A Priori and a Posteriori Dietary Patterns in Women of Childbearing Age in the UK
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sampling Procedure
2.2. Survey
2.3. Anthropometric Measurement
2.4. Food Frequency Questionnaire
2.5. Physical Activity
2.6. Dietary Patterns
2.7. Data Analyses
3. Results
3.1. Intake of MD Individual Food Components and MD Adherence
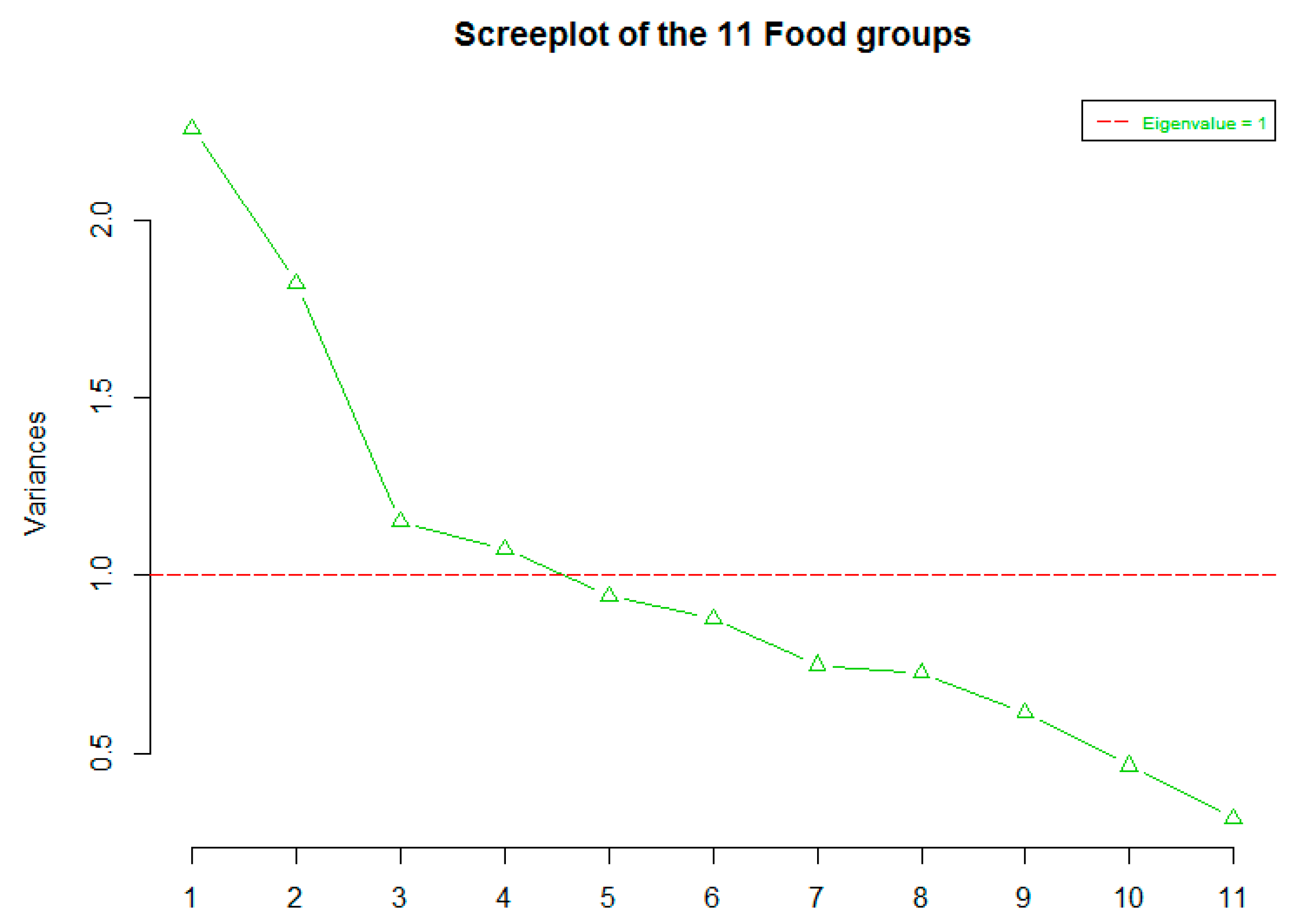
3.2. Factor Analysis
4. Discussion
Strengths and Limitations
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Food Groups | Food Items |
|---|---|
| Cereals | White bread, Brown and wholemeal bread/rolls, Crackers and cheese biscuits, Wholemeal and rye crackers, ‘Bran’ breakfast cereals, Other breakfast cereals, Added bran to foods, Brown and white rice, Pasta and dumplings, Pizza quiches/cheese flans, other puddings, Pastries, Buns, Cakes and gateaux, Biscuits—chocolate, Other biscuits—digestive and ginger. |
| Non-starchy vegetables | Vegetables, Tinned vegetables, Carrots, Parsnips, swede and turnip, Sweet-corn and mixed veg, Tomatoes, Spinach, Broccoli, Brussels sprouts and spring greens, Cabbage and cauliflower, Peppers and watercress, Onion, Green salad, Side salads in dressing, Courgettes, marrow and leeks, Mushrooms, Vegetable dishes. |
| Legumes | Peas and green beans, Beans and pulse. |
| Fruits | Fruit puddings, Tinned fruit not including grapefruit, prunes, figs or blackcurrants, Cooked fruit not including blackcurrants, Dried fruit, Fresh apples and pears, Fresh oranges and orange juice, Grapefruit and grapefruit juice, Blackcurrants, Ribena and hi-juice blackcurrant drinks, Other fruit juices (not squashes), Bananas, Fresh peaches, plums, cherries and grapes, Strawberries and raspberries, Fresh pineapple, melon, kiwi fruit and other tropical fruit. |
