Anti-Hypertensive Effects of Peptides Derived from Rice Bran Protein
Abstract
1. Introduction
2. Anti-Hypertensive Peptides Isolated from Rice Bran Protein
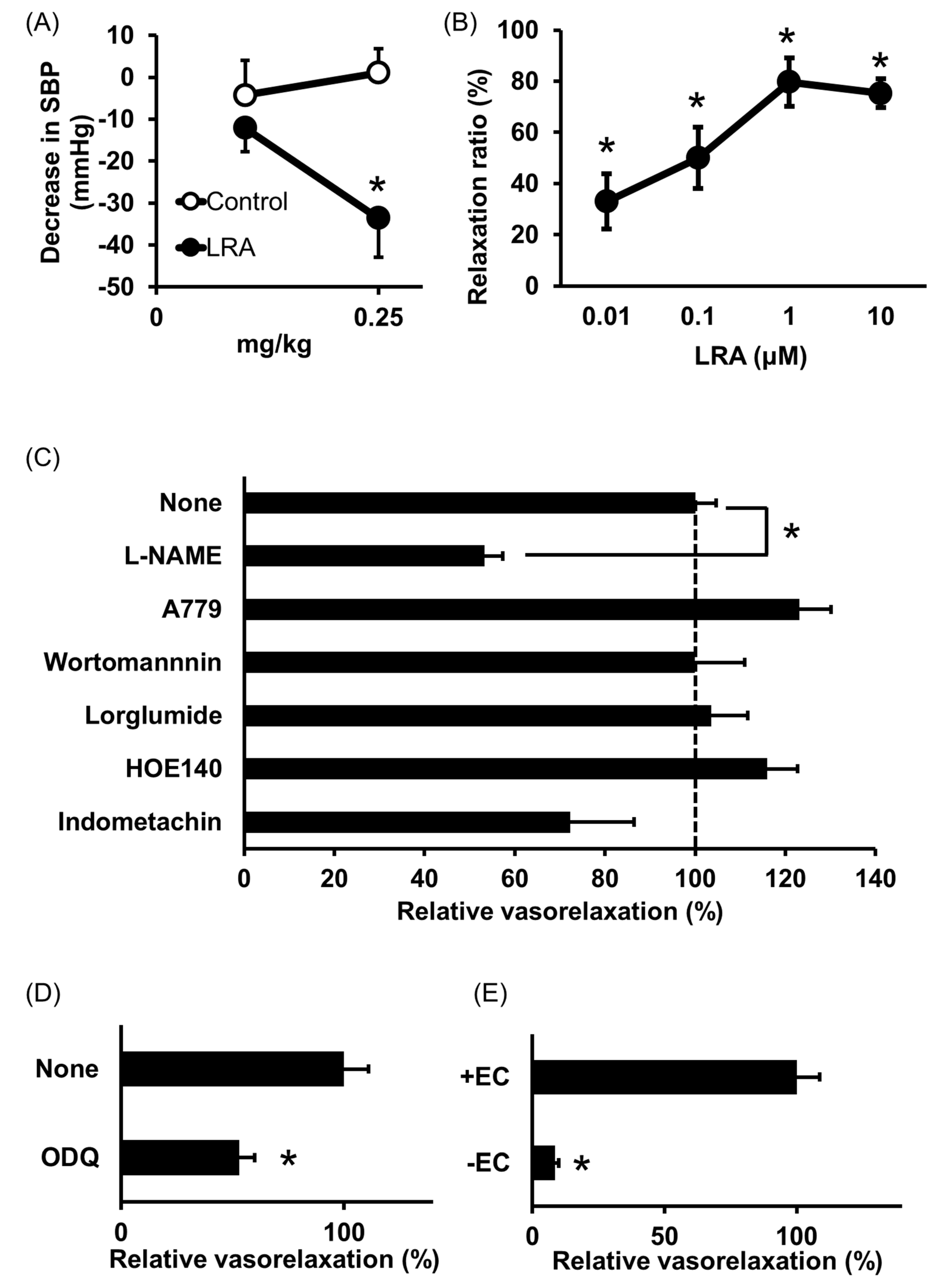
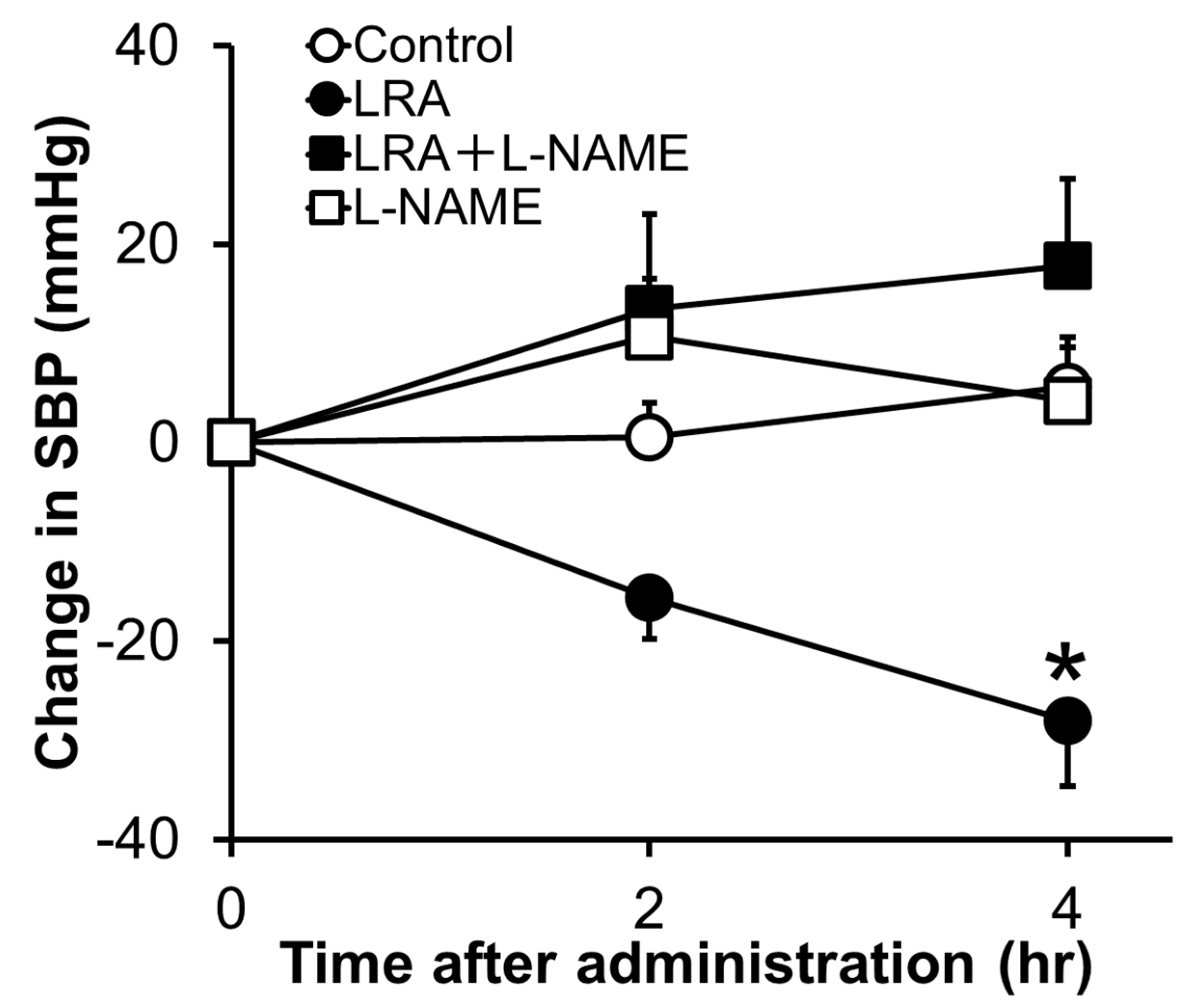
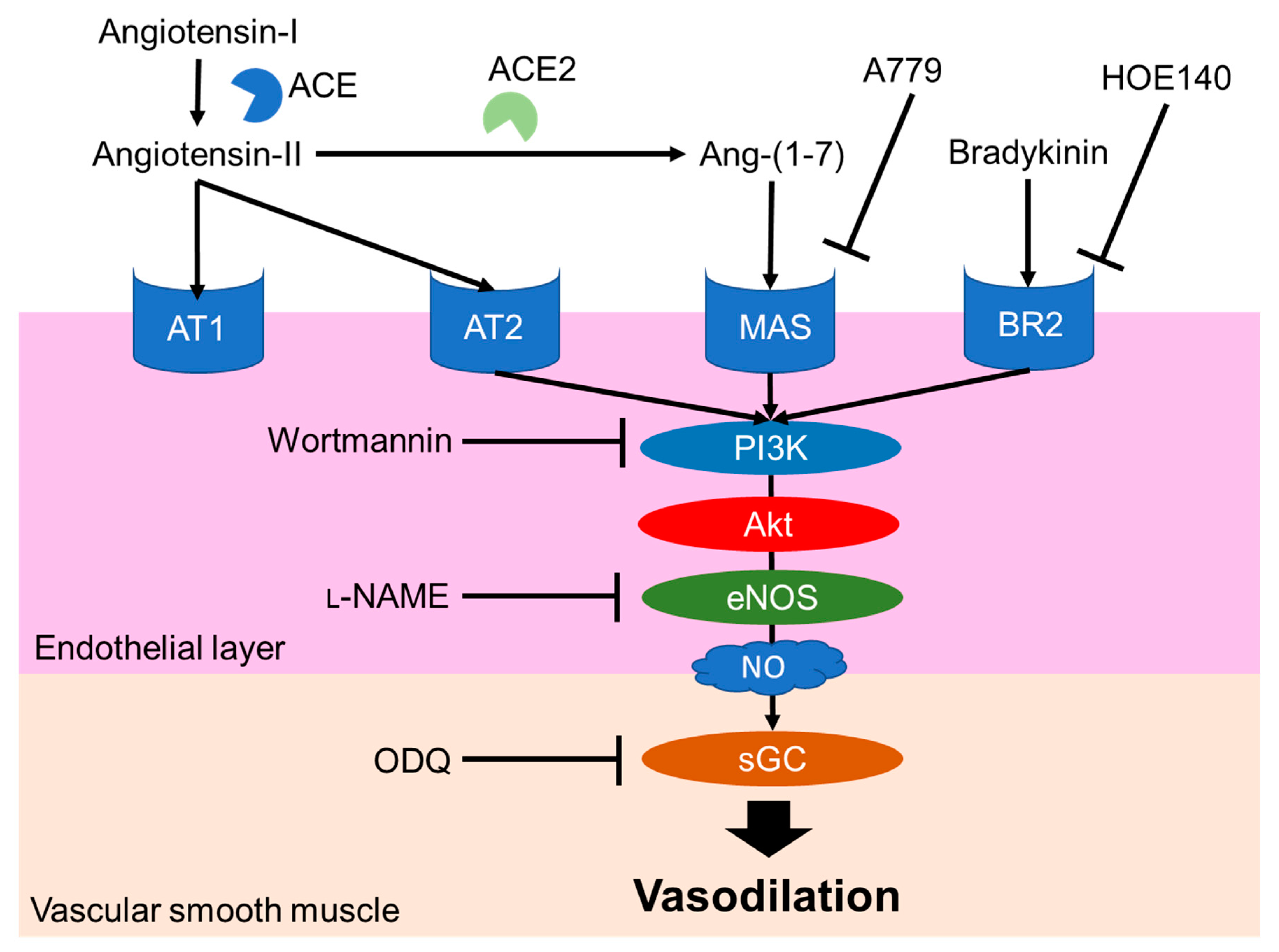
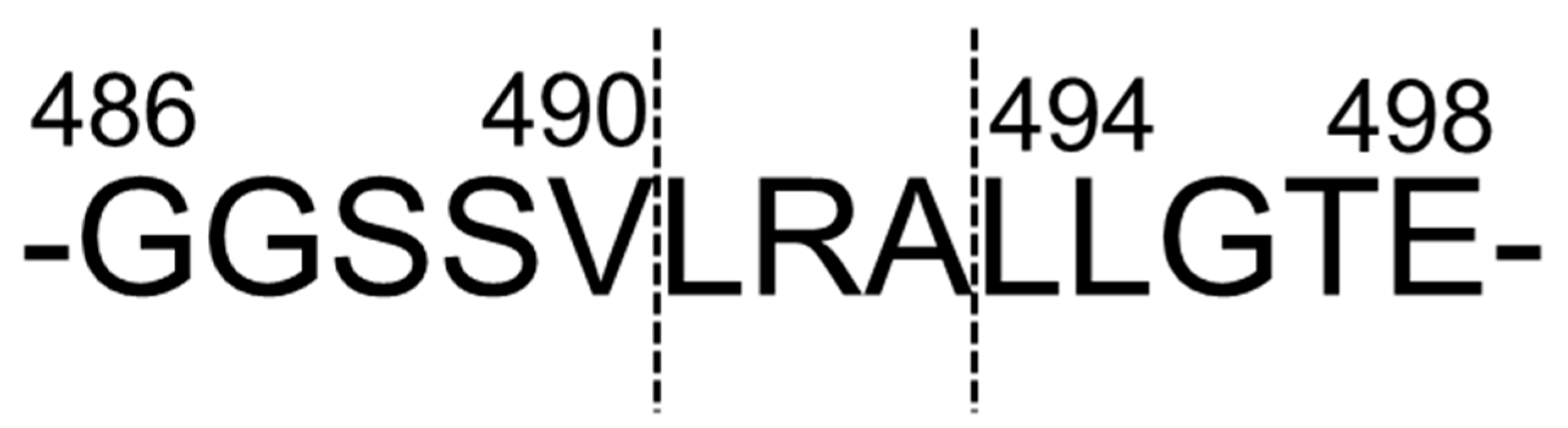
2.1. Leu-Arg-Ala
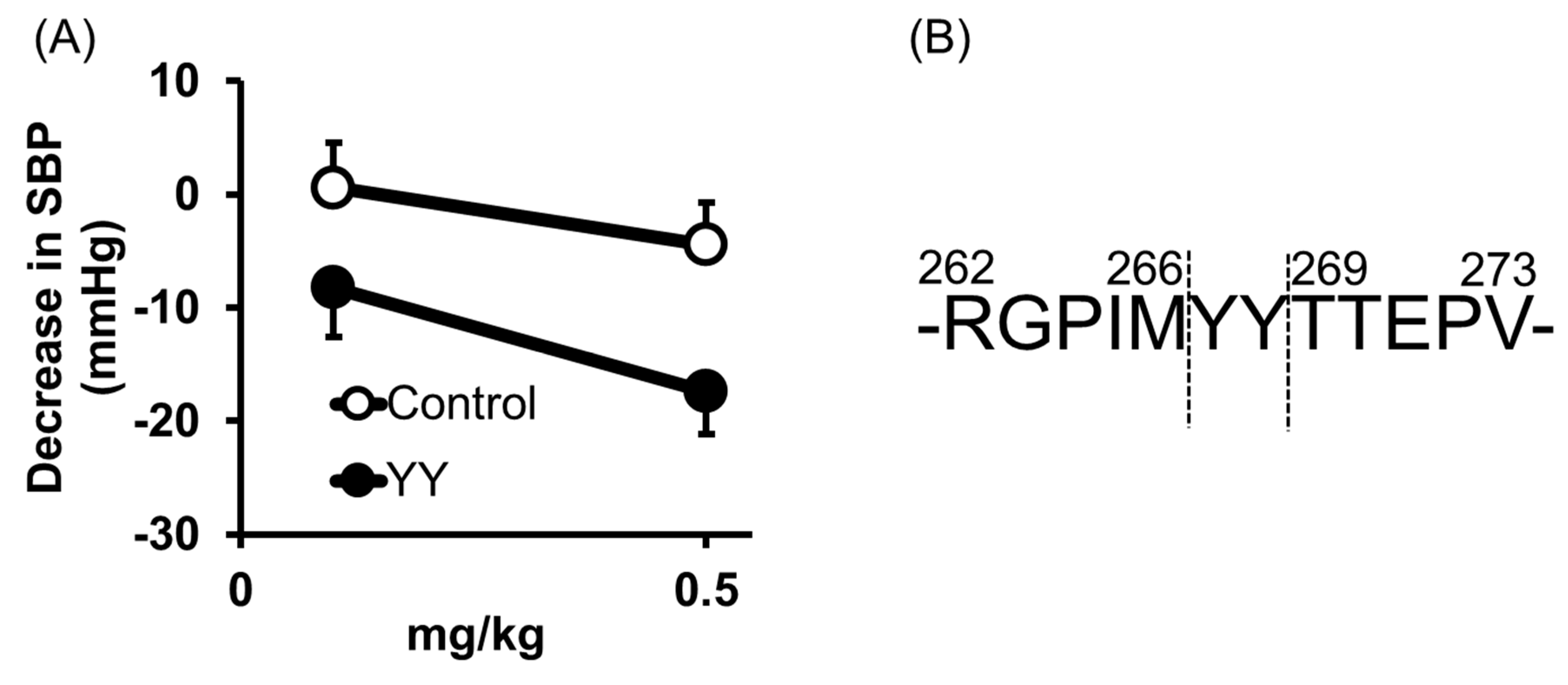
2.2. Tyr-Tyr
2.3. Tyr-Ser-Lys
2.4. Other Peptides
3. Anti-Hypertensive Effects of Protease-Digested Rice Bran
3.1. Thermolysin-Digested Rice Bran (TRB)
3.2. Protease G6-Digested Rice Bran
3.3. Trypsin-Digested Rice Bran
3.4. Fermented Rice Bran
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Milner, J.A. Functional foods and health promotion. J. Nutr. 1999, 129, 1395S–1397S. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Hu, M. Antoine-Laurent Lavoisier (1743–1794): Founder of modern chemistry. Singapore Med. J. 2004, 45, 303–304. [Google Scholar] [PubMed]
- Ryokuerou, S. The backward and future of nutiriology. Jpn. J. Nutr. Diet. 1973, 31, 219–220. [Google Scholar] [CrossRef]
- Yoshida, A. The origin of food nutriology. Kagaku To Seibutsu 1984, 22, 583–590. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Seita, A. Kanehiro Takaki and the control of beriberi in the Japanese Navy. J. R. Soc. Med. 2013, 106, 332–334. [Google Scholar] [CrossRef]
- Stretton, A.O.W. The first sequence: Fred Sanger and insulin. Genetics 2002, 162, 527–532. [Google Scholar]
- Karamanou, M. Milestones in the history of diabetes mellitus: The main contributors. World J. Diabetes 2016, 7, 1. [Google Scholar] [CrossRef]
