Effects of Interval Jump Rope Exercise Combined with Dark Chocolate Supplementation on Inflammatory Adipokine, Cytokine Concentrations, and Body Composition in Obese Adolescent Boys
Abstract
1. Introduction
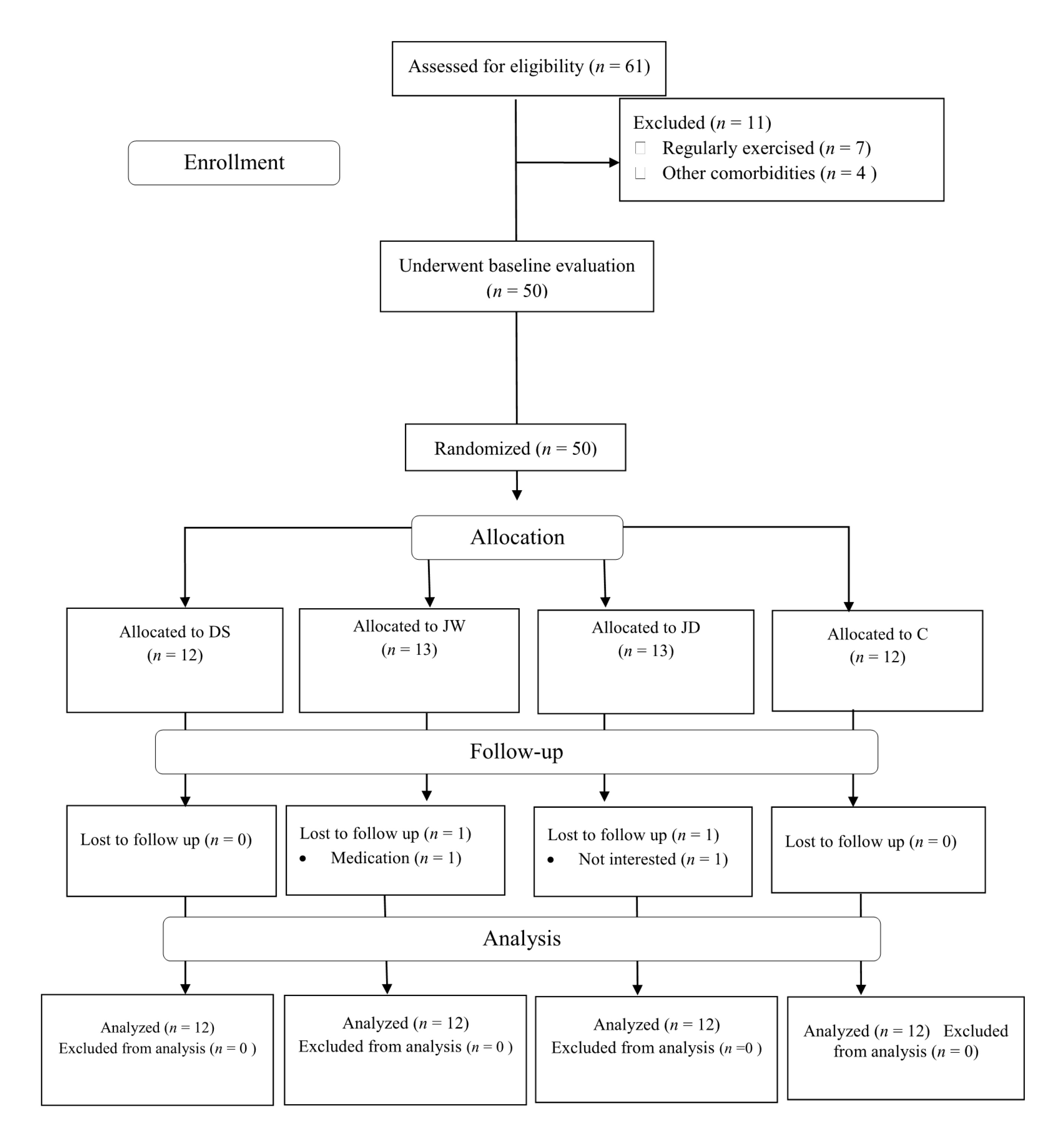
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Anthropometry and Body Composition
2.4. Blood Sampling and Laboratory Analysis
2.5. JRE Protocol
2.6. Dark Chocolate Supplementation
2.7. Nutrient Intake and Dietary Analysis
2.8. Statistical Analysis
3. Results
3.1. Body Composition
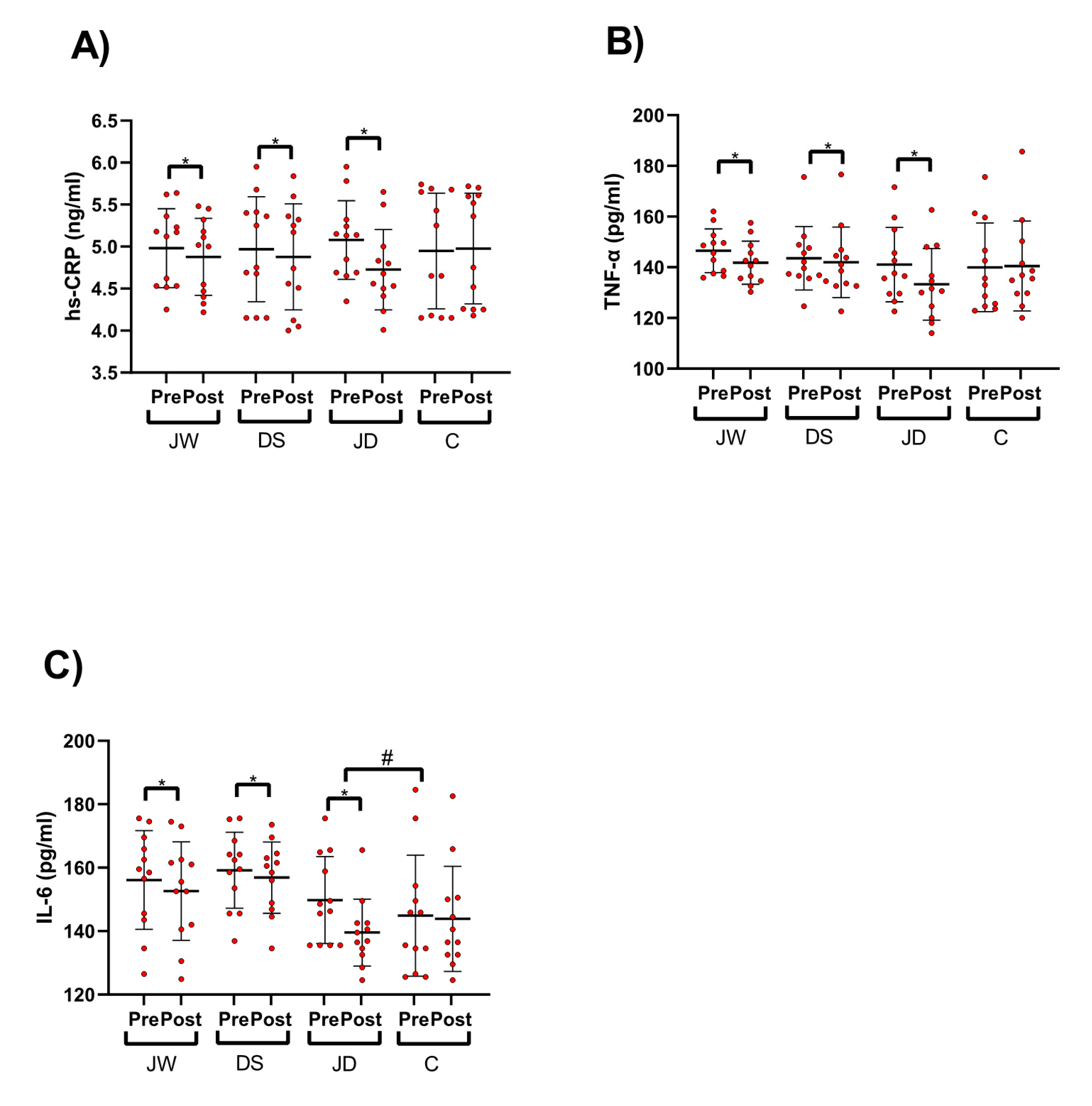
3.2. Pro-Inflammatory Cytokines
3.3. Pro-Inflammatory and Anti-Inflammatory Adipokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Chorin, E.; Hassidim, A.; Hartal, M.; Havakuk, O.; Flint, N.; Ziv-Baran, T.; Arbel, Y. Trends in adolescents obesity and the association between BMI and blood pressure: A cross-sectional study in 714,922 healthy teenagers. Am. J. Hypertens. 2015, 28, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.R.; Martins, F.M.; Souza, A.P.; Carneiro, M.A.; Orsatti, C.L.; Michelin, M.A.; Murta, E.F.; de Oliveira, E.P.; Orsatti, F.L. Effect of high-intensity interval training on body composition and inflammatory markers in obese postmenopausal women: A randomized controlled trial. Menopause 2019, 26, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Im, J.A.; Kim, K.C.; Park, J.H.; Suh, S.H.; Kang, E.S.; Kim, S.H.; Jekal, Y.; Lee, C.W.; Yoon, Y.J. Improved insulin sensitivity and adiponectin level after exercise training in obese Korean youth. Obesity 2007, 15, 3023–3030. [Google Scholar] [CrossRef]
- Tomeleri, C.M.; Ribeiro, A.S.; Souza, M.F.; Schiavoni, D.; Schoenfeld, B.J.; Venturini, D.; Barbosa, D.S.; Landucci, K.; Sardinha, L.B.; Cyrino, E.S. Resistance training improves inflammatory level, lipid and glycemic profiles in obese older women: A randomized controlled trial. Exp. Gerontol. 2016, 84, 80–87. [Google Scholar] [CrossRef]
- Mohamadzadeh Salamat, K.; Bakhtiari, N. The Effects of Endurance and Resistance Training on Systemic Inflammatory Markers and Metabolic Syndrome Parameters in Overweight and Obese Men. Rep. Health Care 2017, 3, 15–26. [Google Scholar]
- Sung, K.-D.; Pekas, E.J.; Scott, S.D.; Son, W.-M.; Park, S.-Y. The effects of a 12-week jump rope exercise program on abdominal adiposity, vasoactive substances, inflammation, and vascular function in adolescent girls with prehypertension. Eur. J. Appl. Physiol. 2019, 119, 577–585. [Google Scholar] [CrossRef]
- Kim, J.; Son, W.-M.; Headid, R.J., III; Pekas, E.J.; Noble, J.M.; Park, S.-Y. The effects of a 12-week jump rope exercise program on body composition, insulin sensitivity, and academic self-efficacy in obese adolescent girls. J. Pediatr. Endocrinol. Metab. 2020, 33, 129–137. [Google Scholar] [CrossRef]
