Evaluating the Memory Enhancing Effects of Angelica gigas in Mouse Models of Mild Cognitive Impairments
Abstract
1. Introduction
2. Results
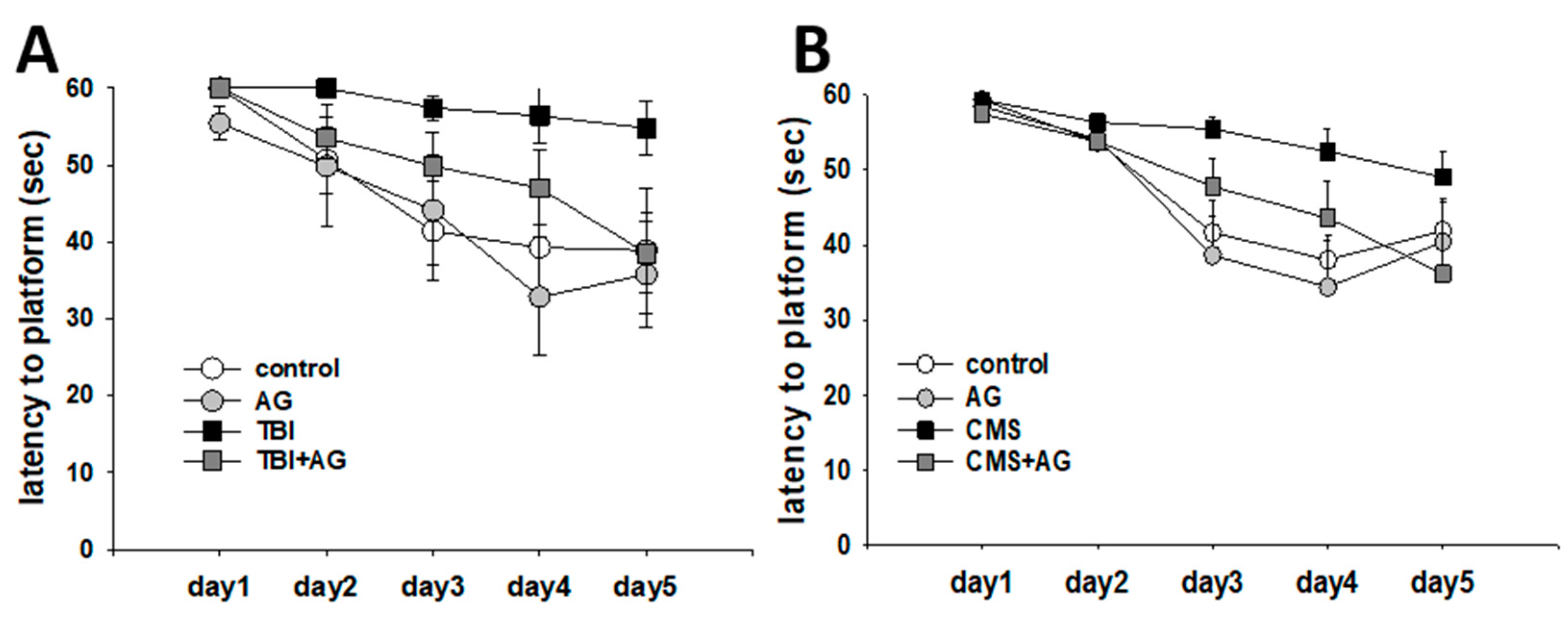
2.1. AG Improved TBI- and CMS-Induced Spatial Learning Deficit
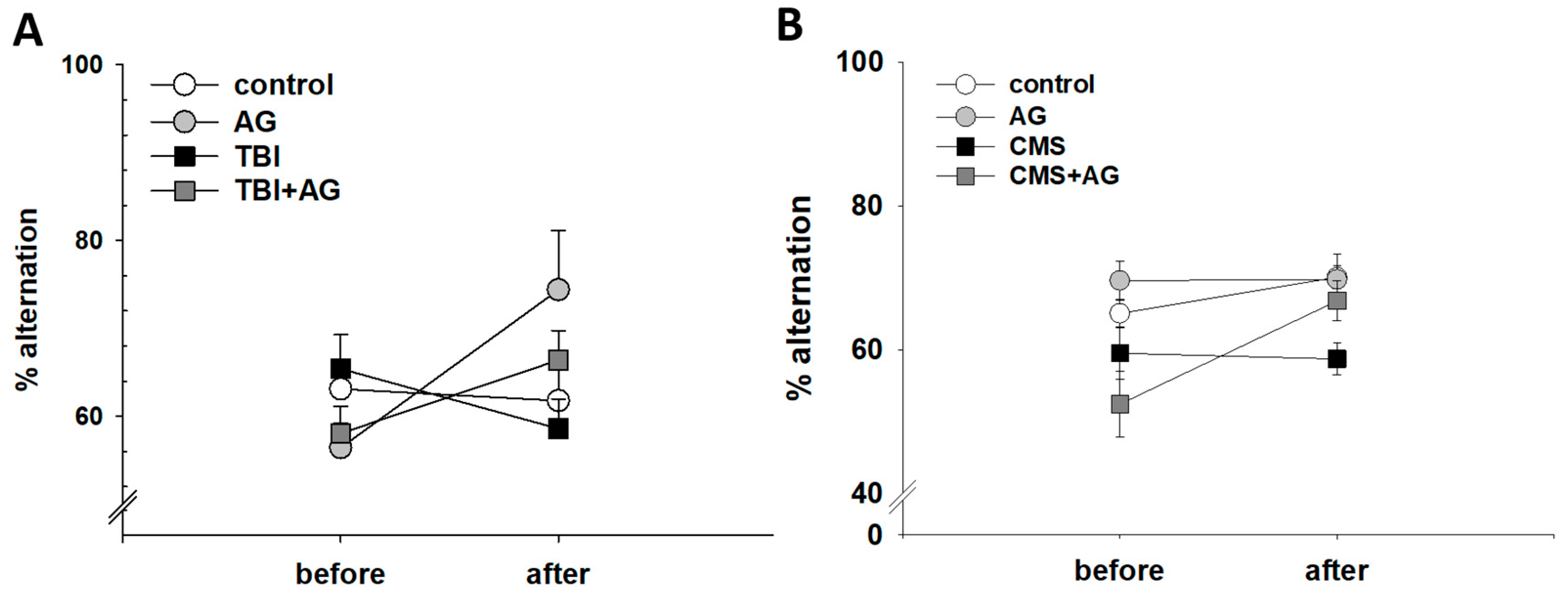
2.2. AG Improved Short-Term Working Memory
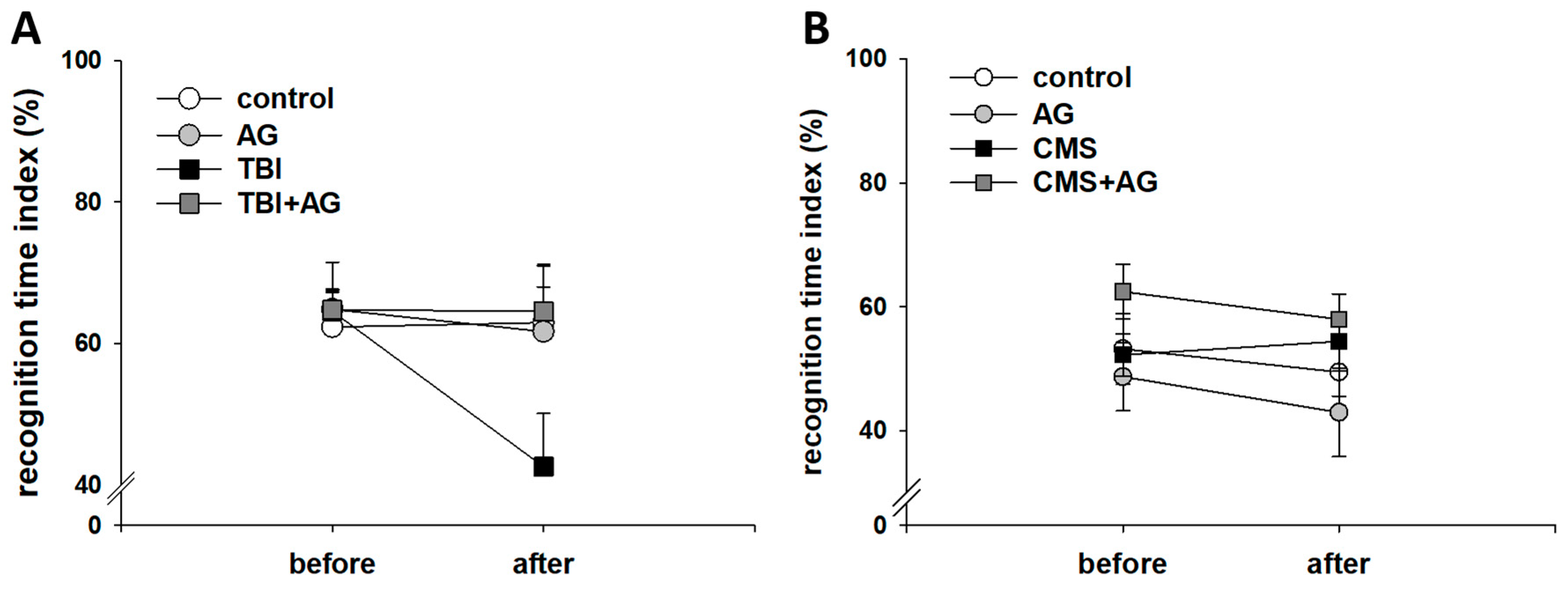
2.3. AG Had No Effect on Object Recognition Memory
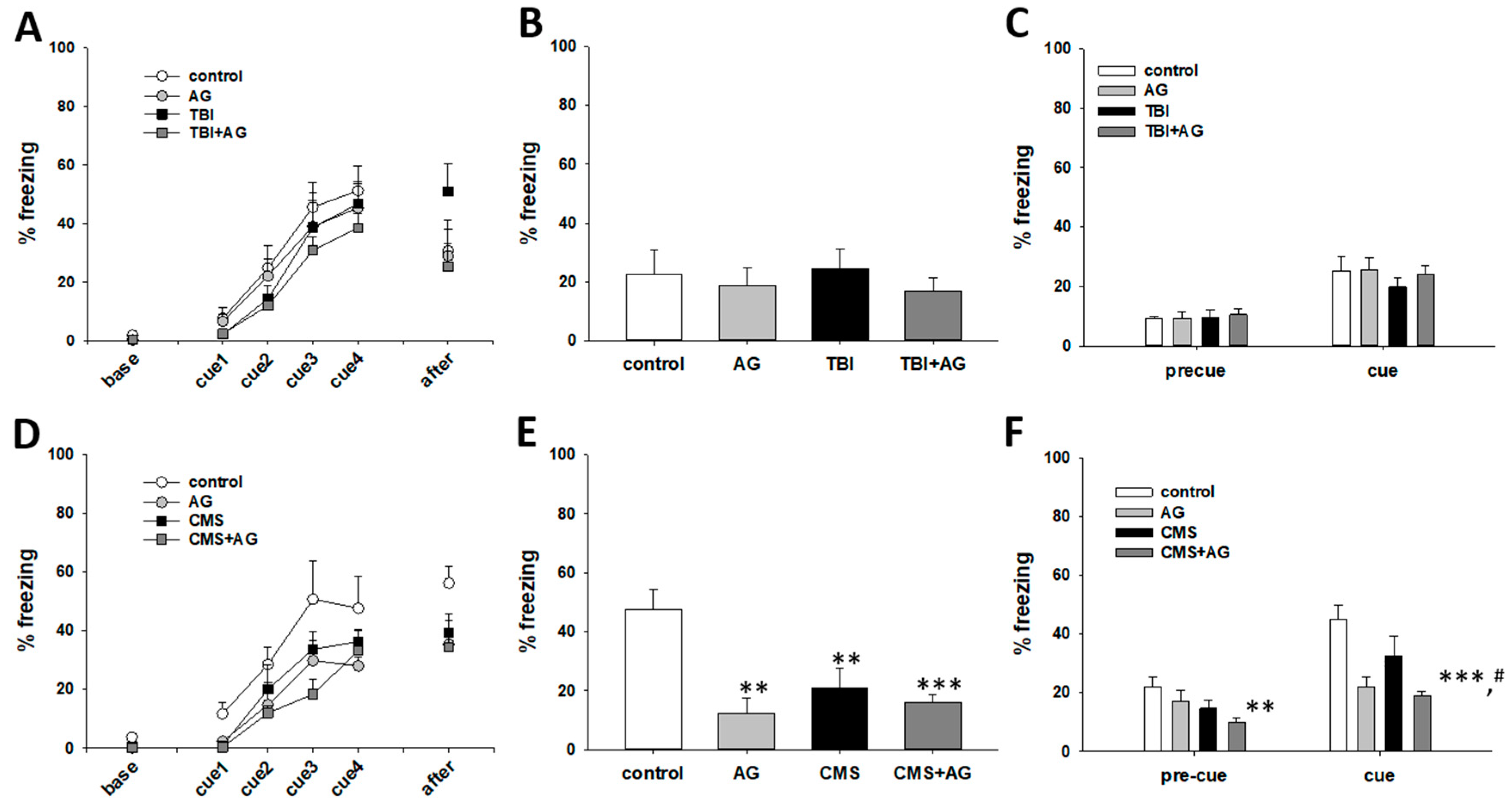
2.4. The Effect of AG on Fear Memory
3. Discussion
4. Materials and Methods
4.1. Preparation of AG Extract
4.2. Animals and Experimental Groups
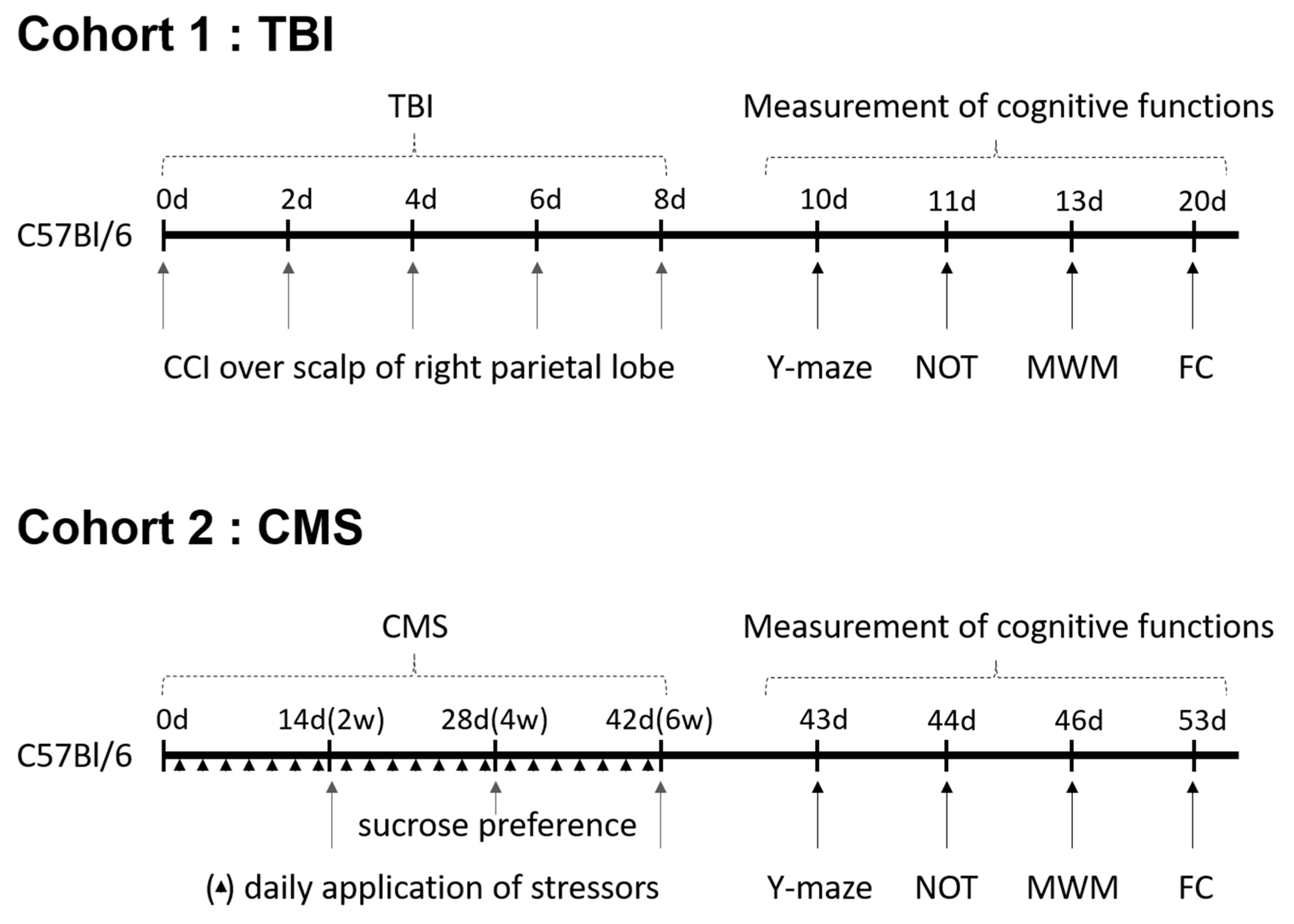
4.3. Creating Animal Models
4.3.1. TBI Model
4.3.2. CMS Model
4.4. Behavioral Tests
4.4.1. Sucrose Preference
4.4.2. Morris Water Maze
4.4.3. Y-Maze
4.4.4. Novel Object Test
4.4.5. Fear Conditioning
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ebly, E.M.; Hogan, D.B.; Parhad, I.M. Cognitive impairment in the nondemented elderly. Results from the Canadian Study of Health and Aging. Arch. Neurol. 1995, 52, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.E.; Rockwood, K.; Beattie, B.L.; Eastwood, R.; Gauthier, S.; Tuokko, H.; McDowell, I. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet 1997, 349, 1793–1796. [Google Scholar] [CrossRef]
- Tuokko, H.; Frerichs, R.; Graham, J.; Rockwood, K.; Kristjansson, B.; Fisk, J.; Bergman, H.; Kozma, A.; McDowell, I. Five-year follow-up of cognitive impairment with no dementia. Arch. Neurol. 