- Brantl, V.; Teschemacher, H.; Henschen, A.; Lottspeich, F. Novel opioid peptides derived from casein (beta-casomorphins). I. Isolation from bovine casein peptone. Hoppe Seylers. Z. Physiol. Chem. 1979, 360, 1211–1216. [Google Scholar] [CrossRef]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive peptides of animal origin: A review. J. Food Sci. Technol. 2015, 52, 5377–5392. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Guang, C.; Phillips, R.D. Plant food-derived angiotensin i converting enzyme inhibitory peptides. J. Agric. Food Chem. 2009, 57, 5113–5120. [Google Scholar] [CrossRef] [PubMed]
- Moser, M. Historical perspectives on the management of hypertension. J. Clin. Hypertens. (Greenwich) 2006, 8 (Suppl. 2), 15–20, quiz 39. [Google Scholar] [CrossRef]
- Laragh, J.H.; Brenner, B.M.; Freis, E.D. Hypertension: Pathophysiology, Diagnosis, and Management, 2nd ed.; Raven Press: New York, NY, USA, 1995. [Google Scholar]
- Moser, M. Evolution of the treatment of hypertension from the 1940s to JNC V. Am. J. Hypertens. 1997, 10, 2S–8S. [Google Scholar] [CrossRef][Green Version]
- Ferreira, S.H.; Bartelt, D.C.; Greene, L.J. Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry 1970, 9, 2583–2593. [Google Scholar] [CrossRef]
- Ondetti, M.A.; Williams, N.J.; Sabo, E.; Pluscec, J.; Weaver, E.R.; Kocy, O. Angiotensin-converting enzyme inhibitors from the venom of Bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochemistry 1971, 10, 4033–4039. [Google Scholar] [CrossRef]
- Ariyoshi, Y. Angiotensin-converting enzyme inhibitors derived from food proteins. Trends Food Sci. Technol. 1993, 4, 139–144. [Google Scholar] [CrossRef]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. NY Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef]
- Shih, F.F. An update on the use of co-products from the milling of rice in value-added food products. J. Am. Oil Chem. Soc. 2012, 89, 1–8. [Google Scholar] [CrossRef]
