DHA-Induced Perturbation of Human Serum Metabolome. Role of the Food Matrix and Co-Administration of Oat β-glucan and Anthocyanins
Abstract
1. Introduction
2. Experimental Section
2.1. Bioactive Enriched Food
2.2. Subjects
2.3. NMR Sample Preparation
2.4. NMR Experiments
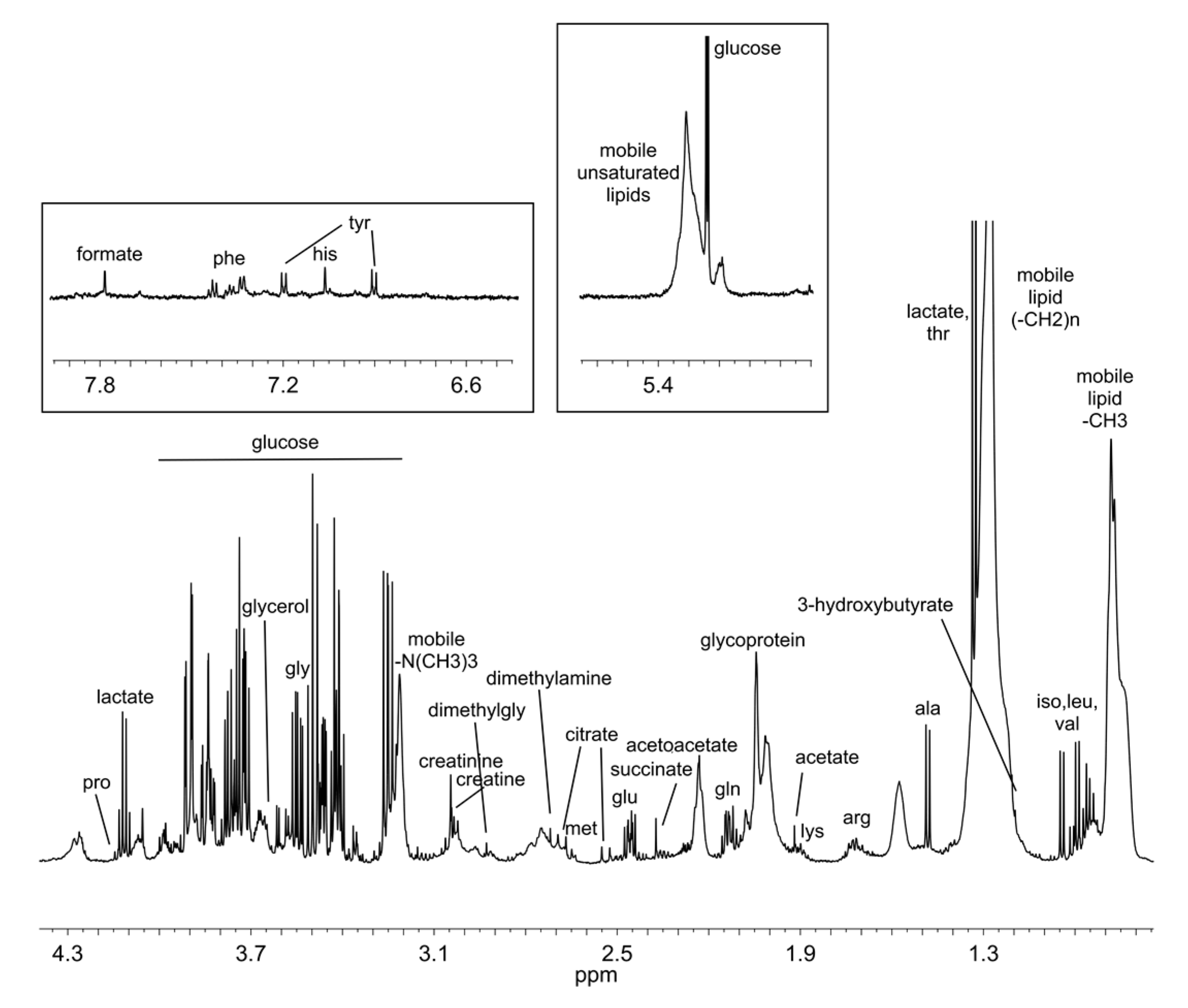
2.5. NMR Spectra Processing and Spectral Analysis
2.6. Statistical Analysis
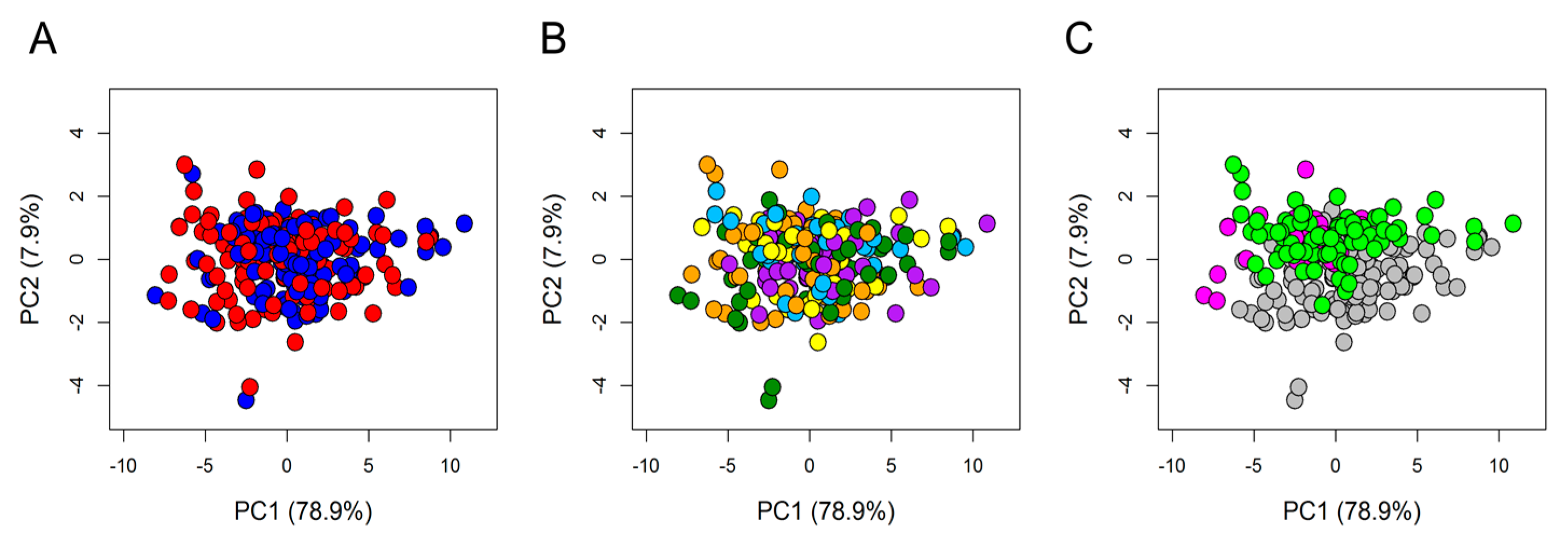
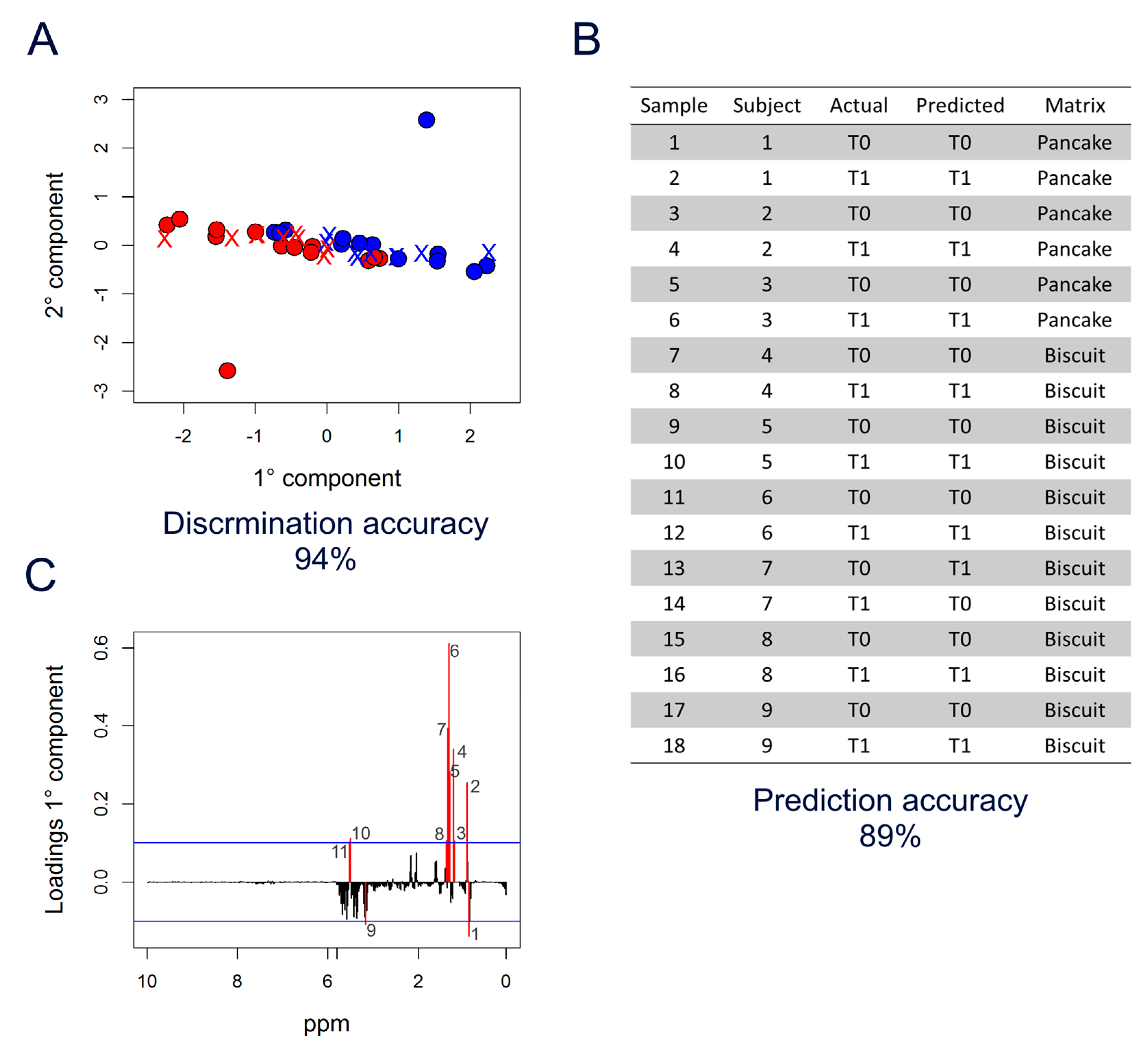
3. Results
4. Discussion
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alissa, E.M.; Ferns, G.A. Functional foods and nutraceuticals in the primary prevention of cardiovascular diseases. J. Nutr. Metab. 2012, 2012, 569486. [Google Scholar] [CrossRef] [PubMed]
- Ozen, A.E.; Pons, A.; Tur, J.A. Worldwide consumption of functional foods: A systematic review. Nutr. Rev. 2012, 70, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, A.; Kalaivani, M. Designer foods and their benefits: A review. J. Food Sci. Technol. 2013, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-F.; Li, X.; Shi, M.; Li, D. n-3 Polyunsaturated Fatty Acids and Metabolic Syndrome Risk: A Meta-Analysis. Nutrients 2017, 9, 703. [Google Scholar] [CrossRef]
- Cloetens, L.; Ulmius, M.; Johansson-Persson, A.; Åkesson, B.; Önning, G. Role of dietary beta-glucans in the prevention of the metabolic syndrome. Nutr. Rev. 2012, 70, 444–458. [Google Scholar] [CrossRef]
- Brown, L.; Poudyal, H.; Panchal, S.K. Functional foods as potential therapeutic options for metabolic syndrome. Obes. Rev. 2015, 16, 914–941. [Google Scholar] [CrossRef]
- Bub, A.; Malpuech-Brugère, C.; Orfila, C.; Amat, J.; Arianna, A.; Blot, A.; Di Nunzio, M.; Holmes, M.; Kertész, Z.; Marshall, L.; et al. A Dietary Intervention of Bioactive Enriched Foods Aimed at Adults at Risk of Metabolic Syndrome: Protocol and Results from PATHWAY-27 Pilot Study. Nutrients 2019, 11, 1814. [Google Scholar] [CrossRef]
- Gibney, M.J.; Walsh, M.; Brennan, L.; Roche, H.-M.; German, B.; Van Ommen, B. Metabolomics in human nutrition: Opportunities and challenges. Am. J. Clin. Nutr. 2005, 82, 497–503. [Google Scholar] [CrossRef]