2003, 60, 577–582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chertkow, H.; Massoud, F.; Nasreddine, Z.; Belleville, S.; Joanette, Y.; Bocti, C.; Drolet, V.; Kirk, J.; Freedman, M.; Bergman, H. Diagnosis and treatment of dementia: 3. Mild cognitive impairment and cognitive impairment without dementia. CMAJ Can. Med. Assoc. J. J. De L’association Med. Can. 2008, 178, 1273–1285. [Google Scholar] [CrossRef]
- Pepeu, G. Mild cognitive impairment: Animal models. Dialogues Clin. Neurosci. 2004, 6, 369–377. [Google Scholar]
- Hernandez, C.M.; Hoifodt, H.; Terry, A.V., Jr. Spontaneously hypertensive rats: Further evaluation of age-related memory performance and cholinergic marker expression. J. Psychiatry Neurosci. JPN 2003, 28, 197–209. [Google Scholar]
- Terry, A.V., Jr.; Hernandez, C.M.; Buccafusco, J.J.; Gattu, M. Deficits in spatial learning and nicotinic-acetylcholine receptors in older, spontaneously hypertensive rats. Neuroscience 2000, 101, 357–368. [Google Scholar] [CrossRef]
- Kibby, M.Y.; Long, C.J. Minor head injury: Attempts at clarifying the confusion. Brain Inj. 1996, 10, 159–186. [Google Scholar] [CrossRef]
- Stern, R.A.; Riley, D.O.; Daneshvar, D.H.; Nowinski, C.J.; Cantu, R.C.; McKee, A.C. Long-term consequences of repetitive brain trauma: Chronic traumatic encephalopathy. PM R J. Inj. Funct. Rehabil. 2011, 3, S460–S467. [Google Scholar] [CrossRef]
- Arciniegas, D.; Adler, L.; Topkoff, J.; Cawthra, E.; Filley, C.M.; Reite, M. Attention and memory dysfunction after traumatic brain injury: Cholinergic mechanisms, sensory gating, and a hypothesis for further investigation. Brain Inj. 1999, 13, 1–13. [Google Scholar] [CrossRef]