| Nuts | Hazelnuts, Cashews, Almonds, Brazil nuts, Walnuts. |
| Meat | Bacon and gammon, Pork, Lamb, Beef, Minced meat dishes, Meat pies, Liver and kidney, Pate and liver sausage, Faggots and black pudding, Sausages, Ham and luncheon meat, Chicken and turkey. |
| Fish | White fish, Fish fingers and fish dishes, Oily-fish, Shellfish. |
| Dairy Products | Cottage Cheese, Cheese, Pizza, quiches and cheese flans, Milk based puddings and sauces, Yogurt and fruit fools, Ice cream and chocolate desserts, Cream, Drinking chocolate and milk shakes not including McDonald style milkshakes. |
| MUFA+PUFA: SFA | Ratio of monounsaturated lipids and polyunsaturated lipids to saturated lipids. |
| Eggs | Mayonnaise/salad cream, Boiled/poached eggs, Omelette and fried eggs. |
| Potatoes | Potatoes boiled/jacket, Roast potatoes/chips, Crisps savoury snacks. |
| Sweets | Added sugar, chocolate, other sweets and sweet spreads. |
| Drinks | Diet coke/Pepsi not caffeine free, Coke and Pepsi, Soft drinks not diet drinks, drinking chocolate/milk shakes, decaffeinated coffee/tea, tea and coffee. |
| Miscellaneous | Gravy granules and powders, pickles chutney, ketchup, brown sauce, stock cubes, marmite, soup. |
References
- Nguyen, N.; Champion, J.; Ponce, J.; Quebbemann, B.; Patterson, E.; Pham, B.; Raum, W.; Buchwald, J.; Segato, G.; Favretti, F. A Review of Unmet Needs in Obesity Management. Obes. Surg. 2012, 22, 956–966. [Google Scholar] [CrossRef] [PubMed]
- World Health Statistics 2016: Monitoring Health for the SDGs. Available online: https://www.who.int/gho/publications/world_health_statistics/2016/Annex_B/en/ (accessed on 29 August 2020).