- Brennan, L. Metabolomics in nutrition research: Current status and perspectives. Biochem. Soc. Trans. 2013, 41, 670–673. [Google Scholar] [CrossRef]
- O’Sullivan, A.; Gibney, M.J.; Brennan, L. Dietary intake patterns are reflected in metabolomic profiles: Potential role in dietary assessment studies. Am. J. Clin. Nutr. 2011, 93, 314–321. [Google Scholar] [CrossRef]
- Scalbert, A.; Brennan, L.; Manach, C.; Andres-Lacueva, C.; Dragsted, L.O.; Draper, J.; Rappaport, S.M.; Van Der Hooft, J.J.; Wishart, D.S. The food metabolome: A window over dietary exposure. Am. J. Clin. Nutr. 2014, 99, 1286–1308. [Google Scholar] [CrossRef] [PubMed]
- Trimigno, A.; Khakimov, B.; Savorani, F.; Tenori, L.; Hendrixson, V.; Čivilis, A.; Glibetic, M.; Gurinovic, M.; Pentikäinen, S.; Sallinen, J.; et al. Investigation of Variations in the Human Urine Metabolome amongst European Populations: An Exploratory Search for Biomarkers of People at Risk-of-Poverty. Mol. Nutr. Food Res. 2019, 63, 1800216. [Google Scholar] [CrossRef] [PubMed]
- Trimigno, A.; Münger, L.; Picone, G.; Freiburghaus, C.; Pimentel, G.; Vionnet, N.; Pralong, F.; Capozzi, F.; Badertscher, R.; Vergères, G. GC-MS Based Metabolomics and NMR Spectroscopy Investigation of Food Intake Biomarkers for Milk and Cheese in Serum of Healthy Humans. Metabolites 2018, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Mínger, L.-H.; Trimigno, A.; Picone, G.; Freiburghaus, C.; Pimentel, G.; Burton, K.-J.; Pralong, F.-P.; Vionnet, N.; Capozzi, F.; Badertscher, R.; et al. Identification of Urinary Food Intake Biomarkers for Milk, Cheese, and Soy-Based Drink by Untargeted GC-MS and NMR in Healthy Humans. J. Proteome Res. 2017, 16, 3321–3335. [Google Scholar] [CrossRef]
- Beckonert, O.; Keun, H.-C.; Ebbels, T.-M.; Bundy, J.; Holmes, E.; Lindon, J.-C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High-Throughput Metabolomics by 1D NMR. Angew. Chem. Int. Ed. 2019, 58, 968–994. [Google Scholar] [CrossRef]
- Takis, P.G.; Ghini, V.; Tenori, L.; Turano, P.; Luchinat, C. Uniqueness of the NMR approach to metabolomics. TrAC Trends Anal. Chem. 2018, 120, 115300. [Google Scholar] [CrossRef]