- Loayza, D.; De Lange, T. POT1 as a terminal transducer of TRF1 telomere length control. Nature 2003, 423, 1013–1018. [Google Scholar] [CrossRef]
- Khan, N.; Khymenets, O.; Urpí-Sardà, M.; Tulipani, S.; Garcia-Aloy, M.; Monagas, M.; Mora-Cubillos, X.; Llorach, R.; Andres-Lacueva, C. Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients 2014, 6, 844–880. [Google Scholar] [CrossRef]
- Jafarirad, S.; Ayoobi, N.; Karandish, M.; Jalali, M.-T.; Haghighizadeh, M.H.; Jahanshahi, A. Dark chocolate effect on serum adiponectin, biochemical and inflammatory parameters in diabetic patients: A randomized clinical trial. Int. J. Prev. Med. 2018, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, N.; Rostami, A.; Eghtesadi, S.; Shidfar, F.; Heidari, I.; Hoseini, A. The effects of dark chocolate on lipid profile, apo-lipoprotein A-1, apo-lipoprotein B and inflammation in type-2 diabetic patients: A randomized clinical trial. Iran. J. Nutr. Sci. Food Technol. 2013, 8, 21–30. [Google Scholar]
- Ellinger, S.; Stehle, P. Impact of cocoa consumption on inflammation processes—A critical review of randomized controlled trials. Nutrients 2016, 8, 321. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Arsenis, N.C.; Disanzo, B.L.; LaMonte, M.J. Effects of exercise training on chronic inflammation in obesity. Sports Med. 2013, 43, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Paltoglou, G.; Schoina, M.; Valsamakis, G.; Salakos, N.; Avloniti, A.; Chatzinikolaou, A.; Margeli, A.; Skevaki, C.; Papagianni, M.; Kanaka-Gantenbein, C. Interrelations among the adipocytokines leptin and adiponectin, oxidative stress and aseptic inflammation markers in pre-and early-pubertal normal-weight and obese boys. Endocrine 2017, 55, 925–933. [Google Scholar] [CrossRef]
- Sirico, F.; Bianco, A.; D’Alicandro, G.; Castaldo, C.; Montagnani, S.; Spera, R.; Di Meglio, F.; Nurzynska, D. Effects of physical exercise on adiponectin, leptin, and inflammatory markers in childhood obesity: Systematic review and meta-analysis. Child. Obes. 2018, 14, 207–217. [Google Scholar] [CrossRef]
- Izadpanah, A.; Barnard, R.J.; Almeda, A.J.E.; Baldwin, G.C.; Bridges, S.A.; Shellman, E.R.; Burant, C.F.; Roberts, C.K. A short-term diet and exercise intervention ameliorates inflammation and markers of metabolic health in overweight/obese children. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E542–E550. [Google Scholar] [CrossRef]