- Kadowaki, M.; Kubota, M.; Watanabe, R. Physiological multifunctions of rice proteins of endosperm and bran. J. Nutr. Sci. Vitaminol. (Tokyo) 2019, 65, S42–S47. [Google Scholar] [CrossRef]
- Mansilla, W.D.; Marinangeli, C.P.F.; Cargo-Froom, C.; Franczyk, A.; House, J.D.; Elango, R.; Columbus, D.A.; Kiarie, E.; Rogers, M.; Shoveller, A.K. Comparison of methodologies used to define the protein quality of human foods and support regulatory claims. Appl. Physiol. Nutr. Metab. 2020, 45, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Chee, K.M.; Cho, S.J. Nutritional quality of rice bran protein in comparison to animal and vegetable protein. Food Chem. 2015, 172, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Yasunori, N.; Osamu, M.; Toshiaki, T. Decrease of tissue angiotensin I-converting enzyme activity upon feeding sour milk in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 1996, 60, 488–489. [Google Scholar] [CrossRef]
- Shobako, N.; Ogawa, Y.; Ishikado, A.; Harada, K.; Kobayashi, E.; Suido, H.; Kusakari, T.; Maeda, M.; Suwa, M.; Matsumoto, M.; et al. A novel antihypertensive peptide identified in thermolysin-digested rice bran. Mol. Nutr. Food Res. 2018, 62, 1–7. [Google Scholar] [CrossRef]
- Shobako, N.; Ishikado, A.; Ogawa, Y.; Sono, Y.; Kusakari, T.; Suwa, M.; Matsumoto, M.; Ohinata, K. Vasorelaxant and antihypertensive effects that are dependent on the endothelial NO system exhibited by rice bran-derived tripeptide. J. Agric. Food Chem. 2019, 67, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Iwasaki, M.; Usui, H.; Ohinata, K.; Marczak, E.D.; Lipkowski, A.W.; Yoshikawa, M. Rapakinin, an anti-hypertensive peptide derived from rapeseed protein, dilates mesenteric artery of spontaneously hypertensive rats via the prostaglandin IP receptor followed by CCK1receptor. Peptides 2010, 31, 909–914. [Google Scholar] [CrossRef]
