Dairy Consumption and Metabolic Health
Abstract
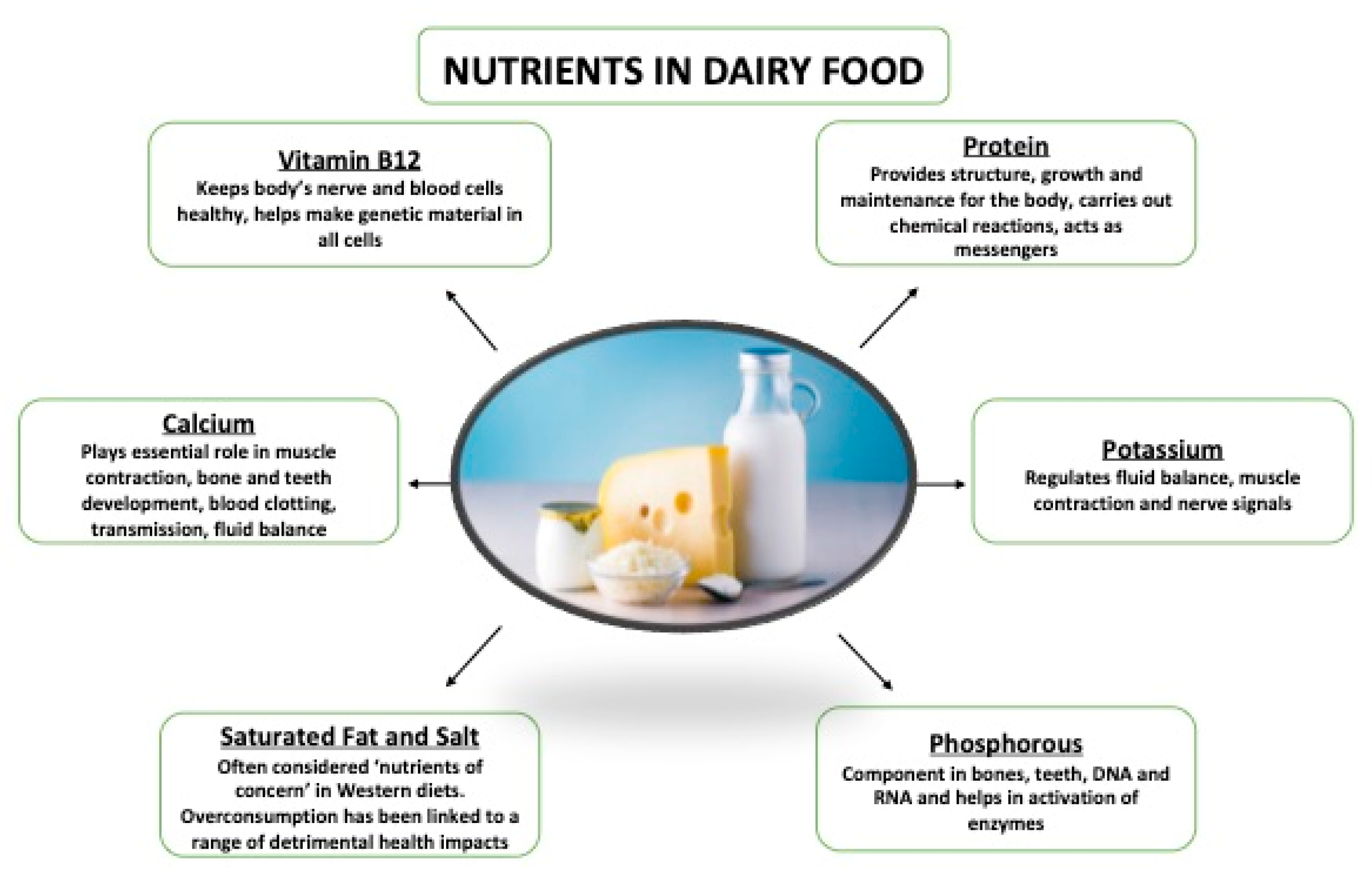
1. Contribution of Dairy to a Balanced Diet
2. Dairy Fat and the Link to Health
2.1. Dietary Guidelines—Nutrient-Based vs. Food-Based
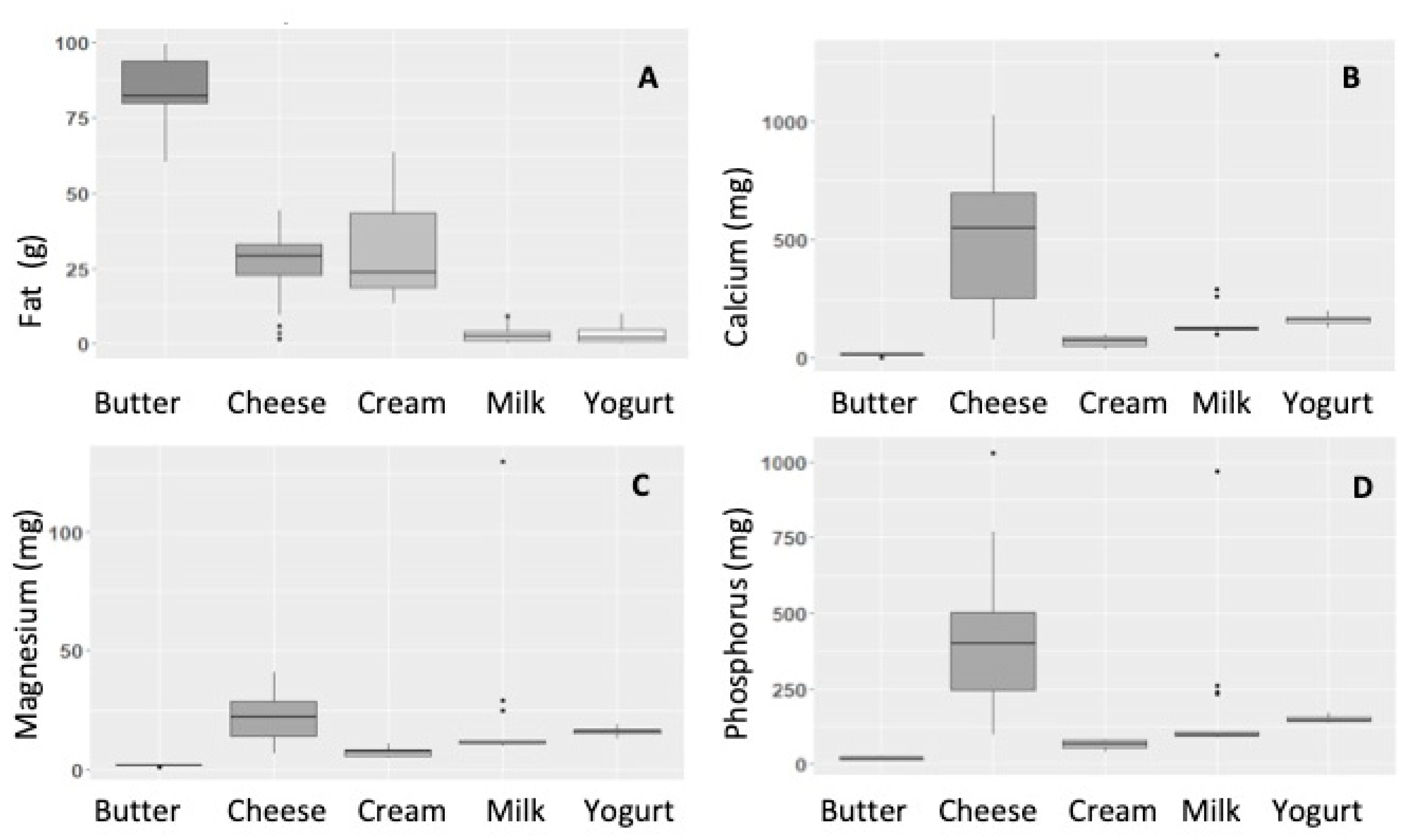
2.2. Total Dairy vs. Specific Products
2.3. Cheese
2.4. Milk
2.5. Yoghurt
3. The ‘Dairy Matrix’
4. Conclusions/Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Pellegrino, L.; Verduci, E.; Ghiselli, A.; Bernabei, R.; Calvani, R.; Cetin, I.; Giampietro, M.; Perticone, F.; Piretta, L. Cow’s milk consumption and health: A health professional’s guide. J. Am. Coll. Nutr. 2019, 38, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Smedman, A.; Lindmark-Månsson, H.; Paulsson, M.; Petrus, P.; Straniero, S.; Rudling, M.; Dahlman, I.; Risérus, U. Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: A randomized study. Am. J. Clin. Nutr. 2015, 102, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Committee to Review Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Gueguen, L.; Pointillart, A. The bioavailability of dietary calcium. J. Am. Coll. Nutr. 2000, 19, 119S–136S. [Google Scholar] [CrossRef]
- Zhao, Y.; Martin, B.R.; Weaver, C.M. Calcium bioavailability of calcium carbonate fortified soymilk is equivalent to cow’s milk in young women. J. Nutr. 2005, 135, 2379–2382. [Google Scholar] [CrossRef] [PubMed]
- Fulgoni, V.L., III; Keast, D.R.; Auestad, N.; Quann, E.E. Nutrients from dairy foods are difficult to replace in diets of Americans: Food pattern modeling and an analyses of the National Health and Nutrition Examination Survey. Nutr. Res. 2011, 31, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Dairy product intake in children and adolescents in developed countries: Trends, nutritional contribution, and a review of association with health outcomes. Nutr. Rev. 2014, 72, 68–81. [Google Scholar] [CrossRef]
- Vissers, P.A.; Streppel, M.T.; Feskens, E.J.; de Groot, L.C. Contribution of dairy products to micronutrient intake in The Netherlands. J. Am. Coll. Nutr. 2011, 30, 415S–421S. [Google Scholar] [CrossRef]
- Coudray, B. Contribution of dairy products to micronutrient intake in France. J. Am. Coll. Nutr. 2011, 30, 410S–414S. [Google Scholar] [CrossRef]
- Kalkwarf, H.J.; Khoury, J.C.; Lanphear, B.P. Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. Am. J. Clin. Nutr. 2003, 77, 257–265. [Google Scholar] [CrossRef]
- Ma, D.F.; Zheng, W.; Ding, M.; Zhang, Y.M.; Wang, P.Y. Milk intake increases bone mineral content through inhibiting bone resorption: Meta-analysis of randomized controlled trials. e-SPEN J. 2013, 8, E1–E7. [Google Scholar] [CrossRef]
- Dror, D.K. Dairy consumption and pre-school, school-age and adolescent obesity in developed countries: A systematic review and meta-analysis. Obes. Rev. 2014, 15, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Spence, L.A.; Cifelli, C.J.; Miller, G.D. The Role of Dairy Products in Healthy Weight and Body Composition in Children and Adolescents. Curr. Nutr. Food. Sci. 2011, 7, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Brantsæter, A.L.; Olafsdottir, A.S.; Forsum, E.; Olsen, S.F.; Thorsdottir, I. Does milk and dairy consumption during pregnancy influence fetal growth and infant birthweight? A systematic literature review. Food Nutr. Res. 2012, 56. [Google Scholar] [CrossRef] [PubMed]
- Geiker, N.R.W.; Mølgaard, C.; Iuliano, S.; Rizzoli, R.; Manios, Y.; van Loon, L.J.C.; Lecerf, J.M.; Moschonis, G.; Reginster, J.Y.; Givens, I.; et al. Impact of whole dairy matrix on musculoskeletal health and aging–current knowledge and research gaps. Osteoporos. Int. 2020, 31, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Hurst, C.; Dismore, L.; Aspray, T.; Stevenson, E.; Witham, M.D.; Sayer, A.A.; Robinson, S. Milk for Skeletal Muscle Health and Sarcopenia in Older Adults: A Narrative Review. Clin. Interv. Aging 2020, 15, 695–714. [Google Scholar] [CrossRef] [PubMed]
- Thorning, T.K.; Raben, A.; Tholstrup, T.; Soedamah-Muthu, S.S.; Givens, I.; Astrup, A. Milk and dairy products: Good or bad for human health? An assessment of the totality of scientific evidence. J. Food Nutr. Res. 2016, 60, 32527. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations. Dairy Development in Asia. Available online: http://www.fao:3/i0588e/I0588E02.htm (accessed on 24 September 2020).