- Blüher, S.; Panagiotou, G.; Petroff, D.; Markert, J.; Wagner, A.; Klemm, T.; Filippaios, A.; Keller, A.; Mantzoros, C.S. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity 2014, 22, 1701–1708. [Google Scholar] [CrossRef]
- Rosner, B.; Prineas, R.; Loggie, J.; Daniels, S.R. Percentiles for body mass index in US children 5 to 17 years of age. J. Pediatr. 1998, 132, 211–222. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Daneghian, S.; Alipour, M.; Rafiei, H.; Ghanavati, M.; Mohammadpour, R.; Kooti, W.; Ashtary-Larky, P.; Afrisham, R. Waist circumference to height ratio: Better correlation with fat mass than other anthropometric indices during dietary weight loss in different rates. Int. J. Endocrinol. Metab. 2018, 16, e55023. [Google Scholar] [CrossRef]
- Patel, R.K.; Brouner, J.; Spendiff, O. Dark chocolate supplementation reduces the oxygen cost of moderate intensity cycling. J. Int. Soc. Sports Nutr. 2015, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.-P.J.; Rezzi, S.; Peré-Trepat, E.; Kamlage, B.; Collino, S.; Leibold, E.; Kastler, J.; Rein, D.; Fay, L.B.; Kochhar, S. Metabolic effects of dark chocolate consumption on energy, gut microbiota, and stress-related metabolism in free-living subjects. J. Proteome Res. 2009, 8, 5568–5579. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, A.; Mahmoodi, M.; Mard, S.A.; karimi Moghaddam, E. The effects of dark chocolate consumption on lipid profile, fasting blood sugar, liver enzymes, inflammation, and antioxidant status in patients with non-alcoholic fatty liver disease: A randomized, placebo-controlled, pilot study. J. Gastroenterol. Hepatol. Res. 2015, 4, 1858–1864. [Google Scholar]
- Ashtary-Larky, D.; Kheirollah, A.; Bagheri, R.; Ghaffari, M.A.; Mard, S.A.; Hashemi, S.J.; Mir, I.; Wong, A. A single injection of vitamin D 3 improves insulin sensitivity and β-cell function but not muscle damage or the inflammatory and cardiovascular responses to an acute bout of resistance exercise in vitamin D-deficient resistance-trained males. Br. J. Nutr. 2020, 123, 394–401. [Google Scholar] [CrossRef]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Alipour, M.; Motevalli, M.S.; Chebbi, A.; Laher, I.; Zouhal, H. Does Green Tea Extract Enhance the Anti-inflammatory Effects of Exercise on Fat Loss? Br. J. Clin. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Lafontan, M.; Viguerie, N. Role of adipokines in the control of energy metabolism: Focus on adiponectin. Curr. Opin. Pharmacol. 2006, 6, 580–585. [Google Scholar] [CrossRef]
- Shirali, S.; Daneghian, S.; Hosseini, S.A.; Ashtary-Larky, D.; Daneghian, M.; Mirlohi, M.-S. Effect of caffeine co-ingested with carnitine on weight, body-fat percent, serum leptin and lipid profile changes in male teen soccer players: A randomized clinical trial. Int. J. Pediatr. 2016, 4, 3685–3698. [Google Scholar]