- Kontani, N.; Omae, R.; Kagebayashi, T.; Kaneko, K.; Yamada, Y.; Mizushige, T.; Kanamoto, R.; Ohinata, K. Characterization of Ile-His-Arg-Phe, a novel rice-derived vasorelaxing peptide with hypotensive and anorexigenic activities. Mol. Nutr. Food Res. 2014, 58, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.S.; Ferreira, A.J.; Verano-Braga, T.; Bader, M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: New players of the renin-angiotensin system. J. Endocrinol. 2013, 216. [Google Scholar] [CrossRef]
- Xia, N.; Förstermann, U.; Li, H. Effects of resveratrol on eNOS in the endothelium and the perivascular adipose tissue. Ann. NY Acad. Sci. 2017, 1403, 132–141. [Google Scholar] [CrossRef]
- Sharma, R.; Randhawa, P.K.; Singh, N.; Jaggi, A.S. Bradykinin in ischemic conditioning-induced tissue protection: Evidences and possible mechanisms. Eur. J. Pharmacol. 2015, 768, 58–70. [Google Scholar] [CrossRef]
- Peiró, C.; Vallejo, S.; Gembardt, F.; Palacios, E.; Novella, S.; Azcutia, V.; Rodríguez-Mañas, L.; Hermenegildo, C.; Sánchez-Ferrer, C.F.; Walther, T. Complete blockade of the vasorelaxant effects of angiotensin-(1-7) and bradykinin in murine microvessels by antagonists of the receptor Mas. J. Physiol. 2013, 591, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Nonaka, A.; Matsushita, A.; Uchida, N.; Ohki, K.; Asakura, M.; Kitakaze, M. Milk casein-derived tripeptides, VPP and IPP induced NO production in cultured endothelial cells and endothelium-dependent relaxation of isolated aortic rings. Heart Vessels 2011, 26, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.J.; Vaughan, D.E. Angiotensin-converting enzyme inhibitors. Circulation 1998, 97. [Google Scholar] [CrossRef] [PubMed]