- Assfalg, M.; Bertini, I.; Colangiuli, D.; Luchinat, C.; Schäfer, H.; Schütz, B.; Spraul, M. Evidence of different metabolic phenotypes in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 1420–1424. [Google Scholar] [CrossRef]
- Bernini, P.; Bertini, I.; Luchinat, C.; Nepi, S.; Saccenti, E.; Schäfer, H.; Schítz, B.; Spraul, M.; Tenori, L. Individual Human Phenotypes in Metabolic Space and Time. J. Proteome Res. 2009, 8, 4264–4271. [Google Scholar] [CrossRef]
- Ghini, V.; Saccenti, E.; Tenori, L.; Assfalg, M.; Luchinat, C. Allostasis and Resilience of the Human Individual Metabolic Phenotype. J. Proteome Res. 2015, 14, 2951–2962. [Google Scholar] [CrossRef]
- Saccenti, E.; Menichetti, G.; Ghini, V.; Remondini, D.; Tenori, L.; Luchinat, C. Entropy-Based Network Representation of the Individual Metabolic Phenotype. J. Proteome Res. 2016, 15, 3298–3307. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Brolsma, E.-M.; Brennan, L.; Drevon, C.A.; van Kranen, H.; Manach, C.; Dragsted, L.-O.; Roche, H.-M.; Andres-Lacueva, C.; Bakker, S.J.; Bouwman, J.; et al. Combining traditional dietary assessment methods with novel metabolomics techniques: Present efforts by the Food Biomarker Alliance. Proc. Nutr. Soc. 2017, 76, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Wallner-Liebmann, S.; Gralka, E.; Tenori, L.; Konrad, M.; Hofmann, P.; Dieber-Rotheneder, M.; Turano, P.; Luchinat, C.; Zatloukal, K. The impact of free or standardized lifestyle and urine sampling protocol on metabolome recognition accuracy. Genes Nutr. 2015, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Gralka, E.; Luchinat, C.; Tenori, L.; Ernst, B.; Thurnheer, M.; Schultes, B. Metabolomic fingerprint of severe obesity is dynamically affected by bariatric surgery in a procedure-dependent manner. Am. J. Clin. Nutr. 2015, 102, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Calabro, A.; De Carli, V.; Luchinat, C.; Nepi, S.; Porfirio, B.; Renzi, D.; Saccenti, E.; Tenori, L. The Metabonomic Signature of Celiac Disease. J. Proteome Res. 2008, 8, 170–177. [Google Scholar] [CrossRef]