- Levin, H.S.; Mattis, S.; Ruff, R.M.; Eisenberg, H.M.; Marshall, L.F.; Tabaddor, K.; High, W.M., Jr.; Frankowski, R.F. Neurobehavioral outcome following minor head injury: A three-center study. J. Neurosurg. 1987, 66, 234–243. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; Marshall, S.W.; Bailes, J.; McCrea, M.; Cantu, R.C.; Randolph, C.; Jordan, B.D. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 2005, 57, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef]
- Mouzon, B.C.; Bachmeier, C.; Ferro, A.; Ojo, J.O.; Crynen, G.; Acker, C.M.; Davies, P.; Mullan, M.; Stewart, W.; Crawford, F. Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann. Neurol. 2014, 75, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Creeley, C.E.; Wozniak, D.F.; Bayly, P.V.; Olney, J.W.; Lewis, L.M. Multiple episodes of mild traumatic brain injury result in impaired cognitive performance in mice. Acad. Emerg. Med. 2004, 11, 809–819. [Google Scholar] [CrossRef]
- Willner, P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology 1997, 134, 319–329. [Google Scholar] [CrossRef]
- Song, L.; Che, W.; Min-Wei, W.; Murakami, Y.; Matsumoto, K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol. Biochem. Behav. 2006, 83, 186–193. [Google Scholar] [CrossRef]
- Cuadrado-Tejedor, M.; Ricobaraza, A.; Del Rio, J.; Frechilla, D.; Franco, R.; Perez-Mediavilla, A.; Garcia-Osta, A. Chronic mild stress in mice promotes cognitive impairment and CDK5-dependent tau hyperphosphorylation. Behav. Brain Res. 2011, 220, 338–343. [Google Scholar] [CrossRef]
- Sattler, C.; Toro, P.; Schonknecht, P.; Schroder, J. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Res. 2012, 196, 90–95. [Google Scholar] [CrossRef]