- He, H.L.; Liu, D.; Ma, C.B. Review on the Angiotensin-I-Converting Enzyme (ACE) inhibitor peptides from marine proteins. Appl. Biochem. Biotechnol. 2013, 169, 738–749. [Google Scholar] [CrossRef]
- Maruyama, H.; Tokunaga, K.; Suzuki, K.M.; Yoshida, C.; Futamura, Y.; Araki, Y.; Mishima, S. Purification and identification of angiotensin I-converting enzyme inhibitory peptides from royal jelly treated with protease. Nippon Shokuhin Kagaku Kogaku Kaishi 2003, 50, 310–315. [Google Scholar] [CrossRef]
- Sultana, A.; Nabi, A.H.M.N.; Nasir, U.M.; Maruyama, H.; Suzuki, K.M.; Mishima, S.; Suzuki, F. A dipeptide YY derived from royal jelly proteins inhibits renin activity. Int. J. Mol. Med. 2008, 21, 677–681. [Google Scholar] [CrossRef]
- Pantzaris, N.-D.; Karanikolas, E.; Tsiotsios, K.; Velissaris, D. Renin inhibition with aliskiren: A decade of clinical experience. J. Clin. Med. 2017, 6, 61. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT Food Sci. Technol. 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Ochiai, A.; Tanaka, S.; Tanaka, T.; Taniguchi, M. Rice bran protein as a potent source of antimelanogenic peptides with tyrosinase inhibitory activity. J. Nat. Prod. 2016, 79, 2545–2551. [Google Scholar] [CrossRef]
- Hatanaka, T.; Inoue, Y.; Arima, J.; Kumagai, Y.; Usuki, H.; Kawakami, K.; Kimura, M.; Mukaihara, T. Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem. 2012, 134, 797–802. [Google Scholar] [CrossRef]
- Adekoya, O.A.; Sylte, I. The thermolysin family (M4) of enzymes: Therapeutic and biotechnological potential. Chem. Biol. Drug Des. 2009, 73, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Nakano, D.; Ogura, K.; Miyakoshi, M.; Ishii, F.; Kawanishi, H.; Kurumazuka, D.; Kwak, C.