- Meoni, G.; Lorini, S.; Monti, M.; Madia, F.; Corti, G.; Luchinat, C.; Zignego, A.-L.; Tenori, L.; Gragnani, L. The metabolic fingerprints of HCV and HBV infections studied by Nuclear Magnetic Resonance Spectroscopy. Sci. Rep. 2019, 9, 4128. [Google Scholar] [CrossRef]
- Romano, F.; Meoni, G.; Manavella, V.; Baima, G.; Mariani, G.-M.; Cacciatore, S.; Tenori, L.; Aimetti, M. Effect of non-surgical periodontal therapy on salivary metabolic fingerprint of generalized chronic periodontitis using nuclear magnetic resonance spectroscopy. Arch. Oral Biol. 2019, 97, 208–214. [Google Scholar] [CrossRef]
- Denihan, N.M.; Walsh, B.-H.; Reinke, S.-N.; Sykes, B.-D.; Mandal, R.; Wishart, D.-S.; Broadhurst, D.-I.; Boylan, G.-B.; Murray, D.-M. The effect of haemolysis on the metabolomic profile of umbilical cord blood. Clin. Biochem. 2015, 48, 534–537. [Google Scholar] [CrossRef]
- Bernini, P.; Bertini, I.; Luchinat, C.; Nincheri, P.; Staderini, S.; Turano, P. Standard operating procedures for pre-analytical handling of blood and urine for metabolomic studies and biobanks. J. Biomol. NMR 2011, 49, 231–243. [Google Scholar] [CrossRef]
- Ghini, V.; Quaglio, D.; Luchinat, C.; Turano, P. NMR for sample quality assessment in metabolomics. New Biotechnol. 2019, 52, 25–34. [Google Scholar] [CrossRef]
- Mckay, R.T. How the 1D-NOESY suppresses solvent signal in metabonomics NMR spectroscopy: An examination of the pulse sequence components and evolution. Concepts Magn. Reson. 2011, 38A, 197–220. [Google Scholar] [CrossRef]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.-C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.-C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The Human Serum Metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Quantitative metabolomics using NMR. TrAC Trends Anal. Chem. 2008, 27, 228–237. [Google Scholar] [CrossRef]
- Savorani, F.; Kristensen, M.; Larsen, F.-H.; Astrup, A.; Engelsen, S.B. High throughput prediction of chylomicron triglycerides in human plasma by nuclear magnetic resonance and chemometrics. Nutr. Metab. 2010, 7, 43. [Google Scholar] [CrossRef]