- Geda, Y.E.; Roberts, R.O.; Knopman, D.S.; Christianson, T.J.; Pankratz, V.S.; Ivnik, R.J.; Boeve, B.F.; Tangalos, E.G.; Petersen, R.C.; Rocca, W.A. Physical exercise, aging, and mild cognitive impairment: A population-based study. Arch. Neurol. 2010, 67, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Thomas, R.G.; Grundman, M.; Bennett, D.; Doody, R.; Ferris, S.; Galasko, D.; Jin, S.; Kaye, J.; Levey, A.; et al. Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med. 2005, 352, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L. Natural medicine: The genus Angelica. Curr. Med. Chem. 2004, 11, 1479–1500. [Google Scholar] [CrossRef]
- Cha, Y.S.; Choi, D.S.; Oh, S.H. Effects of Angelica gigas Nakai diet on lipid metabolism, alcohol metabolism and liver function of rats administered with chronic ethanol. Appl. Biol. Chem. 1999, 42, 29–33. [Google Scholar]
- Zhao, R.J.; Koo, B.S.; Kim, G.W.; Jang, E.Y.; Lee, J.R.; Kim, M.R.; Kim, S.C.; Kwon, Y.K.; Kim, K.J.; Huh, T.L.; et al. The essential oil from Angelica gigas NAKAI suppresses nicotine sensitization. Biol. Pharm. Bull. 2005, 28, 2323–2326. [Google Scholar] [CrossRef]
- Yan, J.J.; Kim, D.H.; Moon, Y.S.; Jung, J.S.; Ahn, E.M.; Baek, N.I.; Song, D.K. Protection against beta-amyloid peptide-induced memory impairment with long-term administration of extract of Angelica gigas or decursinol in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 25–30. [Google Scholar] [CrossRef]
- Reischies, F.M.; Neu, P. Comorbidity of mild cognitive disorder and depression--a neuropsychological analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2000, 250, 186–193. [Google Scholar] [CrossRef]
