Effect of Daily Ingestion of Quercetin-Rich Onion Powder for 12 Weeks on Visceral Fat: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Subjects
2.3. Preparation of the Test Food
2.4. Physical, Hematological and Biological Assessments
2.5. Measurement of Abdominal Fat Area
2.6. FFQg
2.7. Safety Assessment
2.8. Ethics
2.9. Statistical Analysis
2.10. Sample Size
3. Results
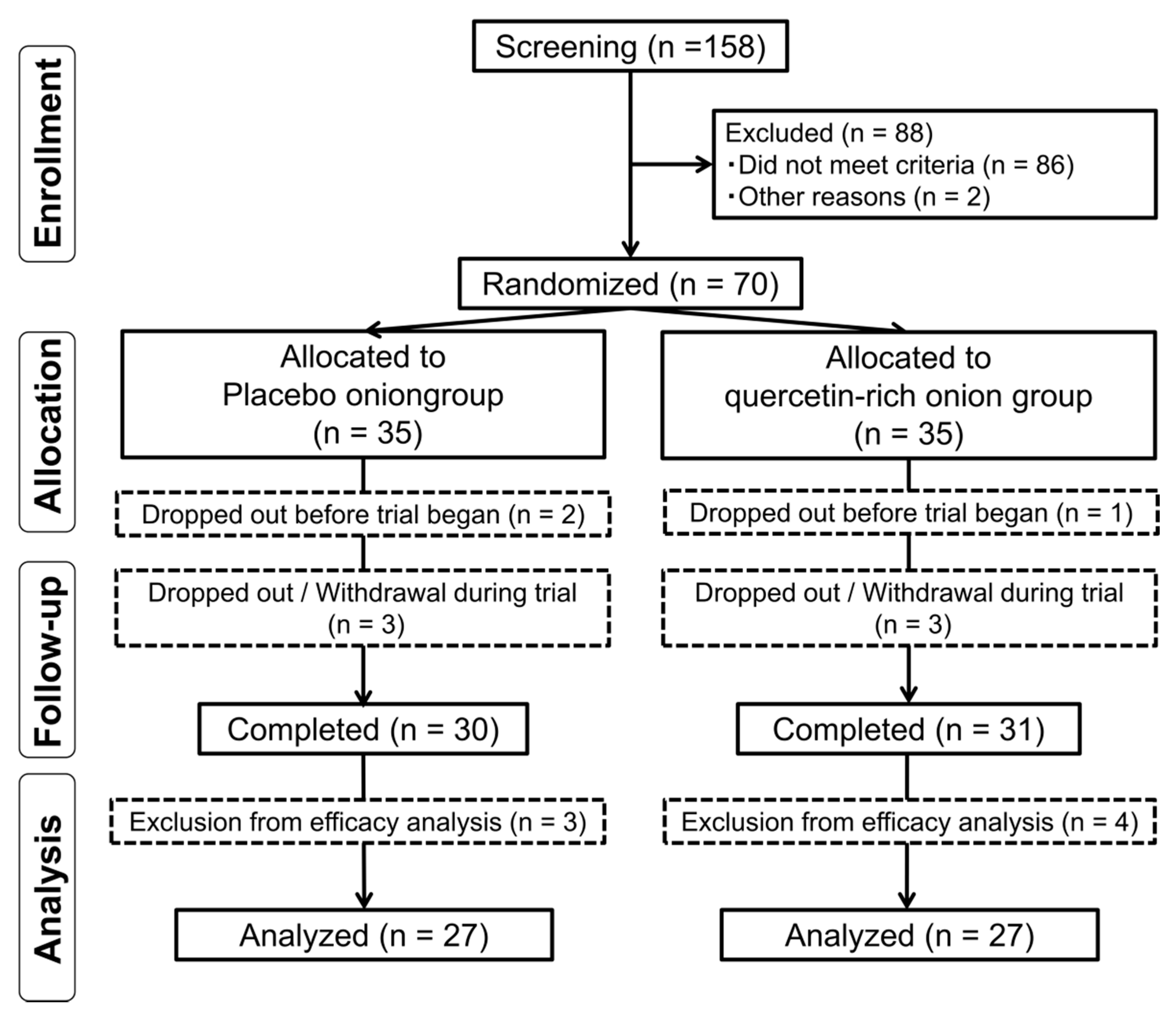
3.1. Flow Chart for Subject Involvement in Trial and Subject Characteristics
3.2. Efficacy of Quercetin-Rich Onion in Case of VFA
3.3. Efficacy of Quercetin-Rich Onion in Case of TFA, SFA and Body Composition
3.4. Efficacy of Quercetin-Rich Onion in Case of BP and the Oxidative Marker
3.5. Assessment of Dietary Nutrients in Case of Liver Marker
3.6. Assessment of Dietary Nutrients of Subjects during the Study
3.7. Safety Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ministry of Health, Labour and Welfare of Japan. The National Health and Nutrition Survey in Japan; Ministry of Health, Labour, and Welfare: Tokyo, Japan, 2015.