- Ala-Korpela, M.; Korhonen, A.; Keisala, J.; Hörkkö, S.; Korpi, P.; Ingman, L.-P.; Jokisaari, J.; Savolainen, M.-J.; Kesäniemi, Y.-A. 1H NMR-based absolute quantitation of human lipoproteins and their lipid contents directly from plasma. J. Lipid Res. 1994, 35, 2292–2304. [Google Scholar]
- Soininen, P.; Kangas, A.J.; Würtz, P.; Tukiainen, T.; Tynkkynen, T.; Laatikainen, R.; Järvelin, M.-R.; Kähönen, M.; Lehtimäki, T.; Viikari, J.; et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst 2009, 134, 1781–1785. [Google Scholar] [CrossRef]
- Jiménez, B.; Holmes, E.; Heude, C.; Tolson, R.F.; Harvey, N.; Lodge, S.L.; Chetwynd, A.J.; Cannet, C.; Fang, F.; Pearce, J.T.; et al. Quantitative Lipoprotein Subclass and Low Molecular Weight Metabolite Analysis in Human Serum and Plasma by 1H NMR Spectroscopy in a Multilaboratory Trial. Anal. Chem. 2018, 90, 11962–11971. [Google Scholar] [CrossRef]
- Ghini, V.; Di Nunzio, M.; Tenori, L.; Valli, V.; Danesi, F.; Capozzi, F.; Luchinat, C.; Bordoni, A. Evidence of a DHA Signature in the Lipidome and Metabolome of Human Hepatocytes. Int. J. Mol. Sci. 2017, 18, 359. [Google Scholar] [CrossRef]
- Oelrich, B.; Dewell, A.; Gardner, C.D. Effect of fish oil supplementation on serum triglycerides, LDL cholesterol and LDL subfractions in hypertriglyceridemic adults. Nutr. Metab. Cardiovasc. Dis. NMCD 2013, 23, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. (n-3) fatty acids and cardiovascular health: Are effects of EPA and DHA shared or complementary? J. Nutr. 2012, 142, 614S–625S. [Google Scholar] [CrossRef] [PubMed]
- Carmena, R.; Duriez, P.; Fruchart, J.-C. Atherogenic lipoprotein particles in atherosclerosis. Circulation 2004, 109, III2–III7. [Google Scholar] [CrossRef] [PubMed]
- Pitsavos, C.; Panagiotakos, D.B.; Skoumas, J.; Papadimitriou, L.; Stefanadis, C. Risk stratification of apolipoprotein B, apolipoprotein A1, and apolipoprotein B/AI ratio on the prevalence of the metabolic syndrome: The ATTICA study. Angiology 2008, 59, 335–341. [Google Scholar] [CrossRef]
- Reynoso-Villalpando, G.-L.; Sevillano-Collantes, C.; Valle, Y.; Moreno-Ruiz, I.; Padilla-Gutiérrez, J.-R.; del Cañizo-Gómez, F.-J. ApoB/ApoA1 ratio and non-HDL-cholesterol/HDL-cholesterol ratio are associated to metabolic syndrome in patients with type 2 diabetes mellitus subjects and to ischemic cardiomyopathy in diabetic women. Endocrinol. Diabetes Nutr. 2019, 66, 502–511. [Google Scholar] [CrossRef]