- Fratiglioni, L.; Wang, H.X.; Ericsson, K.; Maytan, M.; Winblad, B. Influence of social network on occurrence of dementia: A community-based longitudinal study. Lancet 2000, 355, 1315–1319. [Google Scholar] [CrossRef]
- Laurin, D.; Verreault, R.; Lindsay, J.; MacPherson, K.; Rockwood, K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch. Neurol. 2001, 58, 498–504. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Kim, S. Neuroprotective and Cognitive Enhancement Potentials of Angelica gigas Nakai Root: A Review. Sci. Pharm. 2017, 85, 21. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Lee, K.Y.; Sung, S.H.; Park, M.J.; Kim, Y.C. Coumarins isolated from Angelica gigas inhibit acetylcholinesterase: Structure-activity relationships. J. Nat. Prod. 2001, 64, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Visser, P.J.; Verhey, F.R.; Ponds, R.W.; Kester, A.; Jolles, J. Distinction between preclinical Alzheimer’s disease and depression. J. Am. Geriatr. Soc. 2000, 48, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Milman, A.; Rosenberg, A.; Weizman, R.; Pick, C.G. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J. Neurotrauma 2005, 22, 1003–1010. [Google Scholar] [CrossRef]
- Eakin, K.; Baratz-Goldstein, R.; Pick, C.G.; Zindel, O.; Balaban, C.D.; Hoffer, M.E.; Lockwood, M.; Miller, J.; Hoffer, B.J. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS ONE 2014, 9, e90617. [Google Scholar] [CrossRef]
- Cheng, J.S.; Craft, R.; Yu, G.Q.; Ho, K.; Wang, X.; Mohan, G.; Mangnitsky, S.; Ponnusamy, R.; Mucke, L. Tau reduction diminishes spatial learning and memory deficits after mild repetitive traumatic brain injury in mice. PLoS ONE 2014, 9, e115765. [Google Scholar] [CrossRef]
