Mild Dehydration Identification Using Machine Learning to Assess Autonomic Responses to Cognitive Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Stroop Task
2.3. Data Processing
2.3.1. Indices of PRV
2.3.2. Indices of Electrodermal Activity
2.4. Classification Analysis
3. Results
3.1. Dehydration Assessment
3.2. Indices of Autonomic Response
3.3. Classification Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bunn, D.; Hooper, L.; Welch, A. Dehydration and Malnutrition in Residential Care: Recommendations for Strategies for Improving Practice Derived from a Scoping Review of Existing Policies and Guidelines. Geriatrics (Basel) 2018, 3, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncal-Jimenez, C.; Lanaspa, M.A.; Jensen, T.; Sanchez-Lozada, L.G.; Johnson, R.J. Mechanisms by Which Dehydration May Lead to Chronic Kidney Disease. Ann. Nutr. Metab. 2015, 66 (Suppl. 3), 10–13. [Google Scholar] [CrossRef]
- Shirreffs, S.M.; Merson, S.J.; Fraser, S.M.; Archer, D.T. The effects of fluid restriction on hydration status and subjective feelings in man. Br. J. Nutr. 2004, 91, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Ganio, M.S.; Casa, D.J.; Lee, E.C.; McDermott, B.P.; Klau, J.F.; Jimenez, L.; Le Bellego, L.; Chevillotte, E.; Lieberman, H.R. Mild Dehydration Affects Mood in Healthy Young Women, 2. J. Nutr. 2011, 142, 382–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganio, M.S.; Armstrong, L.E.; Casa, D.J.; McDermott, B.P.; Lee, E.C.; Yamamoto, L.M.; Marzano, S.; Lopez, R.M.; Jimenez, L.; Le Bellego, L. Mild dehydration impairs cognitive performance and mood of men. Br. J. Nutr. 2011, 106, 1535–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grucza, R.; Lecroart, J.L.; Carette, G.; Hauser, J.J.; Houdas, Y. Effect of voluntary dehydration on thermoregulatory responses to heat in men and women. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 317–322. [Google Scholar] [CrossRef]
- Armstrong, L.E. Assessing hydration status: The elusive gold standard. J. Am. Coll. Nutr. 2007, 26, 575S–584S. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Maresh, C.M.; Castellani, J.W.; Bergeron, M.F.; Kenefick, R.W.; LaGasse, K.E.; Riebe, D. Urinary indices of hydration status. Int. J. Sport Nutr. 1994, 4, 265–279. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Ely, B.R.; Kenefick, R.W.; Sawka, M.N. Biological variation and diagnostic accuracy of dehydration assessment markers. Am. J. Clin. Nutr. 2010, 92, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Oppliger, R.A.; Magnes, S.A.; Popowski, L.A.; Gisolfi, C.V. Accuracy of urine specific gravity and osmolality as indicators of hydration status. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 236–251. [Google Scholar] [CrossRef]