- Cifelli, C.J.; Houchins, J.A.; Demmer, E.; Fulgoni, V.L. Increasing Plant Based Foods or Dairy Foods Differentially Affects Nutrient Intakes: Dietary Scenarios Using NHANES. Nutrients 2016, 8, 422. [Google Scholar] [CrossRef]
- Weinberger, M.H. Salt Sensitivity of Blood Pressure in Humans. Hypertension 1996, 27, 481–490. [Google Scholar] [CrossRef]
- Strazzullo, P.; D’Elia, L.; Kandala, N.-B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef]
- Ni Mhurchu, C.; Capelin, C.; Dunford, E.K.; Webster, J.L.; Neal, B.C.; Jebb, S.A. Sodium content of processed foods in the United Kingdom: Analysis of 44,000 foods purchased by 21,000 households. Am. J. Clin. Nutr. 2010, 93, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.; Appel, L.J.; Okuda, N.; Brown, I.J.; Chan, Q.; Zhao, L.; Ueshima, H.; Kesteloot, H.; Miura, K.; Curb, J.D.; et al. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: The INTERMAP study. J. Am. Diet. Assoc. 2010, 110, 736–745. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Huth, P.J.; Fulgoni, V.L.; Keast, D.R.; Park, K.; Auestad, N. Major food sources of calories, added sugars, and saturated fat and their contribution to essential nutrient intakes in the U.S. diet: Data from the National Health and Nutrition Examination Survey (2003–2006). Nutr. J. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Feeney, E.L.; Nugent, A.P.; Mc Nulty, B.; Walton, J.; Flynn, A.; Gibney, E.R. An overview of the contribution of dairy and cheese intakes to nutrient intakes in the Irish diet: Results from the National Adult Nutrition Survey. Br. J. Nutr. 2016, 115, 709–717. [Google Scholar] [CrossRef]
- Scientific Advisory Committee on Nutrition. Saturated Fats and Health. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/814995/SACN_report_on_saturated_fat_and_health.pdf (accessed on 24 September 2020).
- Department of Health, Ireland. Your Guide to Healthy Eating Using the Food Pyramid. 2016. Available online: https://www.hse.ie/eng/about/who/healthwellbeing/our-priority-programmes/heal/food-pyramid-images/food-pyramid-simple-version.pdf (accessed on 24 September 2020).
- De Oliveira, M.C.O.; Mozaffarian, D.; Kromhout, D.; Bertoni, A.G.; Sibley, C.T.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary intake of saturated fat by food source and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2012, 96, 397–404. [Google Scholar] [CrossRef]
- O’Sullivan, T.A.; Hafekost, K.; Mitrou, F.; Lawrence, D. Food sources of saturated fat and the association with mortality: A meta-analysis. Am. J. Public Health 2013, 103, e31–e42. [Google Scholar] [CrossRef]
- Feeney, E.L.; Barron, R.; Dible, V.; Hamilton, Z.; Power, Y.; Tanner, L.; Flynn, C.; Bouchier, P.; Beresford, T.; Noronha, N. Dairy matrix effects: Response to consumption of dairy fat differs when eaten within the cheese matrix—A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 667–674. [Google Scholar] [CrossRef]
- Chen, M.; Pan, A.; Malik, V.S.; Hu, F.B. Effects of dairy intake on body weight and fat: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 735–747. [Google Scholar] [CrossRef]
- Menotti, A.; Keys, A.; Aravanis, C.; Blackburn, H.; Dontas, A.; Fidanza, F.; Karvonen, M.J.; Kromhout, D.; Nedeljkovic, S.; Nissinen, A.; et al. Seven Countries Study. First 20-Year Mortality Data in 12 Cohorts of Six Countries. Ann. Med. 1989, 21, 175–179. [Google Scholar] [CrossRef]
- Kromhout, D.; Bloemberg, B.; Feskens, E.; Menotti, A.; Nissinen, A.; Seven Countries Study Group. Saturated fat, vitamin C and smoking predict long-term population all-cause mortality rates in the Seven Countries Study. Int. J. Epidemiol. 2000, 29, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.A.; Maki, K.C.; Orringer, C.E.; Jones, P.H.; Kris-Etherton, P.; Sikand, G.; La Forge, R.; Daniels, S.R.; Wilson, D.P.; Morris, P.B. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 2. J. Clin. Lipidol. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Miller, N.H.; Hubbard, V.S.; Lee, I.-M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2960–2984. [Google Scholar] [CrossRef] [PubMed]
- USDA. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture. Available online: https://health.gov/sites/default/files/2019-09/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf (accessed on 24 September 2020).