- Galland, L. Diet and Inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S. Relationship of Metabolic Risk Factors and Development of Cardiovascular Disease and Diabetes. Obesity 2006, 14, 121S–127S. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, K.; McCusker, C.; Connors, M.; ZuWallack, R.; Lahiri, B. The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chronic Respir. Dis. 2008, 5, 205–209. [Google Scholar] [CrossRef]
- Tanentsapf, I.; Heitmann, B.; Adegboye, A. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy Childbirth 2011, 11, 81. [Google Scholar] [CrossRef]
- Ryckman, K.; Spracklen, C.; Smith, C.; Robinson, J.; Saftlas, A. Maternal lipid levels during pregnancy and gestational diabetes: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 643–651. [Google Scholar] [CrossRef]
- Knight, M.; Bunch, K.; Tuffnell, D.; Shakespeare, J.; Kotnis, R.; Kenyon, S.; Kurinczuk, J.J. (Eds.) Saving Lives, Improving Mothers’ Care—Lessons learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2015–2017; National Perinatal Epidemiology Unit, University of Oxford: Oxford, UK, 2019. [Google Scholar]
- Bartley, K.; Underwood, B.; Deckelbaum, R. A life cycle micronutrient perspective for women’s health. Am. J. Clin. Nutr. 2005, 81, 1188S–1193S. [Google Scholar] [CrossRef]
- Harnisch, J.; Harnisch, P.; Harnisch, D. Family Medicine Obstetrics: Pregnancy and Nutrition. Prim. Care: Clin. Off. Pract. 2012, 39, 39–54. [Google Scholar] [CrossRef]
- Gardiner, P.; Nelson, L.; Shellhaas, C.; Dunlop, A.; Long, R.; Andrist, S.; Jack, B. The clinical content of preconception care: Nutrition and dietary supplements. Am. J. Obstet. Gynecol. 2008, 199, S345–S356. [Google Scholar] [CrossRef]
- Lumley, J.; Watson, L.; Watson, M.; Bower, C. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst. Rev. 2001, 3, CD001056. [Google Scholar]
- Temel, S.; van Voorst, S.; Jack, B.; Denktaş, S.; Steegers, E. Evidence-Based Preconceptional Lifestyle Interventions. Epidemiol. Rev. 2013, 36, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E. Micronutrient Intakes of British Adults Across Mid-Life: A Secondary Analysis of the UK National Diet and Nutrition Survey. Front. Nutr. 2018, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Thangaratinam, S.; Rogozińska, E.; Jolly, K.; Glinkowski, S.; Roseboom, T.; Tomlinson, J.; Kunz, R.; Mol, B.; Coomarasamy, A.; Khan, K. Effects of Interventions in Pregnancy on Maternal Weight and Obstetric Outcomes. Obstet. Gynecol. Surv. 2012, 67, 603–604. [Google Scholar] [CrossRef]
- Gunderson, E.; Sternfeld, B.; Wellons, M.; Whitmer, R.; Chiang, V.; Quesenberry Jr, C.; Lewis, C.; Sidney, S. Childbearing May Increase Visceral Adipose Tissue Independent of Overall Increase in Body Fat. Obesity 2008, 16, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Hoşcan, Y.; Yiğit, F.; Müderrisoğlu, H. Mediterranean Diet and Cardiovascular Diseases in an Turkish Population. J. Am. Coll. Cardiol. 2013, 62, C193–C194. [Google Scholar] [CrossRef][Green Version]
- Hu, F. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Schulze, M.; Martínez-González, M.; Fung, T.; Lichtenstein, A.; Forouhi, N. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef]
- Jacobs, D.; Steffen, L. Nutrients, foods, and dietary patterns as exposures in research: A framework for food synergy. Am. J. Clin. Nutr. 2003, 78, 508S–513S. [Google Scholar] [CrossRef]
- Kant, A. Dietary patterns and health outcomes. J. Am. Diet. Assoc. 2004, 104, 615–635. [Google Scholar] [CrossRef]
- Willett, W.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef]
- Toledo, E.; Lopez-del Burgo, C.; Ruiz-Zambrana, A.; Donazar, M.; Navarro-Blasco, Í.; Martínez-González, M.; de Irala, J. Dietary patterns and difficulty conceiving: A nested case–control study. Fertil. Steril. 