- Popowski, L.A.; Oppliger, R.A.; Patrick, G.L.; Johnson, R.F.; Kim, A.J.; Gisolf, C.V. Blood and urinary measures of hydration status during progressive acute dehydration. Med. Sci. Sports Exerc. 2001, 33, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Oppliger, R.A.; Bartok, C. Hydration testing of athletes. Sports Med. 2002, 32, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Shirreffs, S.M. Markers of hydration status. Eur. J. Clin. Nutr. 2003, 57, S6–S9. [Google Scholar] [CrossRef] [PubMed]
- Verbalis, J.G. How Does the Brain Sense Osmolality? JASN 2007, 18, 3056–3059. [Google Scholar] [CrossRef]
- Suryadevara, N.K.; Mukhopadhyay, S.C.; Barrack, L. Towards a smart non-invasive fluid loss measurement system. J. Med. Syst. 2015, 39, 206. [Google Scholar] [CrossRef]
- Reljin, N.; Malyuta, Y.; Zimmer, G.; Mendelson, Y.; Blehar, D.J.; Darling, C.E.; Chon, K.H. Automatic Detection of Dehydration using Support Vector Machines. In Proceedings of the 2018 14th Symposium on Neural Networks and Applications (NEUREL), Belgrade, Serbia, 20–21 November 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–6. [Google Scholar]
- Stroop, R.J. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, H.; Ju, K.; Shin, K.; Lee, M.; Shelley, K.; Chon, K.H. Can photoplethysmography variability serve as an alternative approach to obtain heart rate variability information? J. Clin. Monit. Comput. 2008, 22, 23–29. [Google Scholar] [CrossRef]
- Posada-Quintero, H.F.; Delisle-Rodríguez, D.; Cuadra-Sanz, M.B.; Fernández de la Vara-Prieto, R.R. Evaluation of pulse rate variability obtained by the pulse onsets of the photoplethysmographic signal. Physiol. Meas. 2013, 34, 179–187. [Google Scholar] [CrossRef]
- Schäfer, A.; Vagedes, J. How accurate is pulse rate variability as an estimate of heart rate variability? A review on studies comparing photoplethysmographic technology with an electrocardiogram. Int. J. Cardiol. 2013, 166, 15–29. [Google Scholar] [CrossRef]
- Challoner, A.V.J. Photoelectric plethysmography for estimating cutaneous blood flow. Non-Invasive Physiol. Meas. 1979, 1, 125–151. [Google Scholar]
