) There were no significant changes in the plasma concentrations of inflammatory markers in the treatment group (p > 0.05) | Yes |
| Schell et al. (2017) [46] United States | n = 17 adults diagnosed with osteoarthritis of the knee Average age: 57 ± 7 years | Randomized, double-blind | 26 weeks | Group 1: 50 g of freeze-dried strawberry beverage consumed twice a day (n = a) Group 2: Placebo powder (n = a) | Pain Inflammatory markers: Interleukin (IL)-6, IL-1β, and MMP-3 and MMP-8 | ICOAP Pain Questionnaire | Significant reduction of pain (all p < 0.05) in group 1 A significant reduction in the biomarkers interleukin (IL) -6, IL-1β after treatment with strawberry versus control (all p < 0.05). | Yes |
| Ghoochani et al. (2016) [47] Iran | n = 38 patients with osteoarthritis of the knee Age: 30–80 years | Randomized clinical trial | 6 weeks | Group 1: pomegranate juice intervention (n = 19). Consumption of 200 mL without added sugar Group 2 (control): Usual lifestyle (n = 19) | Pain Stiffness Inflammatory markers: MMP-1, MMP-13 | WOMAC questionnaire | Group 1 patients reported a significant reduction in stiffness (p = 0.00), but there was no reduction in pain scores in group 1 (p = 0.49) and group 2 (p = 0.13) There were significant differences between the two groups in relation to MMP-1 (p = 0.05) and MMP-13 (p = 0.02) | No |
| Farid et al. (2010) [48] Iran | N = 33 adults with osteoarthritis of the knee Age: 25–65 years | Randomized, double-blind, placebo-controlled study, with parallel group design | 8 weeks | Group 1: Passion fruit peel extract (n = 17) Group 2 (placebo): pills with inactive ingredients without therapeutic activity and identical appearance (n = 16) | Pain | WOMAC questionnaire | There was a significant reduction in physical function after 30 days and pain after 60 days in group 1 (p < 0.001) | Yes |
| Authors/Year/ Country | Population | Type of Study | Follow-up Period | Intervention | Outcomes Assessed | Pain Assessment Instrument | Main Results | Reduction of Pain |
|---|---|---|---|---|---|---|---|---|
| Essouiri J et al. (2017) [9] Morocco | n = 100 patients with osteoarthritis of the knee Average age: 58.24 ± 7.2 years | Randomized clinical trial | 8 weeks | Group 1: argan oil to be consumed each morning (30 mL per day) (n = 51) Group 2: no treatment (n = 49) | Pain | VAS and WOMAC questionnaire | More significant reductions in pain were found by the VAS (p = 0.02) and WOMAC questionnaire (p < 0.0001) in group 1 compared to group 2 | Yes |
| Hill et al. (2016) [15] Australia | n = 202 patients with osteoarthritis of the knee and pain Age: >40 years | Randomized clinical trial | 104 weeks | Group 1. High dose of fish oil (omega-3 fatty acids 15 mL/day) (n = 101) Group 2. Low dose of fish oil (omega-3 fatty acids and canola oil 1: 9, 0.45 g) 15 mL/day. (n = 101) | Pain | WOMAC questionnaire | There was a greater reduction in pain scores at 2 years of follow-up in group 2 compared with group 1 There was no statistically significant difference between the two groups in a year of segment (p = 0.06) | Yes |
| Peanpadungrat (2015) [16] Thailand | n = 75 adults with osteoarthritis Age: 40–75 years | Randomized clinical trial | 12 weeks | - Group 1. Without fish oil supplement (n = 25) - Group 2. Fish oil supplementation 1000 mg daily for 8 weeks (n = 25) - Group 3. Fish oil supplementation 2000 mg daily for 8 weeks (n = 25) | Pain Stiffness | WOMAC questionnaire | There was a significant reduction in pain and stiffness in groups 1 and 2 (p < 0.0001) | Yes |
| Bitler et al. (2007) [17] United States | n = 90 adults with osteoarthritis Age: 55–75 years | Double-blind, placebo-controlled randomized clinical trial Consumption of 2 capsules twice per day (100 mg) | 8 weeks | Group 1: Olive oil capsules rich in polyphenols. (n = 43). Group 2: Placebo (n = 47). | Pain Inflammatory markers: IL-1β, IL-6, IL-8 | VAS | There was a significant reduction of pain in the treatment group (p = 0.05) | Yes |