2011, 96, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.A.J.M.; Soedamah-Muthu, S.S.; Callway, L.K.; Mishra, G. Pre-pregnancy dietary patterns and the risk of developing hypertensive disorders of pregnancy: Results from the Australian Longitudinal Study on Women’s Health. Am. J. Clin. Nutr. 2015, 102, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Melaki, V.; Sarri, K.; Apostolaki, I.; Roumeliotaki, T.; Ibarluzea, J.; Tardon, A.; Amiano, P.; Lertxundi, A.; Iniguez, C.; et al. Dietary patterns during pregnancy and the risk of postpartum depression: The mother-child ‘Rhea’ cohort in Crete, Greece. Public Health Nutr. 2011, 14, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Izadi, V.; Tehrani, H.; Haghighatdoost, F.; Dehghan, A.; Surkan, P.J.; Azadbakht, L. Adherence to the DASH and Mediterranean diets is associated with decreased risk for gestational diabetes mellitus. Nutrition 2016, 32, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Silva-del Valle, M.A.; Sanchez-Villegas, A.; Serra-Majem, L. Association between the adherence to the Mediterranean diet and overweight and obesity in pregnant women in Gran Crania. Nutr. Hosp. 2013, 28, 654–659. [Google Scholar]
- Orio, F.; Muscogiuri, G.; Palomba, S. Could the Mediterranean diet be effective in women with polycystic ovary syndrome? A proof of concept. Eur. J. Clin. Nutr. 2015, 69, 974. [Google Scholar] [CrossRef]
- Tsofliou, F.; Theodoridis, X.; Arvanitidou, I. Chapter 14: ‘Toward a Mediterranean-style diet outside the Mediterranean countries. In The Mediterranean Diet: An Evidence-Based Approach, 2nd ed.; Preedy, V., Watson, R., Eds.; Academic Press Elsevier: Cambridge, MA, USA, 2020. [Google Scholar]
- H. Al Wattar, B.; Dodds, J.; Placzek, A.; Beresford, L.; Spyreli, E.; Moore, A.; Carreras, F.G.; Austin, F.; Murugesu, N.; Roseboom, T.; et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): A pragmatic multicentre randomised trial. PLoS Med. 2019, 16, e1002857. [Google Scholar] [CrossRef]
- Kretowicz, H.; Hundley, V.; Tsofliou, F. Exploring the Perceived Barriers to Following a Mediterranean Style Diet in Childbearing Age: A Qualitative Study. Nutrients 2018, 10, 1694. [Google Scholar] [CrossRef]
- Panagiotakos, D.; Pitsavos, C.; Stefanadis, C. α-Priori and α-Posterior Dietary Pattern Analyses Have Similar Estimating and Discriminating Ability in Predicting 5-Y Incidence of Cardiovascular Disease: Methodological Issues in Nutrition Assessment. J. Food Sci. 2009, 74, H218–H224. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Quatromoni, P. The internal validity of a dietary pattern analysis. The Framingham Nutrition Studies. J. Epidemiol. Commun. Health 2002, 56, 381–388. [Google Scholar] [CrossRef]
- De Coster, J. Overview of Factor Analysis. Available online: http://www.stat-help.com/factor.pdf (accessed on 26 August 2020).
- Moeller, S.; Reedy, J.; Millen, A.; Dixon, L.; Newby, P.; Tucker, K.; Krebs-Smith, S.; Guenther, P. Dietary Patterns: Challenges and Opportunities in Dietary Patterns Research. J. Am. Diet. Assoc. 2007, 107, 1233–1239. [Google Scholar] [CrossRef]
- Slattery, M. Defining dietary consumption: Is the sum greater than its parts? Am. J. Clin. Nutr. 2008, 88, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Tielemans, M.; Erler, N.; Leermakers, E.; van den Broek, M.; Jaddoe, V.; Steegers, E.; Kiefte-de Jong, J.; Franco, O. A Priori and a Posteriori Dietary Patterns during Pregnancy and Gestational Weight Gain: The Generation R Study. Nutrients 2015, 7, 9383–9399. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, E.; van den Hooven, E.; Franco, O.; Jaddoe, V.; Moll, H.; Kiefte-de Jong, J.; Voortman, T. A priori and a posteriori derived dietary patterns in infancy and cardiometabolic health in childhood: The role of body composition. Clin. Nutr. 2018, 37, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Voortman, T.; Leermakers, E.; Franco, O.; Jaddoe, V.; Moll, H.; Hofman, A.; van den Hooven, E.; Kiefte-de Jong, J. A priori and a posteriori dietary patterns at the age of 1 year and body composition at the age of 6 years: The Generation R Study. Eur. J. Epidemiol. 2016, 31, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.; Zimmet, P.; Shaw, J. Metabolic syndrome-a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Robinson, S.; Godfrey, K.; Osmond, C.; Cox, V. Evaluation of a food frequency questionnaire used to assess nutrient intakes in pregnant women. Eur. J. Clin. Nutr. 1996, 50, 302–308. [Google Scholar]
- Food Safety Regulations. Available online: http://adlib.everysite.co.uk/resources/000/122/464/FSA_0421_0202.pdf (accessed on 26 August 2020).