- Bolkhovsky, J.B.; Scully, C.G.; Chon, K.H. Statistical analysis of heart rate and heart rate variability monitoring through the use of smart phone cameras. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 1610–1613. [Google Scholar]
- Mendelson, Y.; Dao, D.K.; Chon, K.H. Multi-channel pulse oximetry for wearable physiological monitoring. In Proceedings of the 2013 IEEE International Conference on Body Sensor Networks, Cambridge, MA, USA, 6–9 May 2013; pp. 1–6. [Google Scholar]
- Peng, R.C.; Zhou, X.L.; Lin, W.H.; Zhang, Y.T. Extraction of heart rate variability from smartphone photoplethysmograms. Comput. Math. Methods Med. 2015, 2015, 516826. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [CrossRef] [Green Version]
- Boucsein, W.; Fowles, D.C.; Grimnes, S.; Ben-Shakhar, G.; Roth, W.T.; Dawson, M.E.; Filion, D.L. Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures Publication recommendations for electrodermal measurements. Psychophysiology 2012, 49, 1017–1034. [Google Scholar] [PubMed]
- Hernando-Gallego, F.; Luengo, D.; Artes-Rodriguez, A. Feature Extraction of Galvanic Skin Responses by Non-Negative Sparse Deconvolution. IEEE J. Biomed. Health Inform. 2017, 22, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Posada-Quintero, H.F.; Florian, J.P.; Orjuela-Cañón, A.D.; Aljama-Corrales, T.; Charleston-Villalobos, S.; Chon, K.H. Power Spectral Density Analysis of Electrodermal Activity for Sympathetic Function Assessment. Ann. Biomed. Eng. 2016, 44, 3124–3135. [Google Scholar] [CrossRef]
- Chon, K.H.; Dash, S.; Ju, K. Estimation of respiratory rate from photoplethysmogram data using time-frequency spectral estimation. IEEE Trans. Biomed. Eng. 2009, 56, 2054–2063. [Google Scholar] [CrossRef]
- Posada-Quintero, H.F.; Florian, J.P.; Orjuela-Cañón, Á.D.; Chon, K.H. Highly sensitive index of sympathetic activity based on time-frequency spectral analysis of electrodermal activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R582–R591. [Google Scholar] [CrossRef] [Green Version]
- Massey, F.J., Jr. The Kolmogorov-Smirnov test for goodness of fit. J. Am. Stat. Assoc. 1951, 46, 68–78. [Google Scholar] [CrossRef]
- Miller, L.H. Table of percentage points of Kolmogorov statistics. J. Am. Stat. Assoc. 1956, 51, 111–121. [Google Scholar] [CrossRef]
- Wang, J.; Tsang, W.W.; Marsaglia, G. Evaluating Kolmogorov’s distribution. J. Stat. Softw. 2003, 8. [Google Scholar] [CrossRef]
- Gibbons, J.D.; Chakraborti, S. Nonparametric statistical inference. In International Encyclopedia of Statistical Science; Springer: Berlin, Germany, 2011; pp. 977–979. [Google Scholar]