- Japan Society for the Study of Obesity. Guidelines for the Management of Obesity Disease 2016; Life Science Publishing Co., Ltd.: Chuo-ku, Japan, 2016. [Google Scholar]
- Perez-Jimenez, J.; Neveu, V.; Vos, F.; Scalbert, A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: An application of the phenol-explorer database. J. Agric. Food Chem. 2010, 58, 4959–4969. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schafer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Meyers, K.J.; Rudolf, J.L.; Mitchell, A.E. Influence of dietary quercetin on glutathione redox status in mice. J. Agric. Food Chem. 2008, 56, 830–836. [Google Scholar] [CrossRef]
- Luo, M.; Tian, R.; Yang, Z.; Peng, Y.Y.; Lu, N. Quercetin suppressed NADPH oxidase-derived oxidative stress via heme oxygenase-1 induction in macrophages. Arch. Biochem. Biophys. 2019, 671, 69–76. [Google Scholar] [CrossRef]
- Brull, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Muller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Saito, K.; Tanaka, T.; Obata, H.; Nakamura, J.; Fukui, N.; Tonozuka, N. Body Fat Reducing Effect and Safety Evaluation of Long‒term Consumption of Tea Containing Quercetin Glucosides in Obese Subjects (in Japanese). Jpn. Pharmacol. Ther. 2015, 43, 181–194. [Google Scholar]
- Kobori, M.; Masumoto, S.; Akimoto, Y.; Oike, H. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western-style diet in C57/BL6J mice. Mol. Nutr. Food Res. 2011, 55, 530–540. [Google Scholar] [CrossRef]
- Kuppusamy, U.R.; Das, N.P. Effects of flavonoids on cyclic AMP phosphodiesterase and lipid mobilization in rat adipocytes. Biochem. Pharmacol. 1992, 44, 1307–1315. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Park, J.; Ha, T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2008, 373, 545–549. [Google Scholar] [CrossRef]
- Quecan, B.X.V.; Santos, J.T.C.; Rivera, M.L.C.; Hassimotto, N.M.A.; Almeida, F.A.; Pinto, U.M. Effect of Quercetin Rich Onion Extracts on Bacterial Quorum Sensing. Front. Microbiol. 2019, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Muro, T. Cultivate a new quercetin-rich onion, variety named “Quergold”. Available online: https://www.naro.affrc.go.jp/publicity_report/press/laboratory/harc/054566.html (accessed on 14 December 2019).