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
- Drouin-Chartier, J.-P.; Brassard, D.; Tessier-Grenier, M.; Côté, J.A.; Labonté, M.-È.; Desroches, S.; Couture, P.; Lamarche, B. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv. Nutr. 2016, 7, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.D.; Bylsma, L.C.; Vargas, A.J.; Cohen, S.S.; Doucette, A.; Mohamed, M.; Irvin, S.R.; Miller, P.E.; Watson, H.; Fryzek, J.P. Dairy consumption and CVD: A systematic review and meta-analysis. Br. J. Nutr. 2016, 115, 737–750. [Google Scholar] [CrossRef]
- Antoni, R.; Griffin, B. Draft reports from the UK’s Scientific Advisory Committee on Nutrition and World Health Organization concur in endorsing the dietary guideline to restrict intake of saturated fat. Nutr. Bull. 2018, 43, 206–211. [Google Scholar] [CrossRef]
- Astrup, A.; Bertram, H.C.; Bonjour, J.-P.; De Groot, L.C.; de Oliveira Otto, M.C.; Feeney, E.L.; Garg, M.L.; Givens, I.; Kok, F.J.; Krauss, R.M. WHO draft guidelines on dietary saturated and trans fatty acids: Time for a new approach? BMJ 2019, 366, l4137. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Rangarajan, S.; Sheridan, P.; Mohan, V.; Iqbal, R.; Gupta, R.; Lear, S.; Wentzel-Viljoen, E.; Avezum, A.; et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): A prospective cohort study. Lancet 2018, 392, 2288–2297. [Google Scholar] [CrossRef]
- Fontecha, J.; Calvo, M.V.; Juarez, M.; Gil, A.; Martínez-Vizcaino, V. Milk and dairy product consumption and cardiovascular diseases: An overview of systematic reviews and meta-analyses. Adv. Nutr. 2019, 10, S164–S189. [Google Scholar] [CrossRef]
- Zemel, M.B.; Sun, X.; Sobhani, T.; Wilson, B. Effects of dairy compared with soy on oxidative and inflammatory stress in overweight and obese subjects. Am. J. Clin. Nutr. 2010, 91, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulou, D.; Markey, O.; Kliem, K.E.; Fagan, C.C.; Grandison, A.S.; Humphries, D.J.; Todd, S.; Jackson, K.G.; Givens, D.I.; Lovegrove, J.A. Reformulation initiative for partial replacement of saturated with unsaturated fats in dairy foods attenuates the increase in LDL cholesterol and improves flow-mediated dilatation compared with conventional dairy: The randomized, controlled REplacement of SaturatEd fat in dairy on Total cholesterol (RESET) study. Am. J. Clin. Nutr. 2020, 111, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Drouin-Chartier, J.-P.; Li, Y.; Ardisson Korat, A.V.; Ding, M.; Lamarche, B.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in dairy product consumption and risk of type 2 diabetes: Results from 3 large prospective cohorts of US men and women. Am. J. Clin. Nutr. 2019, 110, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Markey, O.; Vasilopoulou, D.; Kliem, K.E.; Koulman, A.; Fagan, C.C.; Summerhill, K.; Wang, L.Y.; Grandison, A.S.; Humphries, D.J.; Todd, S. Plasma phospholipid fatty acid profile confirms compliance to a novel saturated fat-reduced, monounsaturated fat-enriched dairy product intervention in adults at moderate cardiovascular risk: A randomized controlled trial. Nutr. J. 2017, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Benatar, J.R.; Jones, E.; White, H.; Stewart, R.A. A randomized trial evaluating the effects of change in dairy food consumption on cardio-metabolic risk factors. Eur. J. Prev. Cardiol. 2014, 21, 1376–1386. [Google Scholar] [CrossRef]
- Nestel, P.J.; Mellett, N.; Pally, S.; Wong, G.; Barlow, C.K.; Croft, K.; Mori, T.A.; Meikle, P.J. Effects of low-fat or full-fat fermented and non-fermented dairy foods on selected cardiovascular biomarkers in overweight adults. Br. J. Nutr. 2013, 110, 2242–2249. [Google Scholar] [CrossRef]
- Crichton, G.E.; Howe, P.R.; Buckley, J.D.; Coates, A.M.; Murphy, K.J. Dairy consumption and cardiometabolic health: Outcomes of a 12-month crossover trial. Nutr. Metab. 2012, 9, 19. [Google Scholar] [CrossRef]
- Palacios, C.; Bertrán, J.J.; Ríos, R.E.; Soltero, S. No effects of low and high consumption of dairy products and calcium supplements on body composition and serum lipids in Puerto Rican obese adults. Nutrition 2011, 27, 520–525. [Google Scholar] [CrossRef]
- Stancliffe, R.A.; Thorpe, T.; Zemel, M.B. Dairy attentuates oxidative and inflammatory stress in metabolic syndrome. Am. J. Clin. Nutr. 2011, 94, 422–430. [Google Scholar] [CrossRef]
- Van Meijl, L.E.; Mensink, R.P. Effects of low-fat dairy consumption on markers of low-grade systemic inflammation and endothelial function in overweight and obese subjects: An intervention study. Br. J. Nutr. 2010, 104, 1523–1527. [Google Scholar] [CrossRef]
- Wennersberg, M.H.; Smedman, A.; Turpeinen, A.M.; Retterstøl, K.; Tengblad, S.; Lipre, E.; Aro, A.; Mutanen, P.; Seljeflot, I.; Basu, S. Dairy products and metabolic effects in overweight men and women: Results from a 6-mo intervention study. Am. J. Clin. Nutr. 2009, 90, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Van Meijl, L.E.; Mensink, R.P. Low-fat dairy consumption reduces systolic blood pressure, but does not improve other metabolic risk parameters in overweight and obese subjects. Nutr. Metab. Cardiovasc. Dis. 2010, 21, 355–361. [Google Scholar] [CrossRef]
- Tricon, S.; Burdge, G.C.; Jones, E.L.; Russell, J.J.; El-Khazen, S.; Moretti, E.; Hall, W.L.; Gerry, A.B.; Leake, D.S.; Grimble, R.F. Effects of dairy products naturally enriched with cis-9, trans-11 conjugated linoleic acid on the blood lipid profile in healthy middle-aged men. Am. J. Clin. Nutr. 2006, 83, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Richards, J.; Milstead, A.; Campbell, P. Effects of calcium and dairy on body composition and weight loss in African-American adults. Obes. Res. 2005, 13, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup, T.; Høy, C.-E.; Andersen, L.N.; Christensen, R.D.; Sandström, B. Does fat in milk, butter and cheese affect blood lipids and cholesterol differently? J. Am. Coll. Nutr. 2004, 23, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Brassard, D.; Tessier-Grenier, M.; Allaire, J.; Rajendiran, E.; She, Y.; Ramprasath, V.; Gigleux, I.; Talbot, D.; Levy, E.; Tremblay, A. Comparison of the impact of SFAs from cheese and butter on cardiometabolic risk factors: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 800–809. [Google Scholar] [CrossRef]
- Thorning, T.K.; Bertram, H.C.; Bonjour, J.-P.; De Groot, L.; Dupont, D.; Feeney, E.; Ipsen, R.; Lecerf, J.M.; Mackie, A.; McKinley, M.C. Whole dairy matrix or single nutrients in assessment of health effects: Current evidence and knowledge gaps. Am. J. Clin. Nutr. 2017, 105, 1033–1045. [Google Scholar] [CrossRef]
- Limongi, F.; Noale, M.; Marseglia, A.; Gesmundo, A.; Mele, M.; Banni, S.; Crepaldi, G.; Maggi, S. Impact of cheese rich in Conjugated Linoleic Acid on low density lipoproteins cholesterol: Dietary Intervention in Older People (CLADIS Study). J. Food. Nutr. Res. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Raziani, F.; Tholstrup, T.; Kristensen, M.D.; Svanegaard, M.L.; Ritz, C.; Astrup, A.; Raben, A. High intake of regular-fat cheese compared with reduced-fat cheese does not affect LDL cholesterol or risk markers of the metabolic syndrome: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 973–981. [Google Scholar] [CrossRef]
- Thorning, T.K.; Raziani, F.; Bendsen, N.T.; Astrup, A.; Tholstrup, T.; Raben, A. Diets with high-fat cheese, high-fat meat, or carbohydrate on cardiovascular risk markers in overweight postmenopausal women: A randomized crossover trial. Am. J. Clin. Nutr. 2015, 102, 573–581. [Google Scholar] [CrossRef]
- Nilsen, R.; Høstmark, A.T.; Haug, A.; Skeie, S. Effect of a high intake of cheese on cholesterol and metabolic syndrome: Results of a randomized trial. Food. Nutr. Res. 2015, 59, 27651. [Google Scholar] [CrossRef] [PubMed]
- Soerensen, K.V.; Thorning, T.K.; Astrup, A.; Kristensen, M.; Lorenzen, J.K. Effect of dairy calcium from cheese and milk on fecal fat excretion, blood lipids, and appetite in young men. Am. J. Clin. Nutr. 2014, 99, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Hjerpsted, J.; Leedo, E.; Tholstrup, T. Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. Am. J. Clin. Nutr. 2011, 94, 1479–1484. [Google Scholar] [CrossRef]
- Intorre, F.; Foddai, M.S.; Azzini, E.; Martin, B.; Montel, M.-C.; Catasta, G.; Toti, E.; Finotti, E.; Palomba, L.; Venneria, E. Differential effect of cheese fatty acid composition on blood lipid profile and redox status in normolipidemic volunteers: A pilot study. Int. J. Food. Sci. Nutr. 2011, 62, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Pintus, S.; Murru, E.; Carta, G.; Cordeddu, L.; Batetta, B.; Accossu, S.; Pistis, D.; Uda, S.; Ghiani, M.E.; Mele, M. Sheep cheese naturally enriched in α-linolenic, conjugated linoleic and vaccenic acids improves the lipid profile and reduces anandamide in the plasma of hypercholesterolaemic subjects. Br. J. Nutr. 2013, 109, 1453–1462. [Google Scholar] [CrossRef]