- Racil, G.; Ounis, O.B.; Hammouda, O.; Kallel, A.; Zouhal, H.; Chamari, K.; Amri, M. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur. J. Appl. Physiol. 2013, 113, 2531–2540. [Google Scholar] [CrossRef]
- Ali, F.; Ismail, A.; Kersten, S. Molecular mechanisms underlying the potential antiobesity-related diseases effect of cocoa polyphenols. Mol. Nutr. Food Res. 2014, 58, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yu, S.; Lambert, J.D. Dietary cocoa ameliorates obesity-related inflammation in high fat-fed mice. Eur. J. Nutr. 2014, 53, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Nagasawa, A.; Suzuki, J.; Hase, T.; Tokimitsu, I. Beneficial effects of tea catechins on diet-induced obesity: Stimulation of lipid catabolism in the liver. Int. J. Obes. 2002, 26, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and dark chocolate polyphenols: From biology to clinical applications. Front. Immunol. 2017, 8, 677. [Google Scholar] [CrossRef] [PubMed]
- Lubkowska, A.; Radecka, A.; Bryczkowska, I.; Rotter, I.; Laszczyńska, M.; Dudzińska, W. Serum adiponectin and leptin concentrations in relation to body fat distribution, hematological indices and lipid profile in humans. Int. J. Environ. Res. Public Health 2015, 12, 11528–11548. [Google Scholar] [CrossRef]
- Tsukinoki, R.; Morimoto, K.; Nakayama, K. Association between lifestyle factors and plasma adiponectin levels in Japanese men. Lipids Health Dis. 2005, 4, 27. [Google Scholar] [CrossRef][Green Version]
- Simpson, K.A.; Singh, M.A.F. Effects of exercise on adiponectin: A systematic review. Obesity 2008, 16, 241–256. [Google Scholar] [CrossRef]
- Nassis, G.P.; Papantakou, K.; Skenderi, K.; Triandafillopoulou, M.; Kavouras, S.A.; Yannakoulia, M.; Chrousos, G.P.; Sidossis, L.S. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism 2005, 54, 1472–1479. [Google Scholar] [CrossRef]
- Ahmadizad, S.; Ghorbani, S.; Ghasemikaram, M.; Bahmanzadeh, M. Effects of short-term nonperiodized, linear periodized and daily undulating periodized resistance training on plasma adiponectin, leptin and insulin resistance. Clin. Biochem. 2014, 47, 417–422. [Google Scholar] [CrossRef]
- Zakavi, I.; Bizhani, B.; Ghaisii, M.B.H.E. The Effect of an Eight-Week Rope Skipping Exercise Program on Interleukin-10 and C-Reactive Protein in Overweight and Obese Adolescents. Jentashapir J. Health Res. 2015, 6. [Google Scholar] [CrossRef]