| Berbert et al. (2005) [13] Brazil | n = 43 patients Age: 20–73 years | Randomized clinical trial | 12 and 24 weeks | Group 1. Placebo (soybean oil) (n = 13) Group 2. Omega-3 fish oil (3 g/d) (n = 13) Group 3. Fish oil - omega-3 fatty acids (3 g/d) and 9.6mL of olive oil (n = 17) | Pain Inflammatory marker: CRP | VAS | A more statistically significant reduction (p < 0.05) in the intensity of joint pain in groups 2 and 3 compared to group 1 was observed There was no statistically significant change in CRP | Yes |
| Authors/Year/ Country | Population | Type of Study | Follow-Up Period | Intervention | Outcomes Assessed | Pain Assessment Instrument | Main Results | Reduction of Pain |
|---|---|---|---|---|---|---|---|---|
| Lugo et al. (2016) [49] United States | n = 190 adults with osteoarthritis of the knee Age: 40–75 years | Randomized, double-blind, placebo-controlled clinical study | 25 weeks | Group 1: undenatured type II collagen (40 mg) (n = 53) Group 2. placebo (n = 54) | Pain Stiffness Physical function Inflammatory markers: CRP, IL-6, MMP-3 | WOMAC questionnaire | Significant reduction for all three WOMAC subscales in group 1: pain (p = 0.0003 vs. placebo), stiffness (p = 0.004 versus placebo), physical function (p = 0.007 vs. placebo) | Yes |
| Solà et al. (2015) [50] Spain | n = 80 adults with osteoarthritis of the knee Average age: 42.52 ± 13.16 years | Randomized, double-blind, placebo-controlled parallel study | 12 weeks | Group 1. skimmed yogurt (125 mL d (1)) supplemented with 80 mg d (−1) of the crest of the rooster “roostercombextract (RCE) rich in hyaluronicacid” (n = 40) Group 2. Placebo-yogurt (n = 40) | Pain | VAS | There were no significant differences between the groups for the inflammatory markers | No |
| Gendelman et al. (2015) [51] Israel | n = 74 patients with musculoskeletal pain > 6 months Age: >18 years Follow-up period: 13 weeks | Randomized, double-blind and controlled study | 13 weeks | Group 1. 4000 IU of vitamin D3 orally (4 gel capsules of 1000 units) (n = 36) Group 2. Placebo (n = 38) Both: regular pain reliever for 3 months | Pain Inflammatory markers: CRP, IL-6, TNF-α | VAS | There were no statistically significant differences between the intervention and control groups in relation to pain (p = not reported) TNFa levels dropped by 54.3% (after 6 weeks, p < 0.026) in the group treated with vitamin D | Yes |
| Schreuder et al. (2012) [52] Netherlands | n = 84 adults with musculoskeletal pain Age: 18–60 years | Randomized controlled study | 6 weeks | Group 1. Vitamin D (150,000 IU of vitamin D (3) orally) (n = 44) Group 2. Placebo (n = 40) | Pain | VAS | Group 1 had significantly lower pain reduction than the placebo group (p < 0.001) | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendonça, C.R.; Noll, M.; Castro, M.C.R.; Silveira, E.A. Effects of Nutritional Interventions in the Control of Musculoskeletal Pain: An Integrative Review. Nutrients 2020, 12, 3075. https://doi.org/10.3390/nu12103075
Mendonça CR, Noll M, Castro MCR, Silveira EA. Effects of Nutritional Interventions in the Control of Musculoskeletal Pain: An Integrative Review. Nutrients. 2020; 12(10):3075. https://doi.org/10.3390/nu12103075
Chicago/Turabian StyleMendonça, Carolina Rodrigues, Matias Noll, Maria Clara Rezende Castro, and Erika Aparecida Silveira. 2020. "Effects of Nutritional Interventions in the Control of Musculoskeletal Pain: An Integrative Review" Nutrients 12, no. 10: 3075. https://doi.org/10.3390/nu12103075
APA StyleMendonça, C. R., Noll, M., Castro, M. C. R., & Silveira, E. A. (2020). Effects of Nutritional Interventions in the Control of Musculoskeletal Pain: An Integrative Review. Nutrients, 12(10), 3075. https://doi.org/10.3390/nu12103075