- Bays, H.E.; Tighe, A.P.; Sadovsky, R.; Davidson, M.H. Prescription omega-3 fatty acids and their lipid effects: Physiologic mechanisms of action and clinical implications. Expert Rev. Cardiovasc. Ther. 2008, 6, 391–409. [Google Scholar] [CrossRef]
- Ramprasath, V.-R.; Thandapilly, S.-J.; Yang, S.; Abraham, A.; Jones, P.-J.; Ames, N. Effect of consuming novel foods consisting high oleic canola oil, barley β-glucan, and DHA on cardiovascular disease risk in humans: The CONFIDENCE (Canola Oil and Fibre with DHA Enhanced) stud-protocol for a randomized controlled trial. Trials 2015, 16, 489. [Google Scholar] [CrossRef][Green Version]
- Bordoni, A.; Capozzi, F. Foodomics for healthy nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 418–424. [Google Scholar] [CrossRef]
- Bernstein, A.M.; Ding, E.L.; Willett, W.C.; Rimm, E.B. A meta-analysis shows that docosahexaenoic acid from algal oil reduces serum triglycerides and increases HDL-cholesterol and LDL-cholesterol in persons without coronary heart disease. J. Nutr. 2012, 142, 99–104. [Google Scholar] [CrossRef]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118. [Google Scholar] [CrossRef]

| DHA (mg) | AC (mg) | OBG (g) | |
|---|---|---|---|
| Biscuits DHA | 292 | 0 | 0 |
| Biscuits DHA + AC | 302 | 19 | 0 |
| Biscuits DHA + OBG | 329 | 0 | 2.9 |
| Biscuits AC | 0 | 17 | 0 |
| Biscuits OBG | 0 | 0 | 3.6 |
| Pancake DHA | 225 | 0 | 0 |
| Pancake DHA + AC | 208 | 57 | 0 |
| Pancake DHA + OBG | 215 | 0 | 4.3 |
| Pancake AC | 0 | 58 | 0 |
| Pancake OBG | 0 | 0 | 3.7 |
| Milkshake DHA | 261 | 0 | 0 |
| Milkshake DHA + AC | 228 | 12 | 0 |
| Milkshake DHA + OBG | 226 | 0 | 3.8 |
| Milkshake AC | 0 | 10 | 0 |
| Milkshake OBG | 0 | 0 | 4.2 |
| Matrix | Subjects (TOT) | DHA | AC | OBG | DHA + AC | DHA + OBG |
|---|---|---|---|---|---|---|
| Milkshake | 66 | 14 | 15 | 12 | 12 | 13 |
| Biscuits | 37 | 7 | 7 | 9 | 8 | 6 |
| Pancake | 14 | 5 | 3 | 1 | 2 | 3 |
| Total | 117 | 26 | 25 | 22 | 22 | 22 |