-J.; Ikemura, K.; Takaoka, M.; Moriguchi, S.; et al. Antihypertensive effect of angiotensin I-converting enzyme inhibitory peptides from a sesame protein hydrolysate in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2006, 70, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Yoshikawa, M. LKPNM: A prodrug-type ACE-inhibitory peptide derived from fish protein. Immunopharmacology 1999, 44, 123–127. [Google Scholar] [CrossRef]
- Ogawa, Y.; Shobako, N.; Fukuhara, I.; Satoh, H.; Kobayashi, E.; Kusakari, T.; Suwa, M.; Matsumoto, M.; Ishikado, A. rice bran supplement containing a functional substance, the novel peptide Leu-Arg-Ala, has anti-hypertensive effects: A double-blind, randomized, placebo-controlled study. Nutrients 2019, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- Ngo-Son, A.; Katekaew, S. Purification and characterization of angiotensin converting enzyme-inhibitory derivefrom crocodile blood hydrolysates. Food Sci. Technol. 2019, 39, 818–823. [Google Scholar] [CrossRef]
- Boonla, O.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P.; Thawornchinsombut, S. Peptides-derived from Thai rice bran improves endothelial function in 2K-1C renovascular hypertensive rats. Nutrients 2015, 7, 5783–5799. [Google Scholar] [CrossRef]
- Senaphan, K.; Sangartit, W.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P.; Thawornchinsombut, S.; Greenwald, S.E.; Kukongviriyapan, U. Rice bran protein hydrolysates reduce arterial stiffening, vascular remodeling and oxidative stress in rats fed a high-carbohydrate and high-fat diet. Eur. J. Nutr. 2016, 1–12. [Google Scholar] [CrossRef]
- Jan-on, G.; Sangartit, W.; Pakdeechote, P.; Kukongviriyapan, V.; Senaphan, K.; Boonla, O.; Thongraung, C.; Kukongviriyapan, U. Antihypertensive effect and safety evaluation of rice bran hydrolysates from sang-yod rice. Plant Foods Hum. Nutr. 2020, 75, 89–95. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamamoto, N.; Sakai, K.; Okubo, A.; Yamazaki, S.; Takano, T. Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. J. Dairy Sci. 1995, 78, 777–783. [Google Scholar] [CrossRef]