- Sofi, F.; Buccioni, A.; Cesari, F.; Gori, A.M.; Minieri, S.; Mannini, L.; Casini, A.; Gensini, G.F.; Abbate, R.; Antongiovanni, M. Effects of a dairy product (pecorino cheese) naturally rich in cis-9, trans-11 conjugated linoleic acid on lipid, inflammatory and haemorheological variables: A dietary intervention study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 117–124. [Google Scholar] [CrossRef]
- Nestel, P.; Chronopulos, A.; Cehun, M. Dairy fat in cheese raises LDL cholesterol less than that in butter in mildly hypercholesterolaemic subjects. Eur. J. Clin. Nutr. 2005, 59, 1059–1063. [Google Scholar] [CrossRef]
- Biong, A.S.; Müller, H.; Seljeflot, I.; Veierød, M.B.; Pedersen, J.I. A comparison of the effects of cheese and butter on serum lipids, haemostatic variables and homocysteine. Br. J. Nutr. 2004, 92, 791–797. [Google Scholar] [CrossRef]
- Karvonen, H.; Tapola, N.; Uusitupa, M.; Sarkkinen, E. The effect of vegetable oil-based cheese on serum total and lipoprotein lipids. Eur. J. Clin. Nutr. 2002, 56, 1094–1101. [Google Scholar] [CrossRef]
- Drouin-Chartier, J.-P.; Tremblay, A.J.; Maltais-Giguère, J.; Charest, A.; Guinot, L.; Rioux, L.-E.; Labrie, S.; Britten, M.; Lamarche, B.; Turgeon, S.L.; et al. Differential impact of the cheese matrix on the postprandial lipid response: A randomized, crossover, controlled trial. Am. J. Clin. Nutr. 2017, 106, 1358–1365. [Google Scholar] [CrossRef]
- Hansson, P.; Holven, K.B.; Øyri, L.K.; Brekke, H.K.; Biong, A.S.; Gjevestad, G.O.; Raza, G.S.; Herzig, K.-H.; Thoresen, M.; Ulven, S.M. Meals with similar fat content from different dairy products induce different postprandial triglyceride responses in healthy adults: A randomized controlled cross-over trial. J. Nutr. 2019, 149, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Messina, M.; Kiazand, A.; Morris, J.L.; Franke, A.A. Effect of Two Types of Soy Milk and Dairy Milk on Plasma Lipids in Hypercholesterolemic Adults: A Randomized Trial. J. Am. Coll. Nutr. 2007, 26, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Seo, J.A.; Yoon, T.; Seo, I.; Lee, J.H.; Im, D.; Lee, J.H.; Bahn, K.-N.; Ham, H.S.; Jeong, S.A.; et al. Effects of low-fat milk consumption on metabolic and atherogenic biomarkers in Korean adults with the metabolic syndrome: A randomised controlled trial. J. Hum. Nutr Diet. 2016, 29, 477–486. [Google Scholar] [CrossRef]
- Hidaka, H.; Takiwaki, M.; Yamashita, M.; Kawasaki, K.; Sugano, M.; Honda, T. Consumption of nonfat milk results in a less atherogenic lipoprotein profile: A pilot study. Ann. Nutr. Metab. 2012, 61, 111–116. [Google Scholar] [CrossRef]
- Rosado, J.L.; Garcia, O.P.; Ronquillo, D.; Hervert-Hernández, D.; Caamaño, M.D.C.; Martínez, G.; Gutiérrez, J.; García, S. Intake of milk with added micronutrients increases the effectiveness of an energy-restricted diet to reduce body weight: A randomized controlled clinical trial in Mexican women. J. Am. Diet. Assoc. 2011, 111, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Venkatramanan, S.; Joseph, S.V.; Chouinard, P.Y.; Jacques, H.; Farnworth, E.R.; Jones, P.J. Milk enriched with conjugated linoleic acid fails to alter blood lipids or body composition in moderately overweight, borderline hyperlipidemic individuals. J. Am. Coll. Nutr. 2010, 29, 152–159. [Google Scholar] [CrossRef]
- Faghih, S.H.; Abadi, A.R.; Hedayati, M.; Kimiagar, S.M. Comparison of the effects of cows’ milk, fortified soy milk, and calcium supplement on weight and fat loss in premenopausal overweight and obese women. Nutr. Metab. Cardiovasc. Dis. 2009, 21, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.I.; McCarron, D.A.; Heaney, R.P.; Dawson-Hughes, B.; Berga, S.L.; Stern, J.S.; Oparil, S. Effects of increased consumption of fluid milk on energy and nutrient intake, body weight, and cardiovascular risk factors in healthy older adults. J. Am. Diet. Assoc. 2000, 100, 810–817. [Google Scholar] [CrossRef]
- El Khoury, D.; Brown, P.; Smith, G.; Berengut, S.; Panahi, S.; Kubant, R.; Anderson, G.H. Increasing the protein to carbohydrate ratio in yogurts consumed as a snack reduces post-consumption glycemia independent of insulin. Clin. Nutr. 2014, 33, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Shab-Bidar, S.; Neyestani, T.R.; Djazayery, A.; Eshraghian, M.-R.; Houshiarrad, A.; Gharavi, A.; Kalayi, A.; Shariatzadeh, N.; Zahedirad, M.; Khalaji, N.; et al. Regular consumption of vitamin D-fortified yogurt drink (Doogh) improved endothelial biomarkers in subjects with type 2 diabetes: A randomized double-blind clinical trial. BMC Med. 2011, 9, 125. [Google Scholar] [CrossRef]
- Sadrzadeh-Yeganeh, H.; Elmadfa, I.; Djazayery, A.; Jalali, M.; Heshmat, R.; Chamary, M. The effects of probiotic and conventional yoghurt on lipid profile in women. Br. J. Nutr. 2010, 103, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V.; Akbarian-Moghari, A. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J. Dairy Sci. 2011, 94, 3288–3294. [Google Scholar] [CrossRef] [PubMed]
- Ataie-Jafari, A.; Larijani, B.; Majd, H.A.; Tahbaz, F. Cholesterol-lowering effect of probiotic yogurt in comparison with ordinary yogurt in mildly to moderately hypercholesterolemic subjects. Ann. Nutr. Metab. 2009, 54, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, G.; Schneider, J.; Jahreis, G. Long-term consumption of fermented dairy products over 6 months increases HDL cholesterol. Eur. J. Clin. Nutr. 2002, 56, 843–849. [Google Scholar] [CrossRef]
- Rizkalla, S.W.; Luo, J.; Kabir, M.; Chevalier, A.; Pacher, N.; Slama, G. Chronic consumption of fresh but not heated yogurt improves breath-hydrogen status and short-chain fatty acid profiles: A controlled study in healthy men with or without lactose maldigestion. Am. J. Clin. Nutr. 2000, 72, 1474–1479. [Google Scholar] [CrossRef]
- Agerholm-Larsen, L.; Raben, A.; Haulrik, N.; Hansen, A.; Manders, M.; Astrup, A. Effect of 8 week intake of probiotic milk products on risk factors for cardiovascular diseases. Eur. J. Clin. Nutr. 2000, 54, 288–297. [Google Scholar] [CrossRef]
- Anderson, J.W.; Gilliland, S.E. Effect of fermented milk (yogurt) containing Lactobacillus acidophilus L1 on serum cholesterol in hypercholesterolemic humans. J. Am. Coll. Nutr. 1999, 18, 43–50. [Google Scholar] [CrossRef]
- Feeney, E.L.; McKinley, M.C. The dairy food matrix: What it is and what it does. In Milk and Dairy Foods: Their Functionality in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2020; pp. 205–225. [Google Scholar] [CrossRef]
- Ayala-Bribiesca, E.; Lussier, M.; Chabot, D.; Turgeon, S.L.; Britten, M. Effect of calcium enrichment of Cheddar cheese on its structure, in vitro digestion and lipid bioaccessibility. Int. Dairy J. 2016, 53, 1–9. [Google Scholar] [CrossRef]
- Lorenzen, J.K.; Nielsen, S.; Holst, J.J.; Tetens, I.; Rehfeld, J.F.; Astrup, A. Effect of dairy calcium or supplementary calcium intake on postprandial fat metabolism, appetite, and subsequent energy intake. Am. J. Clin. Nutr. 2007, 85, 678–687. [Google Scholar] [CrossRef]
- Ditscheid, B.; Keller, S.; Jahreis, G. Cholesterol metabolism is affected by calcium phosphate supplementation in humans. J. Nutr. 2005, 135, 1678–1682. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Semidalas, C.E.; Koussissis, S.; Demopoulos, C.A. Platelet-activating factor (PAF) antagonists in foods: A study of lipids with PAF or anti-PAF-like activity in cow’s milk and yogurt. J. Agric. Food Chem. 1996, 44, 3047–3051. [Google Scholar] [CrossRef]
- Poutzalis, S.; Anastasiadou, A.; Nasopoulou, C.; Megalemou, K.; Sioriki, E.; Zabetakis, I. Evaluation of the in vitro anti-atherogenic activities of goat milk and goat dairy products. Dairy Sci. Technol. 2016, 96, 317–327. [Google Scholar] [CrossRef]
- Meisel, H. Bioactive peptides from milk proteins: A perspective for consumers and producers. Aust. J. Dairy Technol. 2001, 56, 83. [Google Scholar]