- Nishimuro, H.; Ohnishi, H.; Sato, M.; Ohnishi-Kameyama, M.; Matsunaga, I.; Naito, S.; Ippoushi, K.; Oike, H.; Nagata, T.; Akasaka, H.; et al. Estimated daily intake and seasonal food sources of quercetin in Japan. Nutrients 2015, 7, 2345–2358. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, K.; Iwasaki, Y.; Ouchi, A.; Inakuma, T.; Nagaoka, S.; Terao, J.; Mukai, K. Development of singlet oxygen absorption capacity (SOAC) assay method. 2. Measurements of the SOAC values for carotenoids and food extracts. J. Agric. Food Chem. 2011, 59, 3717–3729. [Google Scholar] [CrossRef] [PubMed]
- The-Japanese-Society-of-Chemotherapy. The criteria for evaluation of adverse reactions and clinical laboratory abnormalities in clinical trials with antimicrobial agents. Chemotherapy 1991, 39, 687–689. [Google Scholar]
- Barzi, F.; Woodward, M.; Czernichow, S.; Lee, C.M.; Kang, J.H.; Janus, E.; Lear, S.; Patel, A.; Caterson, I.; Patel, J.; et al. The discrimination of dyslipidaemia using anthropometric measures in ethnically diverse populations of the Asia-Pacific Region: The Obesity in Asia Collaboration. Obes. Rev. 2010, 11, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bujo, H.; Takahashi, K.; Shibasaki, M.; Zhu, Y.; Yoshida, Y.; Otsuka, Y.; Hashimoto, N.; Saito, Y. Visceral fat: Higher responsiveness of fat mass and gene expression to calorie restriction than subcutaneous fat. Exp. Biol. Med. 2003, 228, 1118–1123. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C.; Saran, M. Flavonoid antioxidants: Rate constants for reactions with oxygen radicals. Methods Enzymol. 1994, 234, 420–429. [Google Scholar] [CrossRef]
- Messer, J.G.; Hopkins, R.G.; Kipp, D.E. Quercetin Metabolites Up-Regulate the Antioxidant Response in Osteoblasts Isolated From Fetal Rat Calvaria. J. Cell Biochem. 2015, 116, 1857–1866. [Google Scholar] [CrossRef]
- Duranti, G.; Ceci, R.; Patrizio, F.; Sgro, P.; Di Luigi, L.; Sabatini, S.; Felici, F.; Bazzucchi, I. Chronic consumption of quercetin reduces erythrocytes oxidative damage: Evaluation at resting and after eccentric exercise in humans. Nutr. Res. 2018, 50, 73–81. [Google Scholar] [CrossRef]
- Molina, M.F.; Sanchez-Reus, I.; Iglesias, I.; Benedi, J. Quercetin, a flavonoid antioxidant, prevents and protects against ethanol-induced oxidative stress in mouse liver. Biol. Pharm. Bull. 2003, 26, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Q.; Mo, W.; Feng, J.; Li, S.; Li, J.; Liu, T.; Xu, S.; Wang, W.; Lu, X.; et al. Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-beta1/Smads and PI3K/Akt pathways. Sci. Rep. 2017, 7, 9289. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, O. Hypoxic Signaling and Cholesterol Lipotoxicity in Fatty Liver Disease Progression. Oxid. Med. Cell Longev. 2018, 2018, 2548154. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Guidance and Agreement | Screening | Randomisation | Wash Out Period | Test Food Intake Period | |||
|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 4 | Week 8 | Week 12 | |||||
| Visit | Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | |||
| Informed consent | ● | |||||||
| Medical interview | ● | ● | ● | ● | ● | |||
| Vital sign measurement | ● | ● | ● | ● | ● | |||
| Abdominal fat measurement | ● | ● | ||||||
| Body composition measurement | ● | ● | ● | ● | ● | |||
| Blood sampling | ● | ● | ● | ● | ● | |||
| Home blood pressure measurement | During 1 week before each visit | |||||||