- Kuebler, U.; Arpagaus, A.; Meister, R.E.; von Känel, R.; Huber, S.; Ehlert, U.; Wirtz, P.H. Dark chocolate attenuates intracellular pro-inflammatory reactivity to acute psychosocial stress in men: A randomized controlled trial. Brain Behav. Immun. 2016, 57, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Khan, N.; Andres-Lacueva, C.; Casas, R.; Urpi-Sarda, M.; Llorach, R.; Lamuela-Raventos, R.M.; Estruch, R. Effect of cocoa powder on the modulation of inflammatory biomarkers in patients at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2009, 90, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Ghafourian, M.; Ashtary-Larky, D.; Chinipardaz, R.; Eskandary, N.; Mehavaran, M. Inflammatory biomarkers’ response to two different intensities of a single bout exercise among soccer players. Iran. Red Crescent Med. J. 2016, 18, e21498. [Google Scholar] [CrossRef] [PubMed]
- Al-Suhaimi, E.A.; Shehzad, A. Leptin, resistin and visfatin: The missing link between endocrine metabolic disorders and immunity. Eur. J. Med. Res. 2013, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, A.; Savia, G.; Tagliaferri, M.; Lucantoni, R.; Berselli, M.; Petroni, M.; De Medici, C.; Viberti, G. Serum leptin concentration in moderate and severe obesity: Relationship with clinical, anthropometric and metabolic factors. Int. J. Obes. 1999, 23, 1066–1073. [Google Scholar] [CrossRef]
- Al Maskari, M.Y.; Alnaqdy, A.A. Correlation between serum leptin levels, body mass index and obesity in Omanis. Sultan Qaboos Univ. Med. J. 2006, 6, 27–31. [Google Scholar] [PubMed]
- Degawa-Yamauchi, M.; Bovenkerk, J.E.; Juliar, B.E.; Watson, W.; Kerr, K.; Jones, R.; Zhu, Q.; Considine, R.V. Serum resistin (FIZZ3) protein is increased in obese humans. J. Clin. Endocrinol. Metab. 2003, 88, 5452–5455. [Google Scholar] [CrossRef]
- Heilbronn, L.; Rood, J.; Janderova, L.; Albu, J.; Kelley, D.; Ravussin, E.; Smith, S. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J. Clin. Endocrinol. Metab. 2004, 89, 1844–1848. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, W.; Tang, J.; Yuan, Z. The adipocytokine resistin stimulates the production of proinflammatory cytokines TNF-α and IL-6 in pancreatic acinar cells via NF-κB activation. J. Endocrinol. Investig. 2013, 36, 986–992. [Google Scholar]
- Stofkova, A. Resistin and visfatin: Regulators of insulin sensitivity, inflammation and immunity. Endocr. Regul. 2010, 44, 25–36. [Google Scholar] [CrossRef]
- Sennello, J.A.; Fayad, R.; Morris, A.M.; Eckel, R.H.; Asilmaz, E.; Montez, J.; Friedman, J.M.; Dinarello, C.A.; Fantuzzi, G. Regulation of T cell-mediated hepatic inflammation by adiponectin and leptin. Endocrinology 2005, 146, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Agell, M.; Urpi-Sarda, M.; Sacanella, E.; Camino-López, S.; Chiva-Blanch, G.; Llorente-Cortés, V.; Tobias, E.; Roura, E.; Andres-Lacueva, C.; Lamuela-Raventos, R. Cocoa consumption reduces NF-κB activation in peripheral blood mononuclear cells in humans. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Görgens, S.W.; Eckardt, K.; Jensen, J.; Drevon, C.A.; Eckel, J. Exercise and Regulation of Adipokine and Myokine Production. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2015; Volume 135, pp. 313–336. [Google Scholar]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Ghaedi, E.; Zakerkish, M.; Ghadiri, A.; Ashtary-larky, D.; Safari, M.; Parsanahad, M.; Alipour, M. Effects of ginseng extract on chemerin, apelin and glycemic biomarkers in type 2 diabetic patients. Indian J. Physiol. Pharm. 2017, 61, 152–158. [Google Scholar]
- Neuparth, M.J.; Proença, J.B.; Santos-Silva, A.; Coimbra, S. The positive effect of moderate walking exercise on chemerin levels in Portuguese patients with type 2 diabetes mellitus. J. Investig. Med. 2014, 62, 350–353. [Google Scholar] [CrossRef]
- Saremi, A.; Shavandi, N.; Parastesh, M.; Daneshmand, H. Twelve-Week aerobic training decreases chemerin level and improves cardiometabolic risk factors in overweight and obese men. Asian J. Sports Med. 2010, 1, 151–158. [Google Scholar] [CrossRef]
- Kord-Varkaneh, H.; Ghaedi, E.; Nazary-Vanani, A.; Mohammadi, H.; Shab-Bidar, S. Does cocoa/dark chocolate supplementation have favorable effect on body weight, body mass index and waist circumference? A systematic review, meta-analysis and dose-response of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2018, 59, 2349–2362. [Google Scholar] [CrossRef]
- Matsui, N.; Ito, R.; Nishimura, E.; Yoshikawa, M.; Kato, M.; Kamei, M.; Shibata, H.; Matsumoto, I.; Abe, K.; Hashizume, S. Ingested cocoa can prevent high-fat diet-induced obesity by regulating the expression of genes for fatty acid metabolism. Nutrition 2005, 21, 594–601. [Google Scholar] [CrossRef]
- Min, S.; Yang, H.; Seo, S.; Shin, S.; Chung, M.; Kim, J.; Lee, S.; Lee, H.; Lee, K. Cocoa polyphenols suppress adipogenesis in vitro and obesity in vivo by targeting insulin receptor. Int. J. Obes. 2013, 37, 584–592. [Google Scholar] [CrossRef]
- Massolt, E.T.; van Haard, P.M.; Rehfeld, J.F.; Posthuma, E.F.; van der Veer, E.; Schweitzer, D.H. Appetite suppression through smelling of dark chocolate correlates with changes in ghrelin in young women. Regul. Pept. 2010, 161, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, L.B.; Astrup, A. Eating dark and milk chocolate: A randomized crossover study of effects on appetite and energy intake. Nutr. Diabetes 2011, 1, e21. [Google Scholar] [CrossRef] [PubMed]