- Gobbetti, M.; Stepaniak, L.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Latent bioactive peptides in milk proteins: Proteolytic activation and significance in dairy processing. Crit. Rev. Food Sci. Nutr. 2002, 42, 223–239. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, R.J.; Meisel, H. Milk protein-derived peptide inhibitors of angiotensin-I-converting enzyme. Br. J. Nutr. 2000, 84, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Ohki, K.; Kawagishi, R.; Kajimoto, Y.; Mizuno, S.; Nakamura, Y.; Kitakaze, M. Casein hydrolysate containing the Antihypertensive Tripeptides Val-Pro-Pro and Ile-Pro-Pro improves vascular endothelial function independent of Blood Pressure–Lowering Effects: Contribution of the inhibitory action of Angiotensin-Converting enzyme. Hypertens. Res. 2007, 30, 489–496. [Google Scholar] [CrossRef]
- Uenishi, H.; Kabuki, T.; Seto, Y.; Serizawa, A.; Nakajima, H. Isolation and identification of casein-derived dipeptidyl-peptidase 4 (DPP-4)-inhibitory peptide LPQNIPPL from gouda-type cheese and its effect on plasma glucose in rats. Int. Dairy J. 2012, 22, 24–30. [Google Scholar] [CrossRef]
- Gupta, A.; Mann, B.; Kumar, R.; Sangwan, R.B. ACE-inhibitory activity of cheddar cheeses made with adjunct cultures at different stages of ripening. Adv. Dairy. Res. 2013, 1. [Google Scholar] [CrossRef]
- Gómez-Ruiz, J.Á.; Ramos, M.; Recio, I. Angiotensin-converting enzyme-inhibitory peptides in Manchego cheeses manufactured with different starter cultures. Int. Dairy J. 2002, 12, 697–706. [Google Scholar] [CrossRef]
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212. [Google Scholar] [CrossRef]
- Norris, G.H.; Milard, M.; Michalski, M.-C.; Blesso, C.N. Protective properties of milk sphingomyelin against dysfunctional lipid metabolism, gut dysbiosis, and inflammation. J. Nutr. Biochem. 2019, 73, 108224. [Google Scholar] [CrossRef]
- Millar, C.L.; Jiang, C.; Norris, G.H.; Garcia, C.; Seibel, S.; Anto, L.; Lee, J.-Y.; Blesso, C.N. Cow’s milk polar lipids reduce atherogenic lipoprotein cholesterol, modulate gut microbiota and attenuate atherosclerosis development in LDL-receptor knockout mice fed a Western-type diet. J. Nutr. Biochem. 2020, 79, 108351. [Google Scholar] [CrossRef] [PubMed]
- Singh, H. The milk fat globule membrane—A biophysical system for food applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 154–163. [Google Scholar] [CrossRef]
- Vanderghem, C.; Bodson, P.; Danthine, S.; Paquot, M.; Deroanne, C.; Blecker, C. Milk fat globule membrane and buttermilks: From composition to valorization. Biotechnol. Agron. Soc. Environ. 2010, 14, 485–500. [Google Scholar]
- Demmer, E.; Van Loan, M.D.; Rivera, N.; Rogers, T.S.; Gertz, E.R.; German, J.B.; Smilowitz, J.T.; Zivkovic, A.M. Addition of a dairy fraction rich in milk fat globule membrane to a high-saturated fat meal reduces the postprandial insulinaemic and inflammatory response in overweight and obese adults. J. Nutr. Sci. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Calder, P.C. Plasma cytokine response during the postprandial period: A potential causal process in vascular disease? Br. J. Nutr. 2005, 93, 3–9. [Google Scholar] [CrossRef] [PubMed]
- De Goede, J.; Geleijnse, J.M.; Ding, E.L.; Soedamah-Muthu, S.S. Effect of cheese consumption on blood lipids: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2015, 73, 259–275. [Google Scholar] [CrossRef]
- Hjerpsted, J.B. Cheese and Cardiovascular Health: Evidence from Observational, Intervention and Explorative Studies; University of Copenhagen: Copenhagen, Denmark, 2013. [Google Scholar]
- Hjerpsted, J.; Tholstrup, T. Cheese and cardiovascular disease risk: A review of the evidence and discussion of possible mechanisms. Crit. Rev. Food. Sci. Nutr. 2016, 56, 1389–1403. [Google Scholar] [CrossRef]
- Feeney, E.L.; O’Sullivan, A.; Nugent, A.P.; McNulty, B.; Walton, J.; Flynn, A.; Gibney, E.R. Patterns of dairy food intake, body composition and markers of metabolic health in Ireland: Results from the National Adult Nutrition Survey. Nutr. Diabetes 2017, 7, e243. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Appel, L.J. Health effects of dietary patterns: Critically important but vastly understudied. Am. J. Clin. Nutr. 2018, 108, 207–208. [Google Scholar] [CrossRef]
- Markey, O.; Vasilopoulou, D.; Givens, D.I.; Lovegrove, J.A. Dairy and cardiovascular health: Friend or foe? Nutr. Bull. 2014, 39, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Raziani, F.; Ebrahimi, P.; Engelsen, S.B.; Astrup, A.; Raben, A.; Tholstrup, T. Consumption of regular-fat vs reduced-fat cheese reveals gender-specific changes in LDL particle size—A randomized controlled trial. Nutr. Metab. 2018, 15, 61. [Google Scholar] [CrossRef] [PubMed]
| Author | Country | Study Design | Population | Age (Years) | Intervention | Duration | Main Findings |
|---|---|---|---|---|---|---|---|
| Vasilopoulou et al. 2020 [47] | UK | Crossover | n = 54 (31 male; 23 female), with risk of CVD | 25–70 | 2 arm: (A) monounsaturated fatty acid (MUFA)-modified dairy—340 g UHT milk, 45 g cheese, 25.1 g butter. (B) Control—340 g ultrahigh temperature (UHT) pasteurised milk, 45 g cheese, 25.1 g butter | Two 12-week periods separated by an 8-week washout period | No significant change from baseline in serum total cholesterol (TC) between diets. Group A had a significant beneficial effect in terms of attenuation of the rise of the low-density lipoprotein (LDL) cholesterol. No changes in high-density lipoprotein (HDL) cholesterol between diets. The LDL:HDL ratio decreased significantly after group A, and increased after the control. No significant differences were observed for indexes of insulin sensitivity/resistance. Fasting plasma nitrite concentrations increased after the modified diet, yet decreased after the control. |
| Markey et al. 2017 [49] | UK | Crossover | n = 54 (31 male; 23 female), with risk of CVD | 25–70 | 2 arm: (A) MUFA-modified dairy—340 g UHT milk, 45 g cheese, 25.1 g butter. (B) Control—340 g UHT milk, 45 g cheese, 25.1 g butter. | Two 12-week periods, separated by an 8-week washout period | Group A showed a smaller increase in saturated fatty acids (SFA) and greater increase in MUFA intake when compared with the control. |
| Rosqvist et al. 2015 [3] | Sweden | Parallel | n = 57 (gender split not stated), overweight or obese | 20–70 | 2 arm: (A) milk-fat globule membrane (MFGM) group—100 mL whipping cream (40%fat)/d, 100 mL fat-free milk (0.1% fat)/d and 1 scone/d (baked with wheat flour, water, sodium chloride and baking powder). (B) Fat-free milk, 100 mL, (0.1% fat)/d and 1 scone/d (baked with wheat flour, water, butter oil (98.7% fat), sodium chloride, baking powder and milk protein isolate). | 8 weeks | Control diet increased TC, LDL, apolipoprotein B:apolipoprotein A-I ratio and non-HDL plasma lipids, whereas the MFGM diet did not. HDL, triglyceride, sitosterol, lathosterol, campesterol and proprotein convertase subtilisin/kexin type 9 concentrations and fatty acid compositions did not differ between groups. |
| Benatar et al. 2013 [50] | New Zealand | Parallel | n = 180 (54 male; 126 female), healthy volunteers | >18 | 3 arm: (A) increased dairy—an extra two-to-three servings per day, and to change to high-fat milk and dairy solids. (B) Habitual dairy intake remains unchanged. (C) Decreased dairy were asked to eliminate all possible sources of dairy. | 1 month | No significant change in LDL or HDL, triglycerides, systolic or diastolic BP, C-reactive protein, glucose or insulin across groups. There was a small increase in weight in group A. |
| Nestel et al. 2013 [51] | Australia | Crossover | n = 12 (gender not specified) overweight or obese | 40–70 | 3 arm: (A) low-fat dairy, 1% fat milk (400 mL/d) and 1% fat yoghurt (200 g/d). (B) Full-fat dairy (fermented), cheddar cheese (85 g/d) and full-cream yoghurt (three servings, 600 g/d). (C) Full-fat dairy (non-fermented), butter (30 g/d) and cream (70 mL/d) and small amounts of ice-cream. | Two 3-week periods, for group B + C (full fat diets). Group A diet (low fat) was consumed twice—between and at the end of the full-fat dairy dietary periods, for a duration of 2 weeks. | Lowest LDL and HDL concentrations were observed in group A, but plasma Triacylglycerol (TAG) concentrations did not differ significantly across the groups. Concentrations of plasma sphingomyelin and IL-6 were significantly higher after the non-fermented dairy diet (group C) than the low-fat dairy diet |
| Crichton et al. 2012 [52] | Australia | Crossover | n = 61 (18 male; 43 female), overweight or obese | 18–75 | 2 arm: (A) 4 servings of reduced-fat dairy/d, 1 serving = 250 mL milk, 175–200 g yoghurt and 190 g custard. (B) Control—1 serving of dairy/d, reflecting habitual intake. | Two 6-month periods, no washout period | No significant changes in resting metabolic rate or total energy expenditure, systolic and diastolic BP, fasting blood glucose, TC, HDL or LDL, triglycerides or hs-CRP (high sensitivity C-reactive protein). Additionally no differences between groups for waist circumference (WC), body weight and fat mass. |