- Dywan, J.; Segalowitz, S.J.; Henderson, D.; Jacoby, L. Memory for source after traumatic brain injury. Brain Cogn. 1993, 21, 20–43. [Google Scholar] [CrossRef][Green Version]
- Gauthier, I.; Tarr, M.J.; Anderson, A.W.; Skudlarski, P.; Gore, J.C. Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nat. Neurosci. 1999, 2, 568–573. [Google Scholar] [CrossRef]
- Meyer, D.L.; Davies, D.R.; Barr, J.L.; Manzerra, P.; Forster, G.L. Mild traumatic brain injury in the rat alters neuronal number in the limbic system and increases conditioned fear and anxiety-like behaviors. Exp. Neurol. 2012, 235, 574–587. [Google Scholar] [CrossRef]
- Lifshitz, J.; Witgen, B.M.; Grady, M.S. Acute cognitive impairment after lateral fluid percussion brain injury recovers by 1 month: Evaluation by conditioned fear response. Behav. Brain Res. 2007, 177, 347–357. [Google Scholar] [CrossRef]
- McEwen, B.S.; Sapolsky, R.M. Stress and cognitive function. Curr. Opin. Neurobiol. 1995, 5, 205–216. [Google Scholar] [CrossRef]
- Henningsen, K.; Andreasen, J.T.; Bouzinova, E.V.; Jayatissa, M.N.; Jensen, M.S.; Redrobe, J.P.; Wiborg, O. Cognitive deficits in the rat chronic mild stress model for depression: Relation to anhedonic-like responses. Behav. Brain Res. 2009, 198, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Pothion, S.; Bizot, J.C.; Trovero, F.; Belzung, C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav. Brain Res. 2004, 155, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Haridas, S.; Kumar, M.; Manda, K. Melatonin ameliorates chronic mild stress induced behavioral dysfunctions in mice. Physiol. Behav. 2013, 119, 201–207. [Google Scholar] [CrossRef]