- Krzanowski, W. Principles of Multivariate Analysis; OUP Oxford: Oxford, UK, 2000; Volume 23. [Google Scholar]
- Seber, G.A. Multivariate Observations; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 252. [Google Scholar]
- Le Cessie, S.; Van Houwelingen, J.C. Ridge Estimators in Logistic Regression. J. R. Stat. Soc. Ser. C Appl. Stat. 1992, 41, 191–201. [Google Scholar] [CrossRef]
- Shawe-Taylor, J.; Cristianini, N. An Introduction to Support Vector Machines and Other Kernel-Based Learning Methods; Cambridge University Press: Cambridge, UK, 2000; Volume 204. [Google Scholar]
- Breiman, L. Classification and Regression Trees; Routledge: Abingdon upon Thames, UK, 2017. [Google Scholar]
- Duda, R.O.; Hart, P.E.; Stork, D.G. Pattern Classification; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Friedman, J.H.; Bentley, J.L.; Finkel, R.A. An algorithm for finding best matches in logarithmic time. ACM Trans. Math. Softw. 1976, 3, 209–226. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, C.; Zhang, D. An experimental evaluation of ensemble methods for EEG signal classification. Pattern Recognit. Lett. 2007, 28, 2157–2163. [Google Scholar] [CrossRef]
- Ho, T.K. The random subspace method for constructing decision forests. IEEE Trans. Pattern Anal. Mach. Intell. 1998, 20, 832–844. [Google Scholar]
- Tsymbal, A.; Pechenizkiy, M.; Cunningham, P. Diversity in search strategies for ensemble feature selection. Inf. Fusion 2005, 6, 83–98. [Google Scholar] [CrossRef]
- Peng, H.; Long, F.; Ding, C. Feature selection based on mutual information: Criteria of max-dependency, max-relevance, and min-redundancy. IEEE Trans. Pattern Anal. Mach. Intell. 2005, 1226–1238. [Google Scholar] [CrossRef]
- Meyer, P.E.; Bontempi, G. On the use of variable complementarity for feature selection in cancer classification. In Proceedings of the Workshops on Applications of Evolutionary Computation; Springer: Berlin, Germany, 2006; pp. 91–102. [Google Scholar]
- Yang, H.H.; Moody, J. Data visualization and feature selection: New algorithms for nongaussian data. In Proceedings of the Advances in Neural Information Processing Systems, Denver, CO, USA, 29 November –4 December 1999; pp. 687–693. [Google Scholar]
- Muñoz, C.X.; Johnson, E.C.; Demartini, J.K.; Huggins, R.A.; McKenzie, A.L.; Casa, D.J.; Maresh, C.M.; Armstrong, L.E. Assessment of hydration biomarkers including salivary osmolality during passive and active dehydration. Eur. J. Clin. Nutr. 2013, 67, 1257–1263. [Google Scholar] [CrossRef]