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef]
- Kelaiditi, E.; Jennings, A.; Steves, C.; Skinner, J.; Cassidy, A.; MacGregor, A.; Welch, A. Measurements of skeletal muscle mass and power are positively related to a Mediterranean dietary pattern in women. Osteoporos. Int. 2016, 27, 3251–3260. [Google Scholar] [CrossRef]
- Papadaki, A.; Wood, L.; Sebire, S.; Jago, R. Adherence to the Mediterranean diet among employees in South West England: Formative research to inform a web-based, work-place nutrition intervention. Prev. Med. Rep. 2015, 2, 223–228. [Google Scholar] [CrossRef]
- Boghossian, N.; Yeung, E.; Mumford, S.; Zhang, C.; Gaskins, A.; Wactawski-Wende, J.; Schisterman, E. Adherence to the Mediterranean diet and body fat distribution in reproductive aged women. Eur. J. Clin. Nutr. 2013, 67, 289–294. [Google Scholar] [CrossRef]
- VanKim, N.; Austin, S.; Jun, H.; Hu, F.; Corliss, H. Dietary Patterns during Adulthood among Lesbian, Bisexual, and Heterosexual Women in the Nurses’ Health Study II. J. Acad. Nutr. Diet. 2017, 117, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Thiele, S.; Mensink, G.; Beitz, R. Determinants of diet quality. Public Health Nutr. 2004, 7, 29–37. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, S.; Previdelli, A.; Cesar, C.; Marchioni, D.; Fisberg, R. Trends in diet quality among adolescents, adults and older adults: A population-based study. Prev. Med. Rep. 2016, 4, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Hiza, H.; Casavale, K.; Guenther, P.; Davis, C. Diet Quality of Americans Differs by Age, Sex, Race/Ethnicity, Income, and Education Level. J. Acad. Nutr. Diet. 2013, 113, 297–306. [Google Scholar] [CrossRef]
- Verhoeven, A.; Adriaanse, M.; de Vet, E.; Fennis, B.; de Ridder, D. It’s my party and I eat if I want to. Reasons for unhealthy snacking. Appetite 2015, 84, 20–27. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Bes-Rastrollo, M.; Martínez-González, M.; Serra-Majem, L. Adherence to a Mediterranean dietary pattern and weight gain in a follow-up study: The SUN cohort. Int. J. Obes. 2005, 30, 350–358. [Google Scholar] [CrossRef]
- Woo, J.; Cheung, B.; Ho, S.; Sham, A.; Lam, T. Influence of dietary pattern on the development of overweight in a Chinese population. Eur. J. Clin. Nutr. 2007, 62, 480–487. [Google Scholar] [CrossRef]
- Rossi, M.; Negri, E.; Bosetti, C.; Dal Maso, L.; Talamini, R.; Giacosa, A.; Montella, M.; Franceschi, S.; La Vecchia, C. Mediterranean diet in relation to body mass index and waist-to-hip ratio. Public Health Nutr. 2008, 11, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Naska, A.; Orfanos, P.; Trichopoulos, D. Mediterranean diet in relation to body mass index and waist-to-hip ratio: The Greek European Prospective Investigation into Cancer and Nutrition Study. Am. J. Clin. Nutr. 2005, 82, 935–940. [Google Scholar] [CrossRef]
- Beunza, J.; Toledo, E.; Hu, F.; Bes-Rastrollo, M.; Serrano-Martínez, M.; Sánchez-Villegas, A.; Martínez, J.; Martínez-González, M. Adherence to the Mediterranean diet, long-term weight change, and incident overweight or obesity: The Seguimiento Universidad de Navarra (SUN) cohort. Am. J. Clin. Nutr. 2010, 92, 1484–1493. [Google Scholar] [CrossRef]
- Mendez, M.; Popkin, B.; Jakszyn, P.; Berenguer, A.; Tormo, M.; Sanchéz, M.; Quirós, J.; Pera, G.; Navarro, C.; Martinez, C.; et al. Adherence to a Mediterranean Diet Is Associated with Reduced 3-Year Incidence of Obesity. J. Nutr. 2006, 136, 2934–2938. [Google Scholar] [CrossRef] [PubMed]