- Wright, R.L.; Conrad, C.D. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress 2005, 8, 151–154. [Google Scholar] [CrossRef]
- Morrissey, M.D.; Mathews, I.Z.; McCormick, C.M. Enduring deficits in contextual and auditory fear conditioning after adolescent, not adult, social instability stress in male rats. Neurobiol. Learn. Mem. 2011, 95, 46–56. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Song, M.; Oh, H.-J.; Hui, J.; Bae, W.; Shin, J.; Ji, S.-D.; Koh, Y.H.; Suh, J.W.; Park, H.; et al. Evaluating the Memory Enhancing Effects of Angelica gigas in Mouse Models of Mild Cognitive Impairments. Nutrients 2020, 12, 97. https://doi.org/10.3390/nu12010097
Kim M, Song M, Oh H-J, Hui J, Bae W, Shin J, Ji S-D, Koh YH, Suh JW, Park H, et al. Evaluating the Memory Enhancing Effects of Angelica gigas in Mouse Models of Mild Cognitive Impairments. Nutrients. 2020; 12(1):97. https://doi.org/10.3390/nu12010097
Chicago/Turabian StyleKim, Minsang, Minah Song, Hee-Jin Oh, Jin Hui, Woori Bae, Jihwan Shin, Sang-Dock Ji, Young Ho Koh, Joo Won Suh, Hyunwoo Park, and et al. 2020. "Evaluating the Memory Enhancing Effects of Angelica gigas in Mouse Models of Mild Cognitive Impairments" Nutrients 12, no. 1: 97. https://doi.org/10.3390/nu12010097
APA StyleKim, M., Song, M., Oh, H.-J., Hui, J., Bae, W., Shin, J., Ji, S.-D., Koh, Y. H., Suh, J. W., Park, H., & Maeng, S. (2020). Evaluating the Memory Enhancing Effects of Angelica gigas in Mouse Models of Mild Cognitive Impairments. Nutrients, 12(1), 97. https://doi.org/10.3390/nu12010097