| Food frequency questionnaire | ● | ● | ● | ● | ||||
| Diary records | ● | ● | ● | ● | ● | |||
| Inclusion criteria | 1. Age, ≥35 years and <65 years. |
| 2. BMI, ≥23 kg/m2 and <30 kg/m2. | |
| Exclusion criteria | 1. Subjects under physician’s advice, treatment, and/or medication for obesity and/or hypertension. |
| 2. Subjects with suspected obesity. | |
| 3. Pacemaker or defibrillator users. | |
| 4. Subjects with (or suspected to have) secondary hypertension such as renovascular hypertension, renal parenchymal hypertension, primary aldosteronism, Cushing’s syndrome, hypothyroidism and hyperthyroidism. | |
| 5. Subjects with serious cerebrovascular, cardiac, hepatic, renal or gastrointestinal diseases and/or affected with infectious diseases requiring reports to the authorities. | |
| 6. Subjects with major surgical history relevant to the digestive system, such as gastrectomy, gastrorhaphy and enterectomy. | |
| 7. Subjects with unusually high and/or low BP and/or abnormal hematological data. | |
| 8. Subjects with severe anaemia. | |
| 9. Pre- or post-menopausal women complaining of obvious physical changes. | |
| 10. Subjects at risk of experiencing allergic reactions to drugs or foods, especially those based on onion. | |
| 11. Subjects regularly taking medicine, functional foods and/or supplements which would affect BW and BFR. | |
| 12. Subjects regularly taking medicine, functional foods and/or supplements which would affect BP. | |
| 13. Heavy smokers, alcohol addicts or subjects with disordered lifestyle. | |
| 14. Subjects who had donated 400 mL whole blood within 16 weeks (women) or 12 weeks (men), 200 mL whole blood within 4 weeks (men and women) or blood components within 2 weeks (men and women) before the current study. | |
| 15. Pregnant or lactating women or women who expect to be pregnant during this study. | |
| 16. Subjects currently participating in other clinical trials or who had participated within the last 4 weeks before the current study. | |
| 17. Any other medical and/or health reasons unfavourable to participation in the current study, as judged by the principal investigator. |
| Nutrients | Placebo Onion Powder (9.0 g/day) | Quercetin-Rich Onion Powder (9.0 g/day) |
|---|---|---|
| Calories (kcal) | 34.7 | 34.5 |
| Proteins (g) | 0.6 | 0.8 |
| Lipids (g) | 0.0 | 0.2 |
| Carbohydrates (g) | 7.5 | 7.4 |
| Sodium chloride (mg) | 12.2 | 1.8 |
| Quercetin aglycone (mg) | - | 60 |
| Characteristic | Placebo Onion Powder | Quercetin-Rich Onion Powder | p |
|---|---|---|---|
| Mean | Mean | ||
| Subjects (n) | 27 | 27 | - |
| Male (n) | 2 | 6 | 0.25 |
| Age (years) | 50.4 ± 7.0 | 49.3 ± 8.3 | 0.59 |
| Body weight (kg) | 61.4 ± 6.6 | 63.3 ± 6.9 | 0.31 |
| BFR (%) | 35.3 ± 4.8 | 32.9 ± 5.6 | 0.11 |
| BMI (kg/m2) | 24.8 ± 1.5 | 24.8 ± 1.6 | 0.85 |
| Abdominal circumference (cm) | 85.7 ± 5.8 | 86.0 ± 4.5 | 0.84 |
| Intake rate (%) | 99.5 ± 0.9 | 99.4 ± 1.2 | 0.88 |
| Variable | n | Week 0 | ∆Week 4 | ∆Week 8 | ∆Week 12 | |
|---|---|---|---|---|---|---|
| VFA (cm2) | Placebo | 27 | 62.1 ± 27.9 | - | - | −2.4 ± 10.0 |
| Quercetin-rich onion | 27 | 67.5 ± 27.6 | - | - | −6.0 ± 9.0 | |
| p | 0.47 | - | - | 0.16 | ||
| VFA whose HDL-C is lower (cm2) | Placebo | 18 | 60.9 ± 29.7 | - | - | −0.7 ± 9.1 |
| Quercetin-rich onion | 19 | 66.8 ± 28.8 | - | - | −5.8 ± 5.8 | |
| p | 0.55 | - | - | 0.046 * | ||
| VFA whose HDL-C is higher (cm2) | Placebo | 9 | 64.4 ± 25.5 | - | - | −5.8 ± 11.4 |