- Romaguera, D.; Norat, T.; Vergnaud, A.; Mouw, T.; May, A.; Agudo, A.; Buckland, G.; Slimani, N.; Rinaldi, S.; Couto, E.; et al. Mediterranean dietary patterns and prospective weight change in participants of the EPIC-PANACEA project. Am. J. Clin. Nutr. 2010, 92, 912–921. [Google Scholar] [CrossRef]
- Schröder, H.; Marrugat, J.; Vila, J.; Covas, M.; Elosua, R. Adherence to the Traditional Mediterranean Diet Is Inversely Associated with Body Mass Index and Obesity in a Spanish Population. J. Nutr. 2004, 134, 3355–3361. [Google Scholar] [CrossRef] [PubMed]
- Romaguera, D.; Norat, T.; Mouw, T.; May, A.; Bamia, C.; Slimani, N. Adherence to the Mediterranean diet is associated with lower abdominal adiposity in European men and women. J. Nutr. 2009, 139, 1728–1737. [Google Scholar] [CrossRef]
- Olmedo-Requena, R.; Fernández, J.; Prieto, C.; Moreno, J.; Bueno-Cavanillas, A.; Jiménez-Moleón, J. Factors associated with a low adherence to a Mediterranean diet pattern in healthy Spanish women before pregnancy. Public Health Nutr. 2013, 17, 648–656. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Delgado-Rodríguez, M.; Martínez-González, M.; de Irala-Estévez, J. Gender, age, socio-demographic and lifestyle factors associated with major dietary patterns in the Spanish Project SUN (Seguimiento Universidad de Navarra). Eur. J. Clin. Nutr. 2003, 57, 285–292. [Google Scholar] [CrossRef]
- Lee, I.; Shiroma, E. Using accelerometers to measure physical activity in large-scale epidemiological studies: Issues and challenges. Br. J. Sports Med. 2013, 48, 197–201. [Google Scholar] [CrossRef]
- Roberts, K.; Cade, J.; Dawson, J.; Holdsworth, M. Empirically Derived Dietary Patterns in UK Adults Are Associated with Sociodemographic Characteristics, Lifestyle, and Diet Quality. Nutrients 2018, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Crozier, S.; Robinson, S.; Borland, S.; Inskip, H. Dietary patterns in the Southampton Women’s Survey. Eur. J. Clin. Nutr. 2006, 60, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M.; Bell, R. Fair society, healthy lives. Public Health 2012, 126, S4–S10. [Google Scholar] [CrossRef] [PubMed]
- Flint, E.; Webb, E.; Cummins, S. Change in commute mode and body-mass index: Prospective, longitudinal evidence from UK Biobank. Lancet Public Health 2016, 1, e46–e55. [Google Scholar] [CrossRef]
- Inglis, V.; Ball, K.; Crawford, D. Why do women of low socioeconomic status have poorer dietary behaviours than women of higher socioeconomic status? A qualitative exploration. Appetite 2005, 45, 334–343. [Google Scholar] [CrossRef]
- Prady, S.; Pickett, K.; Croudace, T.; Fairley, L.; Bloor, K.; Gilbody, S.; Kiernan, K.; Wright, J. Psychological Distress during Pregnancy in a Multi-Ethnic Community: Findings from the Born in Bradford Cohort Study. PLoS ONE 2013, 8, e60693. [Google Scholar] [CrossRef]
- Khaled, K.; Tsofliou, F.; Hundley, V.; Helmreich, R.; Almilaji, O. Perceived stress and diet quality in women of reproductive age: A systematic review and meta-analysis. Nutr. J. 2020, 19. [Google Scholar] [CrossRef] [PubMed]
- Milà-Villarroel, R.; Bach-Faig, A.; Puig, J.; Puchal, A.; Farran, A.; Serra-Majem, L.; Carrasco, J. Comparison and evaluation of the reliability of indexes of adherence to the Mediterranean diet. Public Health Nutr. 2011, 14, 2338–2345. [Google Scholar] [CrossRef]
- Barker, M.; Dombrowski, S.; Colbourn, T.; Fall, C.; Kriznik, N.; Lawrence, W.; Norris, S.; Ngaiza, G.; Patel, D.; Skordis-Worrall, J.; et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet 2018, 391, 1853–1864. [Google Scholar] [CrossRef]