- Phillips, P.A.; Bretherton, M.; Risvanis, J.; Casley, D.; Johnston, C.; Gray, L. Effects of drinking on thirst and vasopressin in dehydrated elderly men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1993, 264, R877–R881. [Google Scholar] [CrossRef]
- Phillips, P.A.; Rolls, B.J.; Ledingham, J.G.; Forsling, M.L.; Morton, J.J.; Crowe, M.J.; Wollner, L. Reduced thirst after water deprivation in healthy elderly men. N. Engl. J. Med. 1984, 311, 753–759. [Google Scholar] [CrossRef]
- Posada-Quintero, H.F.; Dimitrov, T.; Moutran, A.; Park, S.; Chon, K.H. Analysis of Reproducibility of Noninvasive Measures of Sympathetic Autonomic Control Based on Electrodermal Activity and Heart Rate Variability. IEEE Access 2019, 7, 22523–22531. [Google Scholar] [CrossRef]
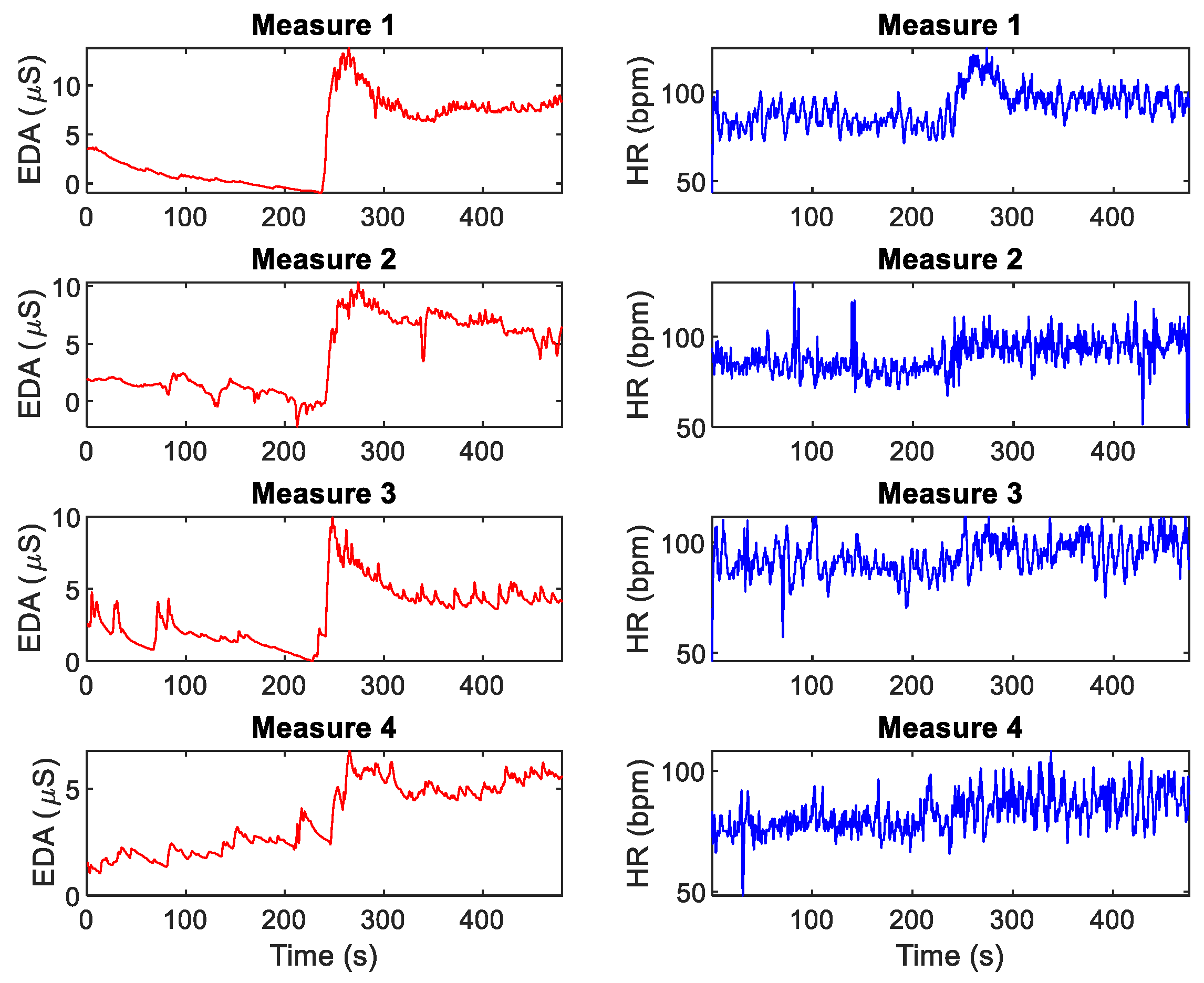
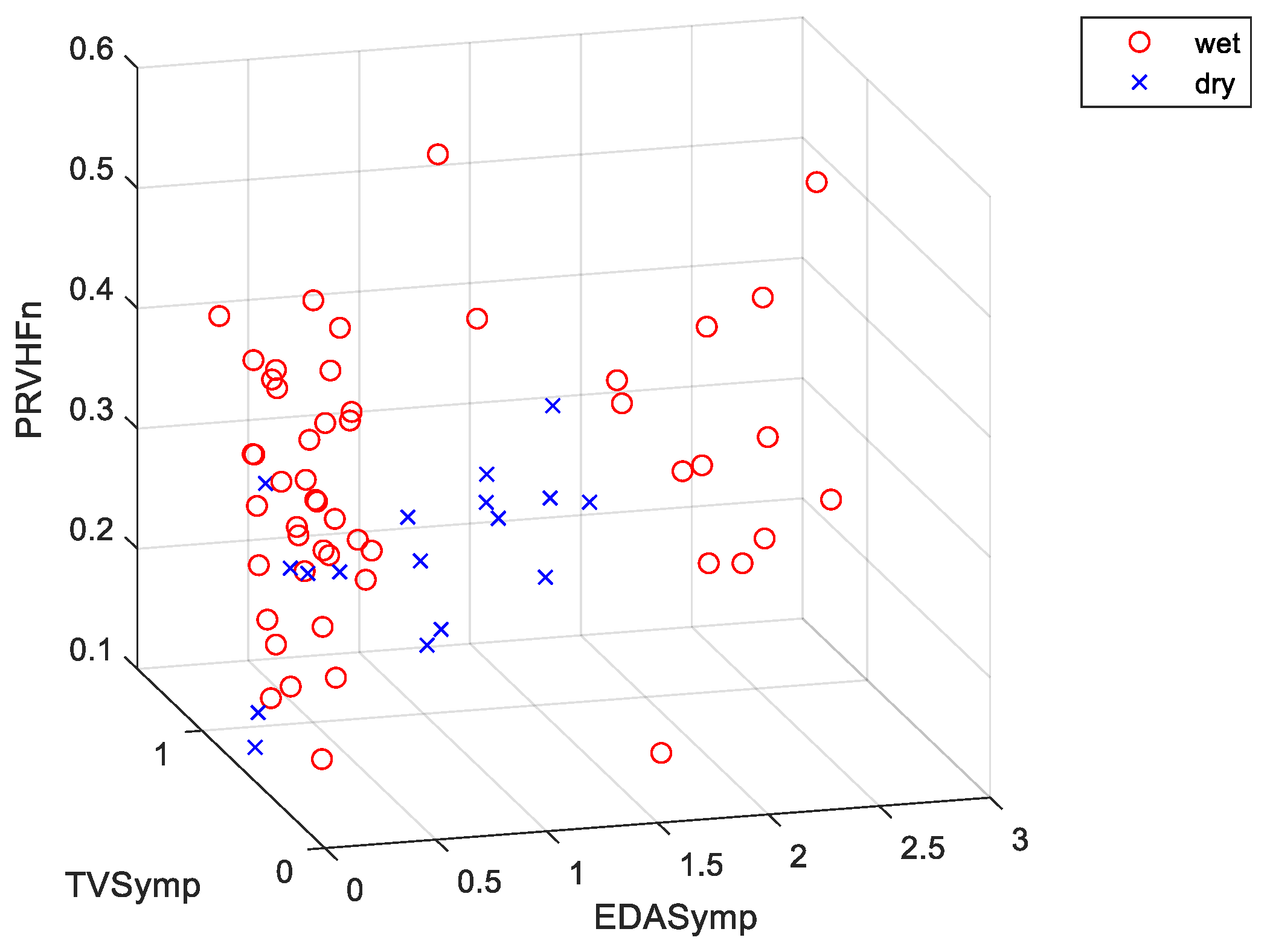
| Day 1 | Day 2 | Day 3 | |
|---|---|---|---|
| Measures taken | Measure 1 | Measure 2 | Measure 3, Measure 4 |
| Activities | Euhydration. Drink and eat normally | Fluid restriction. Drink no fluids and eat dry food | After Measure 3, consume water as desired |
| Name | Description | ||
|---|---|---|---|
| EDA | SCL | Skin conductance level | Mean value of the tonic component |
| NS.SCRs | Non-specific SCRs | Frequency of phasic drivers >0.05 µS | |
| EDASymp | Power spectral index of EDA | Power of EDA in the range of 0.045–0.25 Hz | |
| EDASympn | Normalized EDASymp | EDASymp normalized to total power of EDA | |
| TVSymp | Time-varying index of EDA | Instantaneous amplitude of sympathetic components | |
| PRV | PRVLF | Low frequencies of PRV | Power in the range of 0.045–0.15 Hz |
| PRVLFn | Normalized PRVLF | PRVLF normalized to total power of PRV | |
| PRVHF | High frequencies of PRV | Power in the range of 0.15–0.4 Hz | |
| PRVHFn | Normalized PRVHF | PRVHF normalized to total power of PRV |
| Name | Description |
|---|---|
| LDA | Linear discriminant analysis |
| QDA | Quadratic Discriminant analysis |
| Logistic | Logistic regression model |
| Cubic SVM | SVM with cubic kernel |
| Fine Gaussian SVM | SVM with Gaussian kernel, C = 1, γ = 0.66 |
| Medium Gaussian SVM | SVM with Gaussian kernel, C = 1, γ = 2.6 |
| KNN | k-nearest neighbor classifier |
| DT | Decision trees |
| SE-KNN | Subspace ensemble of KNN classifiers |
| Day 1 (BL) Measure 1 | Day 2 (EU) Measure 2 | Day 3 (FR) Measure 3 | Day 3 (RH) Measure 4 | |
|---|---|---|---|---|