- Alauddin, M.; Shirakawa, H.; Koseki, T.; Kijima, N.; Ardiansyah; Budijanto, S.; Islam, J.; Goto, T.; Komai, M. Fermented rice bran supplementation mitigates metabolic syndrome in stroke-prone spontaneously hypertensive rats. BMC Complement. Altern. Med. 2016, 16, 442. [Google Scholar] [CrossRef]
- Ardiansyah; Shirakawa, H.; Shimeno, T.; Koseki, T.; Shiono, Y.; Murayama, T.; Hatakeyama, E.; Komal, M. Adenosine, an identified active component from the driselase-treated fraction of rice bran, is effective at improving metabolic syndrome in stroke-prone spontaneously hypertensive rats. J. Agric. Food Chem. 2009, 57, 2558–2564. [Google Scholar] [CrossRef]
- Ardiansyah; Ohsaki, Y.; Shirakawa, H.; Koseki, T.; Komai, M. Novel effects of a single administration of ferulic acid on the regulation of blood pressure and the hepatic lipid metabolic profile in stroke-prone spontaneously hypertensive rats. J. Agric. Food Chem. 2008, 56, 2825–2830. [Google Scholar] [CrossRef] [PubMed]
- Boelsma, E.; Kloek, J. Lactotripeptides and antihypertensive effects: A critical review. Br. J. Nutr. 2009, 101, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Aihara, K.; Washitani, A.; Kaneko, K.; Mizutani, J.; Nakamura, K.; Shimura, T.; Yagasaki, K.; Nakamura, Y. Safety evaluation of excessive intake of the tablet containing “Lactotripeptide (VPP, IPP)” in subjects with normal blood pressure to mild hypertension. Jpn. Pharmacol. Ther. 2006, 34, 1107–1117. [Google Scholar]