- Cucó, G.; Fernández-Ballart, J.; Sala, J.; Viladrich, C.; Iranzo, R.; Vila, J.; Arija, V. Dietary patterns and associated lifestyles in preconception, pregnancy and postpartum. Eur. J. Clin. Nutr. 2005, 60, 364–371. [Google Scholar] [CrossRef]
| Participants’ Characteristics | Total Sample | Mediterranean Diet Adherence Categories | p Value | ||
|---|---|---|---|---|---|
| Low MDS (0–3) | Medium MDS (4–5) | High MDS (6–8) | |||
| N (%) | 48 (39) | 49 (40) | 26 (21) | ||
| Physical and lifestyle Characteristics | |||||
| Age (years) # | 25.5 (10.0) | 23.0 (10.0) | 24.5 (8.3) | 28.0 (10.5) | 0.006 |
| Age (years) * | |||||
| 18–24 | 56 (47.4) | 28 (23.7) | 24 (20.3) | 4 (3.4) | |
| 25–34 | 38 (32.2) | 9 (7.6) | 16 (13.6) | 13 (11.0) | 0.01 |
| 35–49 | 24 (20.3) | 9 (7.6) | 9 (7.6) | 6 (5.1) | |
| BMI (kg/m2) # | 24.3 (6.8) | 24.2 (6.8) | 24.3 (6.3) | 25.0 (7.4) | 0.53 |
| BMI * | |||||
| Overweight/obese | 59 (48.0) | 25 (20.4) | 21 (17.1) | 13 (10.6) | 0.485 |
| Normal Weight | 64 (52.0) | 23 (18.7) | 28 (22.8) | 13 (10.6) | |
| Waist Circumference (cm) # | 71.6 (48.0) | 71.1 (43.0) | 71.6 (51.0) | 80.0 (65.0) | 0.911 |
| Waist Circumference * | |||||
| Overweight/obese | 64 (62.7) | 26 (25.5) | 25 (24.5) | 13 (12.7) | 0.658 |
| Normal weight | 38 (37.3) | 12 (11.8) | 17 (16.7) | 9 (8.8) | |
| Waist to Height Ratio # | 0.45 (0.30) | 0.44 (0.28) | 0.45 (0.30) | 0.48 (0.41) | 0.797 |
| Waist to Height Ratio * | |||||
| Overweight/obese | 31 (30.4) | 12 (11.8) | 10 (9.8) | 9 (8.8) | |
| Normal weight | 71 (69.6) | 26 (25.5) | 32 (31.4) | 13 (12.7) | 0.361 |
| Physical Activity (MET) # | 2260 (3499.5) | 1443 (2711.25) | 2515 (4690.5) | 2055 (3049.5) | 0.477 |
| Physical Activity * | |||||
| Low (<600 MET minutes/week) | 24 (19.5) | 15 (12.2) | 6 (4.9) | 3 (2.4) | |
| Moderate (>600 MET minutes/week) | 47 (38.2) | 15 (12.2) | 20 (16.3) | 12 (9.8) | 0.126 |
| High (>3000 MET minutes/week) | 52 (42.2) | 18 (14.6) | 23 (18.7) | 11 (8.9) | |
| Socio-demographic characteristics | |||||
| Father’s Educational level * | |||||
| No qualifications | 14 (11.5) | 5 (4.1) | 6 (4.9) | 3 (2.5) | |
| Certificate of secondary education | 13 (10.6) | 6 (4.9) | 6 (4.9) | 1 (0.8) | |
| O-level or GCSE | 39 (32) | 19 (15.6) | 10 (8.2) | 10 (8.2) | 0.471 |
| A-level school examination | 15 (12.3) | 5 (4.1) | 8 (6.6) | 2 (1.6) | |
| Higher education | 41 (33.6) | 12 (9.8) | 19 (15.6) | 10 (8.2) | |
| Mother’s Educational level * | |||||
| No qualifications | 7 (5.7) | 2 (1.6) | 4 (3.3) | 1 (0.8) | |
| Certificate of secondary education | 7 (5.7) | 4 (3.3) | 2 (1.6) | 1 (0.8) | |
| O-level or GCSE | 46 (37.4) | 23 (18.7) | 17 (13.8) | 6 (4.9) | 0.377 |
| A-level school examination | 11 (9) | 4 (3.3) | 5 (4.1) | 2 (1.6) | |
| Higher education | 52 (42.3) | 15 (12.2) | 21 (17.1) | 16 (13) | |
| Household income (Pound/year) * | |||||
| <£13,000 | 17(13.8) | 9 (7.3) | 6 (4.9) | 2 (1.6) | |
| £13,000 to £23,400 | 28 (22.8) | 13 (10.6) | 11 (8.9) | 4 (3.3) | 0.528 |
| £23,400 to £33,800 | 38 (30.9) | 16 (13) | 14 (11.4) | 8 (6.5) | |
| £33,800 to £52,000 | 29 (23.6) | 8 (6.5) | 13 (10.6) | 8 (6.5) | |
| >£52,000 | 11 (8.9) | 2 (1.6) | 5 (4.1) | 4 (3.3) | |
| Marital Status * | |||||
| Single or divorced | 73 (59.8) | 31 (25.4) | 32 (26.2) | 10 (8.2) | 0.027 |
| Married or living together | 49 (40.2) | 16 (13.1) | 17 (14.0) | 16 (13.1) | |
| Smoking * | |||||