| Palacios et al. 2011 [53] | Puerto Rico | Parallel | n = 25 (5 male; 20 female), obese | 22–50 | 3 arm: (A) 4 servings of dairy/d (low-fat milk, low-fat cheese and low-fat yoghurt), with a dairy-calcium intake goal of 1200–1300 mg/d. (B) calcium supplement (600 mg/d, calcium carbonate). (C) Control—habitual diet with placebo tablet. | 21 weeks | No significant group effects were observed for anthropometric measurements or serum lipids such as TC, HDL, LDL and TAG levels. Although TAG levels decreased by 18% in group A (high dairy). |
| Stancliffe et al. 2011 [54] | USA | Parallel | n = 40 (19 male; 21 female), overweight or obese with metabolic syndrome | 37.0 ± 9.9 | 2 arm: (A) low-dairy diet—0.5 servings/d and provided with 3 servings/d of non-dairy foods that is low-sodium luncheon meats, soy-based luncheon meat substitutes, packaged fruit cups, granola bars and peanut butter crackers. (B) adequate dairy diet—3.5 servings/d, of which 2/3 servings were milk and/or yoghurt. | 12 weeks | Group A decreased malondialdehyde and oxidised LDL. Inflammatory markers were suppressed with intake of AD, with decreases in TNF-a (Tumor Necrosis Factor-a); decreases in IL-6 (Interleukin-6) and monocyte chemoattractant protein 1 and an increase in adiponectin. Group B exerted no effect on oxidative or inflammatory markers. Group A significantly reduced waist circumference and trunk fat but group B exerted no effects. |
| van Meijl and Mensink, 2010 [55] | Netherlands | Crossover | n = 35 (10 male; 25 female), overweight or obese | 18–70 | 2 arm: (A) 500 mL low-fat milk and 150 g low-fat yoghurt per day. (B) Control—600 mL fruit juice and 43 g fruit biscuits per day | Two 8-week periods separated by a 2-week washout period | Plasma concentrations of TNF-a decreased, and soluble TNF-a receptor-1 increased after low-fat dairy consumption compared to the control. s-TNFR-2 also increased. Low-fat dairy consumption had no effect on IL-6, monocyte chemoattractant protein-1, intracellular adhesion molecule-1 and vascular cell adhesion molecule-1 concentrations. Lipid profiles were not analysed. |
| Zemel et al. 2010 [46] | USA | Crossover | n = 20 (14 male; 6 female), overweight or obese | Average—31 ± 10.3 | 2 arm: (A) dairy smoothie, 3 times/d, with non-fat dry milk as the protein source, and containing 350 mg calcium per smoothie. (B) Soy smoothie, 3 times/d, with soy protein isolate as the protein source and 50 mg calcium per smoothie. | Two 4-week periods separated by a 4-week washout period | Group A resulted in significant suppression of oxidative stress and lower inflammatory markers; tumour necrosis factor-a; IL-6; monocyte chemoattractant protein-1 and increased adiponectin. Group B exerted no significant effects. Lipid profiles were not analysed. |
| Wennersberg et al. 2009 [56] | Norway | Parallel | n = 121 (41 male; 80 female) | 30–65 | 2 arm: (A) milk group, 3–5 portions of dairy/d. Portion = 200 g milk, 200–250 g yoghurt or sour milk, 75 g cream or crème fraıche, 15–40 g cheese, 3–10 g butter or butter-containing spreads, 50 mL cottage cheese, and ice-cream occasionally. (B) Control—habitual daily diet. | 6 months | No significant differences between changes in body weight or body composition, BP, markers of inflammation, endothelial function, adiponectin or oxidative stress in group A and B. There was a modest unfavourable increase in serum TC concentrations in the group A. |
| van Meijl and Mensink, 2009 [57] | Netherlands | Crossover | n = 35 (10 males; 25 females), overweight or obese | 18–70 | 2 arm: (A) 500 mL low-fat milk and 150 g low-fat yoghurt per day. (B) Control—600 mL fruit juice and 43 g fruit biscuits per day | Two 8-week periods separated by a 2-week washout period | In group A, systolic BP significantly decreased compared with the control, but diastolic BP did not reach significance. Decreases in HDL and apo A-1 concentrations were also observed in group A. Serum TC, LDL, apo B, TAG, non-esterified fatty acids, glucose, insulin, C-reactive protein and plasminogen activator inhibitor-1 remained unchanged. |
| Tricon et al. 2006 [58] | UK | Crossover | n = 32 males, healthy volunteers | 34–60 | 2 arm: (A) 500 mL UHT full-fat milk, 12.5 g butter and 36.3 g cheese per day, naturally enriched with CLA (conjugated linoleic acid). (B) Control, 500 mL UHT full-fat milk, 12.5 g butter and 28 g cheese per day. | Two 6 week periods, separated by a 7 week washout period | Diet A did not significantly affect body weight, inflammatory markers, insulin, glucose, TAG, or TC, LDL and HDL cholesterol but resulted in a small increase in the LDL:HDL ratio. The modified dairy products changed LDL fatty acid composition but had no significant effect on LDL particle size or the susceptibility of LDL to oxidation. |
| Zemel et al. 2005 [59] | America | Parallel | Study 1.n = 34 (11 male; 23 female), Study 2. n = 29 (4 male; 25 female), obese | 26–55 | Study 1. (A) Dairy group—3 servings of dairy/d, at least one serving to be milk (B) Control—low dairy, 0–1 servings of low-fat dairy/d. Study 2. (A) Dairy group—3 servings of dairy/d, at least one serving to be milk and a 500-kcal/d deficit (B) Control—low dairy, 0–1 servings of low-fat dairy/d and 500 kcal/d deficit diet. | 24 weeks (both studies) | Study 1. Body weight remained stable for both groups. Group A resulted in decreases in total body fat, trunk fat, insulin and BP and an increase in lean mass and no significant changes in the control group. Study 2. Both diets produced significant weight and fat loss. Weight and fat loss within group A were 2-fold higher, and loss of lean body mass significantly reduced compared with the control. There were no effects on circulating lipids in either group. |
| Tholstrup et al. 2004 [60] | Denmark | Crossover | n = 14 males, healthy volunteers | 20–31 | 3 arms: (A) 1.5 L of whole milk/10 MJ (54 g of fat and 1779 mg calcium per 10 MJ). (B) Butter 64 g/10 MJ (54 g of fat and 10 mg calcium per 10 MJ) (C) 205 g of hard cheese, “Samsø”, 45% fat of dry weight, i.e., 26% fat/10 MJ (1989 mg calcium/10 MJ). | Three 3-week periods separated by a 4-week washout period | Fasting LDL concentrations were significantly higher after butter than cheese diet, with a borderline significant difference in TC after the experimental periods. Postprandial glucose showed a higher response after cheese diet compared to milk diet. No differences were found between groups for HDL, Very Low-Density Lipoprotein (VLDL), apo A-1 and apo B concentrations. |
| Author | Country | Study Design | Population | Age (Years) | Intervention | Duration | Main Findings |
|---|---|---|---|---|---|---|---|
| Feeney et al. 2018 [32] | Ireland | Parallel | n = 164 (75 male; 89 female), BMI > 25 kg/m2 | >50 | 4 arm: (A) 120 g full-fat cheddar cheese (FFCC). (B) Reduced-fat Irish cheddar cheese, 120 g, +butter (21 g) (RFC + B). (C) Butter (49 g), calcium caseinate powder (30 g), Ca supplement (BCC). (D) Full-fat Irish cheddar cheese, 120 g, (as per “a” but with 6-week run-in period). | 6 weeks | There was a significant difference in total cholesterol (TC) and LDL between groups. Group A had significantly lower TC and LDL compared with other groups. No differences were observed for HDL cholesterol, anthropometry, fasting glucose or insulin. |
| Limongi et al. 2018 [63] | Italy | Crossover | n = 58 (16 male; 42 female), healthy volunteers | >60 | 2 arm: (A) 90 g/d of CLA-enriched Pecorino cheese. (B) Control, 90 g/d of Pecorino cheese. | Two 2-month periods, separated by 1-month washout period | No significant differences found in relation to LDL between diet A + B. Participants consuming enriched cheese had a lower increase in glycaemia compared to control but did not display an increase in lipid levels. |
| Brassard et al. 2017 [61] | Canada | Crossover | n = 92 (43 male; 49 female), abdominally obese | 18–65 | 5 arm: (A) 90 g/2500 kcal cheese (type not specified). (B) Butter 49 g/2500 kcal. (C) MUFA-rich diet (D) PUFA-rich diet. (E) High-cholesterol, low-fat diet. The SFA content was matched in diets A + B. | Five 4-week periods, separated by 4-week washout periods | No changes were evident in HDL after cheese consumption. LDL was lower in group A compared with group B, but higher than groups C, D and E. No significant differences were found in inflammation markers, blood pressure and insulin-glucose homeostasis. |