| All BEF | Enriched Milkshake | |
|---|---|---|
| DHA | 74% ** | 71% * |
| AC | 63% * | 54% |
| OBG | 55% | 55% |
| DHA + AC | 53% | 56% |
| DHA + OBG | 86% ** | 94% ** |
| DHA | DHA + OBG | |||
|---|---|---|---|---|
| T0 | T1 | T0 | T1 | |
| TG | 156.48 | 138.83 | 193.56 | 153.63 * |
| Chol | 226.25 | 240.43 * | 252.82 | 250.90 |
| LDL-Chol | 122.91 | 129.37 | 136.43 | 147.09 * |
| Apo B100 | 100.41 | 108.63 * | 114.74 | 113.23 |
| Apo A2 | 32.71 | 32.84 | 35.42 | 34.98 * |
| Calculated Figures | ||||
| Apo B100/ Apo A1 | 1.42 | 1.26 * | 1.27 | 1.29 |
| Total Apo B100 Particle Number | 1825.71 | 1975.17 * | 2086.31 | 2058.91 |
| VLDL Particle Number | 192.87 | 175.21 | 236.63 | 208.71 * |
| LDL Particle Number | 1496.1 | 1589.47 * | 1703.64 | 1726.34 |
| Lipoprotein Main Fractions | ||||
| TG-VLDL | 105.3 | 92.49 | 130.67 | 112.03 * |
| TG-IDL | 17.61 | 14.07 | 23.67 | 16.52 * |
| TG-LDL | 22.05 | 25.26 * | 24.55 | 22.80 |
| TG-HDL | 10.69 | 10.97 | 11.69 | 10.10 * |
| Chol-VLDL | 27.59 | 22.44 | 34.54 | 28.24 * |
| Chol-IDL | 15.97 | 16.93 | 19.76 | 15.57 * |
| Chol-LDL | 122.91 | 129.37 | 136.43 | 147.09 * |
| Free Chol-VLDL | 12.65 | 11.27 | 15.23 | 12.54 * |
| Free Chol-IDL | 4.48 | 4.95 | 5.48 | 4.47 * |
| Phospholipids-VLDL | 28.25 | 24.50 | 33.71 | 28.06 * |
| Phospholipids-IDL | 8.67 | 8.925 | 12.03 | 10.23 * |
| Phospholipids-LDL | 69.93 | 72.97 * | 75.48 | 81.04 |
| Apo A2-HDL | 33.53 | 33.99 | 36.26 | 35.63 * |
| Apo B-VLDL | 10.61 | 9.63 | 13.01 | 11.48 * |
| Apo B-LDL | 82.28 | 87.42 * | 93.7 | 94.94 |
| VLDL Subfractions | ||||
| TG-VLDL 1 | 49.54 | 42.19 | 62.56 | 55.9 * |
| TG-VLDL 2 | 19.73 | 13.31 | 23.62 | 19.14 * |
| TG-VLDL 3 | 15.46 | 12.54 | 18.71 | 16.97 * |
| TG-VLDL 4 | 10.82 | 9.63 | 13.48 | 12.31 * |
| TG-VLDL 5 | 3.50 | 3.4 | 3.59 | 3.31 * |
| Chol-VLDL 1 | 9.93 | 8.89 | 11.59 | 9.13 * |
| Chol-VLDL 2 | 4.68 | 3.73 | 5.67 | 4.87 * |
| Free Chol-VLDL 1 | 3.51 | 3.32 | 4.11 | 3.98 * |
| Free Chol-VLDL 2 | 1.93 | 1.67 | 2.31 | 1.93 * |
| Free Chol-VLDL 3 | 2.07 | 1.69 | 2.55 | 2.42 * |
| Phospholipids-VLDL 1 | 8.43 | 6.92 | 10.17 | 9.39 * |
| Phospholipids-VLDL 2 | 4.81 | 3.71 | 5.86 | 4.905 * |