- Farhat, G.; Drummond, S.; Fyfe, L.; Al-Dujaili, E.A. Dark chocolate: An obesity paradox or a culprit for weight gain? Phytother. Res. 2014, 28, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Merchant, A.T. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr. J. 2008, 7, 26. [Google Scholar] [CrossRef]
- O’brien, C.; Young, A.; Sawka, M. Bioelectrical impedance to estimate changes in hydration status. Int. J. Sports Med. 2002, 23, 361–366. [Google Scholar] [CrossRef]
- Deurenberg, P. Limitations of the bioelectrical impedance method for the assessment of body fat in severe obesity. Am. J. Clin. Nutr. 1996, 64, 449S–452S. [Google Scholar] [CrossRef]
- Medicine, A.C.o.S. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Ling, C.H.; de Craen, A.J.; Slagboom, P.E.; Gunn, D.A.; Stokkel, M.P.; Westendorp, R.G.; Maier, A.B. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin. Nutr. 2011, 30, 610–615. [Google Scholar] [CrossRef]
- Jackson, A.; Pollock, M.L.; Graves, J.E.; Mahar, M. Reliability and validity of bioelectrical impedance in determining body composition. J. Appl. Physiol. 1988, 64, 529–534. [Google Scholar] [CrossRef]

| Week | Intensity (Jump/min) | Training Duration | ||
|---|---|---|---|---|
| Warm-Up (5 min) | Exercise (30 min) | Cool-Down (5 min) | ||
| 1 | 60 | Stretching | 20 sets of 1 min of exercise, followed by 30 s of rest | Stretching |
| 2 | 60 | 15 sets of 1.5 min of exercise, followed by 30 s of rest | ||
| 3 | 60 | 12 sets of 2 min of exercise, followed by 30 s of rest | ||
| 4 | 90 | 10 sets of 2.5 min of exercise, followed by 30 s of rest | ||
| 5 | 90 | 9 sets of 3 min of exercise, followed by 30 s of rest | ||
| 6 | 90 | 7 sets of 4 min of exercise, followed by 30 s of rest | ||
| Content Per Dose | Dark Chocolate | White Chocolate |
|---|---|---|
| Energy (kcal) | 184.5 | 168.8 |
| Total fat (g) | 14.6 | 10.7 |
| Carbohydrate (g) | 5.1 | 14.7 |
| Protein (g) | 8.2 | 3.4 |
| Cacao polyphenol (mg) | 2650 | 0 |
| Epicatechin (mg) | 160 | 0 |
| Caffeine (mg) | 130 | 0 |
| Theobromine (mg) | 960 | 0 |
| Cocoa (%) | 83 | 0 |
| Variable | Group | Baseline | Post-Intervention | P |
|---|---|---|---|---|
| Energy (kcal/d) | JW | 1969.8 ± 45.1 | 1957 ± 42.3 | 0.384 |
| DS | 1958.7 ± 71.2 | 1953.1 ± 69.7 | 0.540 | |
| JD | 1982.5 ± 58.6 | 1968.8 ± 43 | 0.428 | |
| C | 1959.1 ± 44.3 | 1954 ± 49.2 | 0.721 | |
| Protein (g/d) | JW | 94.2 ± 5.1 | 94.3 ± 6 | 0.959 |
| DS | 92.5 ± 5.6 | 93 ± 5.7 | 0.702 | |
| JD | 94.8 ± 4.9 | 94.2 ± 5.7 | 0.749 | |
| C | 93.2 ± 4.3 | 93.1 ± 4.2 | 0.925 | |
| Fat (g/d) | JW | 69.1 ± 3.2 | 68 ± 3.5 | 0.370 |
| DS | 66.4 ± 3 | 66.1 ± 4.3 | 0.782 | |
| JD | 68.2 ± 3.2 | 66.8 ± 2.3 | 0.173 | |
| C | 68.1 ± 3.7 | 68 ± 3.5 | 0.944 | |