| Raziani et al. 2016 [64] | Denmark | Parallel | n = 139 (47 male; 92 female), risk of metabolic syndrome | 18–70 | 3 arm: (A) 40 g equal parts regular-fat Danbo and cheddar cheese (REG). (B) Reduced-fat Danbo and cheddar cheese, 40 g, (RED). (C) Noncheese, carbohydrate control (CHO40 g/d)—90 g bread, 25 g jam. | 12 weeks | No differences were evident on lipid profile between groups. In addition, Insulin, glucose, and triacylglycerol concentrations as well as blood pressure and waist circumference did not differ. |
| Thorning et al. 2015 [65] | Denmark | Crossover | n = 14 female, overweight, post-menopausal | 45–68 | 3 arm: (A) 96–120 g of equal parts Dando and cheddar cheese per 8–10 MJ diet. (B) High fat meat—164 g per 10-MJ diet. (C) Non-dairy, low-fat control (CHO)—fruit (84 g), white bread, pasta and rice (58 g), marmalade (20 g), and cake, sweetened biscuits and chocolate (13 g) per 10-MJ diet. | Three 2-week periods, separated by 2-week washout periods | Group A caused higher levels of circulating HDL levels and apo A-I concentrations, and a lower apoB:apo A-I ratio compared to group C. Faecal fat excretion was also higher in group A. TC and LDL was similar across all groups. |
| Nilsen et al. 2015 [66] | Norway | Parallel | n = 153 (73 male; 80 female), normotensive and hypertensive | >18 | 3 arm: (A) 50 g/d Gamalost. (B) Norvegia 80 g/d. (C) Control—limited intake of Gamalost and Norvegia. | 8 weeks | There were no changes in MetS (metabolic equivalents) factors between the intervention groups and control. Significant reductions were noted for TC in those with MetS in group B. Those in group A with high TC also showed significant decreases compared with control. |
| Soerensen et al. 2014 [67] | Denmark | Crossover | n = 15 males, healthy volunteers | 18–50 | 3 arm: (A) 500 mg Ca/d-non-dairy control. (B) Semi-skimmed milk, 670 mL per 10 MJ. (C) Semi hard cheese, 120 g per 10 MJ (45% fat). | Three 2-week periods, separated by 2-week washout period | Significantly lower increases in TC and LDL were found in the group B and C compared with control. Faecal fat excretion also increased group B and C compared with control. No changes were found in blood pressure, high-density lipoprotein cholesterol, triglycerides and lipid ratios. |
| Hjerpsted et al. 2011 [68] | Denmark | Crossover | n = 49 (28 male; 21 female), healthy volunteers | 22–69 | Test-food amounts were dependent on participants’ energy levels. 2 arm: (A) 143 g/d (based on medium energy level) hard cheese “Samsø” (27 g fat/100 g). (B) Salted butter, 47 g/d (based on medium energy level). | Two 6-week periods, separated by 2-week washout period | Group A had significantly lower serum total, LDL and HDL cholesterol, and increased glucose concentrations compared with group B. Faecal fat excretion did not differ between groups. |
| Intorre et al. 2011 [69] | Italy | Crossover | n = 30 (11 male; 19 female), healthy volunteers | 20–40 | 2 arm: (A) 150 g of hard cheese per week (milk from cows fed a grass and maize silage-based diet with 5% of linseed oil added). (B) Control, 150 g of hard cheese per week (from normal cow milk) | Two 4-week periods, separated by a 4-week washout | The blood lipid profile did not change after diet A. Although it led to higher levels of vitamin C and E and stearic acid in blood, while myristic acid and oxidised LDL concentrations were significantly lower. |
| Pintus et al. 2013 [70] | Italy | Crossover | n = 42 (19 male; 23 female), mildly hypercholesterolaemic | 30–60 | 2 arm: (A) control, 90 g/d sheep cheese. (B) Sheep cheese, 90 g/d, naturally enriched with CLA | Two 3-week periods, separated by a 6-week washout | The findings confirmed an association between anandamide and adiposity. Diet B significantly increased the plasma levels of fatty acid hydrocyperoxidases and LDL decreased. However, no changes were detected in levels of inflammatory markers. |
| Sofi et al. 2010 [71] | Italy | Crossover | n = 10 (4 male; 6 female) healthy volunteers | 30–65 | 2 arm: (A) 200 g/week (3 times a week) of pecorino cheese, naturally rich in CLA. (B) Placebo cheese—control, 200 g/week (3 times a week). | Two 10-week periods, separated by a 10-week washout period | Consumption of cheese naturally rich in CLA determined a significant reduction in some inflammatory parameters as well as some haemorheological, appearing to cause favourable biochemical changes of atherosclerotic markers, albeit limited. No significant effects on lipid profile were evident. |
| Nestel et al. 2005 [72] | Australia | Crossover | n = 19 (14 male; 5 female), overweight and mildly hypercholesterolaemic | Average— 56.3 ± 7.8 | 2 arm: (A) 120 g/d mature cheddar (40 g fat). (B) Similar amount of butter fat to group A, from preweighed portions of butter and one butter-rich muffin. | Two 4-week periods, separated by a 2-week washout period | Lipid values did not differ significantly between the group A and run-in periods, but TC and LDL were significantly higher with group B. Group B (butter) also raised total and LDL cholesterol significantly. This was not evident for cheese. |
| Biong et al. 2004 [73] | Norway | Crossover | n = 22 (9 male; 13 female), healthy volunteers | 21–54 | 3 arm: (A) 150 g/d (per 8 MJ diet) Jarlsberg ‘Swiss-type’ cheese. (B) Butter, 52 g/d (per 8 MJ diet) + casein (as calcium caseinate). (C) Butter, 52 g/d (per 8 MJ diet) + egg white. | Three 3-week periods, separated by a 1-week washout periods | TC was significantly lower after diet A compared to diet B. While LDL was lower after diet A, this was not statistically significant. There were also no significant differences in HDL-cholesterol, triacylglycerols, apo A-I, apo B or lipoprotein (a), haemostatic variables and homocysteine between groups. |
| Karvonen et al. 2002 [74] | Finland | Crossover | n = 31 (17 male; 14 female), hyperlipidaemic | 25–65 | 2 arm: (A) 65 g/d low-fat rapeseed oil-based cheese (11 g fat, of which 1 g was SFA). (B) Hard cheese, 65 g/d (15 g fat, of which 10 g was SFA). | Two 4-week periods, washout period not specified | Serum TC and LDL concentration was lower in group A, 2 and 4 weeks after use of rapeseed oil-based cheese compared to group B (control). |
| Author | Country | Study Design | Population | Age (Years) | Intervention | Duration | Main Findings |
|---|---|---|---|---|---|---|---|
| Lee et al. 2016 [78] | Korea | Parallel | n = 58 (29 male; 29 female), overweight and obese with metabolic syndrome | 35–65 | 2 arm: (A) 400 mL per day (200 mL twice daily) of low-fat milk. (B) Control—maintain habitual diet. | 6 weeks | No significant differences in body mass index, blood pressure, lipid profile and adiponectin levels, as well as levels of inflammatory markers, oxidative stress markers and atherogenic markers were found between groups. |
| Hidaka et al. 2012 [79] | Japan | Parallel | n = 14 (8 male; 6 female), healthy volunteers | Mean: 28.6 ± 6.0 S.D | 2 arm: (A) 500 mL/d whole milk. (B) non-fat milk, 500 mL/d. | 4 weeks | Group B showed lowering of plasma triglyceride (TG), phospholipid levels, TG level in HDL and increased plasma apolipoprotein (apo) C-III level. TG/cholesterol ratios in HDL and LDL also significantly decreased in group B. Whole milk consumption showed increases in plasma levels of apoC-III and apoE. C. |
| Rosado et al. 2011 [80] | Mexico | Parallel | n = 139 females, obese | 25–45 | 3 arm: (A) 250 mL of low-fat milk, 3 times per day, and an energy-restricted diet (500 kcal/day). (B) Low-fat milk, 250 mL, with added micronutrients, 3 times per day, an energy-restricted diet (500 kcal/day). (C) Control—an energy-restricted diet (500 kcal/day) with no intake of milk. | 16 weeks | Group B lost significantly more weight compared with group A + C. BMI and body fat changes were also significantly greater in the group B compared with group A + C. No differences were found between groups in glucose level, blood lipid profile, C-reactive protein level or blood pressure. |
| Venkatramanan et al. 2010 [81] | Canada | Crossover | n = 18 (11 male; 7 female) moderately overweight and borderline hyperlipidaemic | 30–60 | 3 arm: (A) 1000 mL/d milk naturally enriched CLA. (B) Milk enriched with synthetic CLA, 1000 mL/d. (C) Control—1000 mL/d untreated milk. | Three 8-week periods separated by 4-week washout periods | Group A + B failed to alter plasma TC, LDL, HDL or triacylglycerol concentrations; body weight; or fat composition compared with the control group. CLA consumption did not significantly affect plasma ALT (alanine transaminase), TBIL (total bilirubin in plasma), CRP (C-reactive protein) or TNF-a (tumor necrosis factor) concentrations. |