| Phospholipids-VLDL 3 | 4.83 | 4.09 | 5.91 | 5.74 * |
| LDL Subfractions | ||||
| TG-LDL 1 | 6.57 | 6.42 | 6.84 | 6.1 * |
| TG-LDL 4 | 2.76 | 2.88 * | 3.00 | 3.03 |
| TG-LDL 5 | 3.13 | 3.79 * | 3.94 | 4.01 |
| Apo A2-HDL 2 | 2.98 | 3.19 | 3.74 | 3.57 * |
| Apo A2-HDL 3 | 6.49 | 6.68 | 7.43 | 6.95 * |
| HDL Subfractions | ||||
| Free Chol-HDL 2 | 1.73 | 1.68 | 1.8 | 1.69 * |
| Free Chol-HDL 3 | 2.33 | 2.47 | 2.70 | 2.29 * |
| Free Chol-HDL 4 | 4.57 | 4.475 | 4.97 | 4.41 * |
| Phospholipids-HDL 3 | 15.59 | 15.15 | 15.94 | 16.25 * |
| Apo A1-HDL 2 | 16.43 | 17.87 * | 18.35 | 17.33 |
| Apo A1-HDL 3 | 25.77 | 26.76 | 26.60 | 26.46 * |
| TG-HDL 2 | 1.69 | 1.79 | 1.67 | 1.415 * |
| TG-HDL 3 | 2.33 | 2.5 | 2.7 | 2.11 * |
| TG-HDL 4 | 4.24 | 4.105 | 4.61 | 4.01 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghini, V.; Tenori, L.; Capozzi, F.; Luchinat, C.; Bub, A.; Malpuech-Brugere, C.; Orfila, C.; Ricciardiello, L.; Bordoni, A. DHA-Induced Perturbation of Human Serum Metabolome. Role of the Food Matrix and Co-Administration of Oat β-glucan and Anthocyanins. Nutrients 2020, 12, 86. https://doi.org/10.3390/nu12010086
Ghini V, Tenori L, Capozzi F, Luchinat C, Bub A, Malpuech-Brugere C, Orfila C, Ricciardiello L, Bordoni A. DHA-Induced Perturbation of Human Serum Metabolome. Role of the Food Matrix and Co-Administration of Oat β-glucan and Anthocyanins. Nutrients. 2020; 12(1):86. https://doi.org/10.3390/nu12010086
Chicago/Turabian StyleGhini, Veronica, Leonardo Tenori, Francesco Capozzi, Claudio Luchinat, Achim Bub, Corinne Malpuech-Brugere, Caroline Orfila, Luigi Ricciardiello, and Alessandra Bordoni. 2020. "DHA-Induced Perturbation of Human Serum Metabolome. Role of the Food Matrix and Co-Administration of Oat β-glucan and Anthocyanins" Nutrients 12, no. 1: 86. https://doi.org/10.3390/nu12010086
APA StyleGhini, V., Tenori, L., Capozzi, F., Luchinat, C., Bub, A., Malpuech-Brugere, C., Orfila, C., Ricciardiello, L., & Bordoni, A. (2020). DHA-Induced Perturbation of Human Serum Metabolome. Role of the Food Matrix and Co-Administration of Oat β-glucan and Anthocyanins. Nutrients, 12(1), 86. https://doi.org/10.3390/nu12010086