- Fekete, Á.A.; Ian Givens, D.; Lovegrove, J.A. Casein-derived lactotripeptides reduce systolic and diastolic blood pressure in a meta-analysis of randomised clinical trials. Nutrients 2015, 7, 659–681. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Maeda, S.; Miyaki, A.; Misono, M.; Choi, Y.; Shimojo, N.; Ajisaka, R.; Tanaka, H. Additive beneficial effects of lactotripeptides intake with regular exercise on endothelium-dependent dilatation in postmenopausal women. Am. J. Hypertens. 2010, 23, 368–372. [Google Scholar] [CrossRef]
- Vita, J.A. Endothelial function. Circulation 2011, 124, 906–913. [Google Scholar] [CrossRef]
| Process | Functional Peptide | Mechanisms | Animals/Human | Reference |
|---|---|---|---|---|
| Thermolysin digestion | LRA, YY | 1.NO-mediated vasodilation 2.ACE inhibition | SHR model Human clinical trial | [26,27,46] |
| Protease G6 digestion | Not identified | 1.Upregulation of NOS expression 2.ACE inhibition | SD rat (with L-NAME,2K1C) model | [47,48,49,50] |
| Trypsin digestion | YSK? | ACE inhibition | SHR model | [40] |
| Fermentation | Not identified | ACE inhibition | SHRSP model | [52,53,54] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shobako, N.; Ohinata, K. Anti-Hypertensive Effects of Peptides Derived from Rice Bran Protein. Nutrients 2020, 12, 3060. https://doi.org/10.3390/nu12103060
Shobako N, Ohinata K. Anti-Hypertensive Effects of Peptides Derived from Rice Bran Protein. Nutrients. 2020; 12(10):3060. https://doi.org/10.3390/nu12103060
Chicago/Turabian StyleShobako, Naohisa, and Kousaku Ohinata. 2020. "Anti-Hypertensive Effects of Peptides Derived from Rice Bran Protein" Nutrients 12, no. 10: 3060. https://doi.org/10.3390/nu12103060
APA StyleShobako, N., & Ohinata, K. (2020). Anti-Hypertensive Effects of Peptides Derived from Rice Bran Protein. Nutrients, 12(10), 3060. https://doi.org/10.3390/nu12103060