| Smoker | 26 (21.5) | 11 (9.1) | 9 (7.4) | 6 (5) | 0.616 |
| Non-smoker | 95 (78.5) | 37 (30.6) | 39 (32.2) | 19 (15.7) | |
| Occupation * | |||||
| Student | 117 (95.1) | 45 (36.6) | 48 (39.0) | 24 (19.5) | 0.391 |
| Associate professional or professional | 2 (1.6) | 0 (0) | 1 (0.8) | 1 (0.8) | |
| Clerical, sales or service worker | 4 (3.2) | 3 (2.4) | 0 (0) | 1 (0.8) | |
| Ethnicity * | |||||
| White | 71 (58.1) | 32 (26.2) | 27 (22.1) | 12 (9.8) | 0.353 |
| Other | 51 (41.7) | 16 (13.1) | 21 (17.2) | 14 (11.4) | |
| MD Food Components (Median (IQR)) | Total Sample (n = 123) | MD Adherence Categories | p Value | ||
|---|---|---|---|---|---|
| Low MDS (0–3) | Medium MDS (4–5) | High MDS (6–8) | |||
| Cereals (g/d) | 280.4 (239.4) | 270.0 (237.3) | 279.1 (258.7) | 316.1 (199.4) | 0.748 |
| Legumes (g/d) | 53.6 (87.9) | 30.7 (45.3) | 102.9 (68.6) | 97.5 (107.1) | <0.001 * |
| Vegetables (g/d) | 603.5 (432.4) | 381.5 (288.8) | 708.9 (395.9) | 809.3 (376.0) | <0.001 * |
| Fruits & Nuts (g/d) | 350.2 (364.1) | 217.9 (255.0) | 350.9 (322.3) | 568.8 (453.9) | <0.001 * |
| Meat (g/d) | 125.3 (139.9) | 137.2 (165.1) | 122.3 (109.5) | 112.1 (156.6) | 0.696 |
| Fish & seafood (g/d) | 32.1 (71.9) | 16.0 (25.3) | 42.2 (69.5) | 67.0 (79.5) | <0.001 * |
| Dairy products (g/d) | 343.6 (334.1) | 504.0 (440.3) | 311.9 (295.9) | 253.9 (243.5) | <0.001 * |
| (MUFA+PUFA)/SFA | 1.58 (0.63) | 1.42 (0.29) | 1.60 (0.70) | 2.07 (0.76) | <0.001 * |
| MD Score | 4.0 (2.0) | 2.5 (1.0) | 5.0 (1.0) | 6.0 (1.0) | <0.001 * |
| Food Groups | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| Cereals (g/d) | 0.83 | ||
| Legumes (g/d) | 0.48 | ||
| Vegetables (g/d) | 0.98 | ||
| Fruits and Nuts (g/d) | 0.40 | ||
| Meat (g/d) | 0.33 | ||
| Fish and seafood (g/d) | 0.79 | ||
| Eggs (g/d) | 0.42 | ||
| Potatoes (g/d) | 0.44 | ||
| Dairy Products (g/d) | 0.36 | ||
| Sweets (g/d) | 0.39 |
| Model | Predictor | Coefficient Estimate | p Value |
|---|---|---|---|
| 1 (Vegetarian-style dietary pattern) | Intercept | −0.3 | 0.2 |
| Physical Activity (METs- h/wk) | 0.00003 | 0.01 | |
| Parental Income (above average) | 0.3 | 0.09 | |
| Ethnicity (white) | −0.5 | <0.01 | |
| Mother’s education (A-level/higher) | 0.5 | <0.01 | |
| 2 (Dairy, sweets and starchy foods dietary pattern) | Intercept | 0.5 | 0.1 |
| Physical Activity (METs- h/wk) | −0.00002 | 0.06 | |
| Ethnicity (white) | 0.4 | 0.03 | |
| Age | −0.02 | 0.03 | |
| 3 (Protein-rich dietary pattern) | Intercept | −0.8 | <0.01 |
| Physical Activity (METs- h/wk) | −0.00002 | 0.01 | |
| Age | 0.03 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaled, K.; Hundley, V.; Almilaji, O.; Koeppen, M.; Tsofliou, F. A Priori and a Posteriori Dietary Patterns in Women of Childbearing Age in the UK. Nutrients 2020, 12, 2921. https://doi.org/10.3390/nu12102921
Khaled K, Hundley V, Almilaji O, Koeppen M, Tsofliou F. A Priori and a Posteriori Dietary Patterns in Women of Childbearing Age in the UK. Nutrients. 2020; 12(10):2921. https://doi.org/10.3390/nu12102921
Chicago/Turabian StyleKhaled, Karim, Vanora Hundley, Orouba Almilaji, Mareike Koeppen, and Fotini Tsofliou. 2020. "A Priori and a Posteriori Dietary Patterns in Women of Childbearing Age in the UK" Nutrients 12, no. 10: 2921. https://doi.org/10.3390/nu12102921
APA StyleKhaled, K., Hundley, V., Almilaji, O., Koeppen, M., & Tsofliou, F. (2020). A Priori and a Posteriori Dietary Patterns in Women of Childbearing Age in the UK. Nutrients, 12(10), 2921. https://doi.org/10.3390/nu12102921