| Faghih et al. 2009 [82] | Iran | Parallel | n = 100 females, premenopausal and overweight or obese | 20–50 | 4 arm: (A) control—500 kcal/d deficit (500–600 mg/d dietary calcium). (B) Calcium-supplemented diet identical to control diet (800 mg/d of calcium carbonate). (C) Servings of low-fat milk, 220 mL, (1.5%) and 500 kcal/d deficit. (D) Three servings of calcium-fortified soy milk and 500 kcal/d deficit. | 8 weeks | Body weight, BMI, waist circumference (WC), waist-to-hip ratio (WHR), body fat mass and percent body fat decreased significantly across all groups. The changes in WC and WHR were significantly higher in groups C and D compared to controls. Reductions in weight and BMI were significantly greater in the group C compared to controls. Lipid profiles were not analysed. |
| Gardner et al. 2007 [77] | USA | Crossover | n = 28 (6 male; 22 female), hypercholesteraemic | 30–65 | 3 arm: 32 oz/d whole soy bean drink. (B) Soy protein isolate drink, 28 oz/d. (C) Dairy milk, 18.5 oz/d, (all volumes were standardised to yield 25 g protein/d. | Three 4-week periods, separated by 4-week washout periods | LDL was significantly lower after consuming soy milk in groups A + B compared to dairy milk (group C). No significant differences between groups were observed for HDL, triacylglycerols, insulin or glucose. |
| Barr et al. 2000 [83] | USA | Parallel | n = 200 (70 male; 130 female), healthy volunteers | 55–85 | 2 arm: (A) three 8 oz/d of skimmed 1% milk. (B) Maintain habitual diet and consuming <1.5 dairy servings per day. | 12 weeks | Similar decreases in blood pressure were apparent across both groups. TC, LDL and the ratio of TC:HDL remained unchanged. Triglyceride levels increased within the normal range in group A. |
| Author | Country | Study Design | Population | Age (Years) | Intervention | Duration | Main Findings |
|---|---|---|---|---|---|---|---|
| El Khoury et al. 2014 [84] | Canada | Crossover | n = 20 males, BMI 20–24.9 kg/m² | 20–30 | 5 arm: (A) 250 g non-fat yoghurt—plain. (B) Non-fat yoghurt with honey—plain, 250 g. (C) Non-fat yoghurt, strawberry flavoured, 250 g. (D) Skimmed milk, 250 g. (E) Orange Juice, 250 g. | Postprandial | Pre-meal glucose responses were dose-dependent to increasing protein and decreasing sugars in dairy. Protein:carbohydrate ratio correlated negatively with pre-meal glucose due to improved efficacy of insulin action. Compared with treatment E, blood glucose was lower after dairy snack treatments, contribution of dairy products to post-meal glucose was independent of their protein:carbohydrate ratio |
| Shab-Bidar et al. 2011 [85] | Iran | Parallel | n = 100 (43 male; 57 female), type 2 diabetes | 29–67 | 2 arm: (A) 250 mL vitamin D3 fortified yoghurt drink, twice a day (B) 250 mL plain yoghurt fortified drink, twice a day. | 12 weeks | Diet A showed significant improvement in fasting glucose, glycated haemoglobin (HbA1c), TAG, HDL cholesterol, endothelin-1, E-selectin and MMP-9 compared with the control (diet B). |
| Sadrzadeh-Yeganeh et al. 2010 [86] | Iran | Parallel | n = 90 females, healthy volunteers | 19–49 | 3 arm: (A) 300 g/d probiotic yoghurt. (B) Conventional yoghurt, 300 g/d. (C) Control, no consumption of fermented products | 6 weeks | No significant difference in lipid profile within any group. No difference in TAG and LDL across the groups. There was a decrease in cholesterol in both group A + B compared with the control as well as a decrease in TC:HDL ratio. HDL increased in group A compared with the control. |
| Ejtahed et al. 2010 [87] | Iran | Parallel | n = 60 (23 male; 37 female), type 2 diabetes | 30–60 | 2 arm: (A) 300 g/d probiotic yoghurt. (B) Control, 300 g/d conventional yoghurt | 6 weeks | Diet A caused a decrease in TC and LDL compared with the control. No significant changes from baseline were shown in TAG and HDL in diet A. The TC:HDL ratio and LDL:HDL ratio as atherogenic indices significantly decreased in after diet A compared with the control. |
| Ataie-Jafari et al. 2009 [88] | Iran | Crossover | n = 14 (4 male; 10 female), mild to moderate hypercholesteraemic | 40–64 | 2 arm: (A) 3 × 100 g/d probiotic yoghurt, Lactobacillus acidophilus and Bifidobacterium lactis (B) Three × 100 g/day control yoghurt. Both yoghurts contain 2.5% fat. | two 6-week periods, separated by a 4-week washout period | Consumption of diet A caused a significant decrease in serum TC compared with the control. No differences were reported for remaining blood lipids examined between the two diets. |
| Kiessling et al. 2002 [89] | Germany | Crossover | n = 29 females, normo- and hypercholesterolaemic | 19–56 | 2 arm: (A) 300 g/d control yoghurt streptococcus thermophilus and L. lactis. (B) Probiotic yoghurt, 300 g/d, enriched with L. acidophilus 145, B. longum 913 and 1% oligofructose. | 6 weeks (all on control diet), followed by two 6-week periods, separated by a 9-day washout periods. | Serum TC and LDL concentrations were not influenced by diet A. The HDL concentration increased significantly after diet A. The ratio of LDL:HDL cholesterol decreased. Long-term consumption of 300 g yoghurt increased HDL and lead to desired improvement of LDL:HDL ratio in both diets. |
| Rizkalla et al. 2000 [90] | France | Crossover | n = 24 males, healthy volunteers | 20–60 | 2 arm: (A) 500 g/d fresh yoghurt w/ live bacterial cultures. (B) Heated yoghurt, 500 g/d | Two 15-day periods, separated by a 15-day washout period | No changes detected in fasting plasma glucose, insulin, fatty acid, TAG or cholesterol concentrations in both groups. Plasma butyrate was higher and plasma propionate tended to be higher in subjects without lactose malabsorption after diet A than B. Subjects with lactose malabsorption increased propionate production after fresh yoghurt consumption compared with baseline measures. |
| Agerholm-Larson et al. 2000 [91] | Denmark | Parallel | n = 70 (20 male; 50 female), healthy volunteers | 18–55 | 5 arm: (A) 450 mL/d yoghurt fermented with two strains of Streptococcus thermophilus and two strains of Lactobacillus acidophilus. (B) Placebo yoghurt, 450 mL/d, fermented with delta-acid-lactone. (C) Yoghurt, 450 mL/d, fermented with two strains of Streptococcus thermophilus and one strain of Lactobacillus rhamnosus. (D) Yoghurt, 450 mL/d, fermented with one strain of Enterococcus faecium and two strains of Streptococcus thermophilus. (E) Two placebo pills per day. | 8 weeks | Comparing all 5 groups, no statistical effects on LDL were observed after consumption of diet D. After adjusting for small changes in body weight, LDL decreased by 8.4% and fibrinogen increased. This was significantly different from the control groups, B + E. After diets A + D, systolic blood pressure reduced significantly more compared to diet C. |
| Anderson and Gilliland, 1999 [92] | USA | Study 1: single-blind, parallel. Study 2: double-blind, crossover. | Study 1: n = 29 (9 male; 20 female). Study 2: n = 40 (18 male; 22 female), all hypercholesterolaemic | 49–55 | Study 1: 2 arm: (A1) 200 g/d fermented milk yoghurt containing human L. acidophilus L1. (B1) Fermented milk yoghurt, 200 g/d, containing swine L. acidophilus strain ATCC 43121 Study 2: 2 arm: (A2) 200 g/d fermented milk yoghurt containing L. acidophilus L1 strain. (B) Fermented milk yoghurt, 200 g/d, without these active bacteria. | Study 1: 3 weeks Study 2: Two 4-week periods with a 2-week washout period | Study 1: Diet A1 showed a significant 2.4% reduction in TC. LDL was lower after both treatment groups although not significant. HDL decreased significantly in both groups. Study 2: diet A2 reduced TC in the 1st treatment period but not in the 2nd. A combined analysis of the two treatment study interventions demonstrated a significant reduction in TC. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timon, C.M.; O’Connor, A.; Bhargava, N.; Gibney, E.R.; Feeney, E.L. Dairy Consumption and Metabolic Health. Nutrients 2020, 12, 3040. https://doi.org/10.3390/nu12103040
Timon CM, O’Connor A, Bhargava N, Gibney ER, Feeney EL. Dairy Consumption and Metabolic Health. Nutrients. 2020; 12(10):3040. https://doi.org/10.3390/nu12103040
Chicago/Turabian StyleTimon, Claire M., Aileen O’Connor, Nupur Bhargava, Eileen R. Gibney, and Emma L. Feeney. 2020. "Dairy Consumption and Metabolic Health" Nutrients 12, no. 10: 3040. https://doi.org/10.3390/nu12103040
APA StyleTimon, C. M., O’Connor, A., Bhargava, N., Gibney, E. R., & Feeney, E. L. (2020). Dairy Consumption and Metabolic Health. Nutrients, 12(10), 3040. https://doi.org/10.3390/nu12103040