| Quercetin-rich onion | 8 | 69.4 ± 26.3 | - | - | −6.6 ± 14.7 | |
| p | 0.70 | - | - | 0.91 | ||
| TFA (cm2) | Placebo | 27 | 282.8 ± 69.7 | - | - | −8.5 ± 28.4 |
| Quercetin-rich onion | 27 | 273.8 ± 59.4 | - | - | −16.5 ± 29.7 | |
| p | 0.61 | - | - | 0.32 | ||
| SFA (cm2) | Placebo | 27 | 220.7 ± 55.4 | - | - | −6.1 ± 26.3 |
| Quercetin-rich onion | 27 | 206.3 ± 48.9 | - | - | −10.5 ± 26.0 | |
| p | 0.32 | - | - | 0.54 | ||
| BW (kg) | Placebo | 27 | 61.1 ± 6.3 | 0.1 ± 0.8 | 0.3 ± 0.8 | 0.4 ± 1.1 |
| Quercetin-rich onion | 25 | 62.8 ± 7.2 | 0.2 ± 0.7 | 0.2 ± 1.2 | 0.3 ± 1.5 | |
| p | 0.36 | 0.59 | 0.73 | 0.75 | ||
| BFR (%) | Placebo | 27 | 35.5 ± 4.9 | 0.5 ± 0.8 | 0.9 ± 0.9 | 1.2 ± 0.9 |
| Quercetin-rich onion | 25 | 33.4 ± 5.4 | 0.4 ± 0.9 | 0.6 ± 1.1 | 0.5 ± 2.2 | |
| p | 0.15 | 0.65 | 0.30 | 0.14 | ||
| BMI (kg/m2) | Placebo | 27 | 24.7 ± 1.5 | 0.0 ± 0.4 | 0.1 ± 0.3 | 0.2 ± 0.5 |
| Quercetin-rich onion | 25 | 24.5 ± 1.6 | 0.1 ± 0.3 | 0.1 ± 0.5 | 0.1 ± 0.6 | |
| p | 0.58 | 0.67 | 0.84 | 0.72 | ||
| Abdominal circumference (cm) | Placebo | 27 | 87.4 ± 6.5 | −0.5 ± 2.6 | −1.0 ± 3.1 | −2.0 ± 3.1 |
| Quercetin-rich onion | 25 | 86.9 ± 4.7 | 0.6 ± 2.9 | −0.3 ± 2.7 | −2.1 ± 3.1 | |
| p | 0.75 | 0.15 | 0.35 | 0.99 |
| Variable | n | Week 0 | ∆Week 4 | ∆Week 8 | ∆Week 12 | |
|---|---|---|---|---|---|---|
| AST (U/L) | Placebo | 27 | 21.1 ± 4.8 | 0.9 ± 4.3 | 1.1 ± 3.4 | 3.2 ± 9.2 |
| Quercetin-rich onion | 25 | 22.4 ± 8.6 | 0.3 ± 7.0 | −0.8 ± 5.8 | −0.4 ± 5.9 | |
| p | 0.50 | 0.70 | 0.15 | 0.11 | ||
| ALT (U/L) | Placebo | 27 | 19.2 ± 7.9 | 2.4 ± 6.0 | 2.9 ± 6.5 | 6.2 ± 13.3 |
| Quercetin-rich onion | 25 | 20.6 ± 9.0 | 3.5 ± 11.4 | −0.2 ± 6.6 | 0.1 ± 5.2 | |
| p | 0.55 | 0.67 | 0.10 | 0.035 * | ||
| γ-GTP (U/L) | Placebo | 27 | 21.3 ± 8.0 | 2.8 ± 6.3 | 3.7 ± 6.2 | 2.9 ± 7.0 |
| Quercetin-rich onion | 25 | 27.7 ± 19.6 | 5.4 ± 12.6 | 5.2 ± 16.0 | 3.3 ± 13.6 | |
| p | 0.14 | 0.37 | 0.66 | 0.88 | ||
| ALP (U/L) | Placebo | 27 | 190.3 ± 50.1 | 4.6 ± 16.1 | 3.3 ± 19.9 | 4.4 ± 21.4 |
| Quercetin-rich onion | 25 | 175.0 ± 36.9 | 11.5 ± 17.0 | 10.6 ± 16.5 | 8.2 ± 14.6 | |
| p | 0.22 | 0.14 | 0.16 | 0.46 | ||
| LDH (U/L) | Placebo | 27 | 186.4 ± 31.1 | −4.4 ± 12.1 | 6.3 ± 19.4 | −4.0 ± 15.7 |
| Quercetin-rich onion | 25 | 192.4 ± 56.0 | −7.7 ± 20.1 | −4.9 ± 29.2 | −13.1 ± 31.0 | |
| p | 0.63 | 0.48 | 0.11 | 0.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, M.; Muro, T.; Kobori, M.; Nishihira, J. Effect of Daily Ingestion of Quercetin-Rich Onion Powder for 12 Weeks on Visceral Fat: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients 2020, 12, 91. https://doi.org/10.3390/nu12010091
Nishimura M, Muro T, Kobori M, Nishihira J. Effect of Daily Ingestion of Quercetin-Rich Onion Powder for 12 Weeks on Visceral Fat: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients. 2020; 12(1):91. https://doi.org/10.3390/nu12010091
Chicago/Turabian StyleNishimura, Mie, Takato Muro, Masuko Kobori, and Jun Nishihira. 2020. "Effect of Daily Ingestion of Quercetin-Rich Onion Powder for 12 Weeks on Visceral Fat: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Study" Nutrients 12, no. 1: 91. https://doi.org/10.3390/nu12010091
APA StyleNishimura, M., Muro, T., Kobori, M., & Nishihira, J. (2020). Effect of Daily Ingestion of Quercetin-Rich Onion Powder for 12 Weeks on Visceral Fat: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients, 12(1), 91. https://doi.org/10.3390/nu12010091