| CHO (g/d) | JW | 242.5 ± 9.3 | 241.9 ± 7.1 | 0.812 |
| DS | 247.6 ± 10.2 | 246.4 ± 7.7 | 0.578 | |
| JD | 247.2 ± 10.2 | 247.5 ± 10.1 | 0.915 | |
| C | 243.1 ± 10.4 | 242.1 ± 7 | 0.745 |
| Variables | Group | Baseline | Post-Intervention | Mean Change |
|---|---|---|---|---|
| Body mass (kg) | JW | 87.7 ± 5.6 | 86.4 ± 5.7 * | −1.2 ± 0.5 |
| DS | 88 ± 4.4 | 87.5 ± 4.5 * | −0.5 ± 0.3 | |
| JD | 89.7 ± 5.6 | 86.9 ± 5.6 * | −2.8 ± 0.5 | |
| C | 90.2 ± 5.7 | 90.3 ± 5.7 | 0.02 ± 0.2 | |
| BMI (kg/m2) | JW | 32 ± 2.2 | 31.5 ± 2.3 * | −0.4 ± 0.2 |
| DS | 31.3 ± 2.3 | 31.1 ± 2.2 * | −0.2 ± 0.1 | |
| JD | 32.4 ± 2.2 | 31.4 ± 2.3 * | −1 ± 0.1 | |
| C | 33.2 ± 2.9 | 33.2 ± 2.9 | 0.02 ± 0.1 | |
| WHR (m) | JW | 0.99 ± 0.03 | 0.98 ± 0.03 * | −0.006 ± 0.006 |
| DS | 0.98 ± 0.04 | 0.98 ± 0.04 * | −0.003 ± 0.004 | |
| JD | 0.99 ± 0.03 | 0.97 ± 0.03 * | −0.01 ± 0.004 | |
| C | 1 ± 0.02 | 1 ± 0.02 | 0.001 ± 0.007 | |
| FM (kg) | JW | 32 ± 1.8 | 31.7 ± 1.9 * | −0.2 ± 0.1 |
| DS | 32.2 ± 2.6 | 32.1 ± 2.5 * | −0.1 ± 0.06 | |
| JD | 32.8 ± 1.8 | 32.1 ± 1.8 * | −0.6 ± 0.1 | |
| C | 33 ± 1.9 | 33.1 ± 1.9 | 0.02 ± 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eskandari, M.; Hooshmand Moghadam, B.; Bagheri, R.; Ashtary-Larky, D.; Eskandari, E.; Nordvall, M.; Dutheil, F.; Wong, A. Effects of Interval Jump Rope Exercise Combined with Dark Chocolate Supplementation on Inflammatory Adipokine, Cytokine Concentrations, and Body Composition in Obese Adolescent Boys. Nutrients 2020, 12, 3011. https://doi.org/10.3390/nu12103011
Eskandari M, Hooshmand Moghadam B, Bagheri R, Ashtary-Larky D, Eskandari E, Nordvall M, Dutheil F, Wong A. Effects of Interval Jump Rope Exercise Combined with Dark Chocolate Supplementation on Inflammatory Adipokine, Cytokine Concentrations, and Body Composition in Obese Adolescent Boys. Nutrients. 2020; 12(10):3011. https://doi.org/10.3390/nu12103011
Chicago/Turabian StyleEskandari, Mozhgan, Babak Hooshmand Moghadam, Reza Bagheri, Damoon Ashtary-Larky, Elham Eskandari, Michael Nordvall, Frédéric Dutheil, and Alexei Wong. 2020. "Effects of Interval Jump Rope Exercise Combined with Dark Chocolate Supplementation on Inflammatory Adipokine, Cytokine Concentrations, and Body Composition in Obese Adolescent Boys" Nutrients 12, no. 10: 3011. https://doi.org/10.3390/nu12103011
APA StyleEskandari, M., Hooshmand Moghadam, B., Bagheri, R., Ashtary-Larky, D., Eskandari, E., Nordvall, M., Dutheil, F., & Wong, A. (2020). Effects of Interval Jump Rope Exercise Combined with Dark Chocolate Supplementation on Inflammatory Adipokine, Cytokine Concentrations, and Body Composition in Obese Adolescent Boys. Nutrients, 12(10), 3011. https://doi.org/10.3390/nu12103011