| Urinary loss (liters) | - | 1.9 ± 1.1 | 0.83 ± 0.28 * | - |
| Blood osmolality | 277.8 ± 10.8 | 275.8 ± 21.9 | 286.6 ± 7.2 * | 281.6 ± 9.7 * |
| Body-mass (kg) | 81 ± 7.3 | 81.1 ± 7.2 | 79.7 ± 7.1 * | 80.9 ± 7.3 * |
| Body-mass loss (%) | - | 0.1 ± 0.8 | −1.78 ± 0.48 | −0.31 ± 0.66 |
| Day 1 (BL) Measure 1 | Day 2 (EU) Measure 2 | Day 3 (FR) Measure 3 | Day 3 (RH) Measure 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Rest | Test | Rest | Test | Rest | Test | Rest | Test | |
| SCL (µS) | 2.2 ± 2.4 | 7.1 ± 4.4 * | 2.3 ± 3.6 | 5.5 ± 6.4 * | 3.8 ± 4.7 | 7.9 ± 6.4 * | 3.8 ± 5.5 | 6.3 ± 6.6 * |
| NS.SCRs (#/min) | 3.8 ± 1.9 | 8.1 ± 2.1 * | 4.2 ± 2.6 | 6.6 ± 3.8 | 3.9 ± 2.5 | 8.2 ± 3 * | 3.9 ± 2.2 | 7.3 ± 3.6 * |
| EDASymp (µS2) | 0.2 ± 0.48 | 6.6 ± 24 * | 0.19 ± 0.33 | 0.97 ± 3.1 | 1.3 ± 4.8 | 0.61 ± 1.1 | 0.092 ± 0.17 | 0.096 ± 0.086 |
| EDASympn (n.u.) | 0.36 ± 0.21 | 0.27 ± 0.21 | 0.35 ± 0.18 | 0.24 ± 0.21 | 0.3 ± 0.17 | 0.2 ± 0.16 | 0.36 ± 0.2 | 0.27 ± 0.15 |
| TVSymp (dimensionless) | 0.52 ± 0.31 | 1.5 ± 0.42 * | 0.69 ± 0.35 | 1.2 ± 0.48 * | 0.55 ± 0.35 | 1.3 ± 0.44 * | 0.72 ± 0.43 | 1.2 ± 0.48 * |
| PRVLF (mS2) | 14 ± 12 | 15 ± 12 | 13 ± 15 | 15 ± 15 | 170 ± 650 | 120 ± 420 | 13 ± 12 | 19 ± 28 |
| PRVLFn (n.u.) | 0.35 ± 0.16 | 0.3 ± 0.14 | 0.29 ± 0.11 | 0.35 ± 0.14 | 0.34 ± 0.18 | 0.46 ± 0.15 * | 0.32 ± 0.12 | 0.39 ± 0.18 |
| PRVHF (mS2) | 15 ± 23 | 16 ± 18 | 12 ± 10 | 17 ± 22 | 37 ± 110 | 55 ± 140 | 17 ± 22 | 27 ± 69 |
| PRVHFn (n.u.) | 0.29 ± 0.17 | 0.26 ± 0.13 | 0.34 ± 0.19 | 0.3 ± 0.13 | 0.29 ± 0.18 | 0.25 ± 0.14 | 0.35 ± 0.17 | 0.29 ± 0.13 |
| Data Used | Classifier | Indices | Accuracy | Error Rate | Sensitivity | FPR | Specificity | Precision |
|---|---|---|---|---|---|---|---|---|
| Only rest | SE-KNN | EDASymp, TVSymp, PRVHFn | 91.2% | 8.8% | 76.5% | 3.9% | 96.1% | 86.7% |
| Only test | Cubic SVM | NS.SCRs, EDASymp, EDASympn, PRVLF, PRVLFn, PRVHFn | 91.2% | 8.8% | 100.0% | 11.8% | 88.2% | 73.9% |
| (Test-rest) | KNN | SCL, NS.SCRs, TVSymp, PRVLF, PRVLFn, PRVHFn | 86.8% | 13.2% | 88.2% | 13.7% | 86.3% | 68.2% |
| Rest and test | QDA | SCL, NS.SCRs, PRVLF, PRVLFn | 86.80% | 13.2% | 52.9% | 2.0% | 98.0% | 90.0% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posada-Quintero, H.F.; Reljin, N.; Moutran, A.; Georgopalis, D.; Lee, E.C.-H.; Giersch, G.E.W.; Casa, D.J.; Chon, K.H. Mild Dehydration Identification Using Machine Learning to Assess Autonomic Responses to Cognitive Stress. Nutrients 2020, 12, 42. https://doi.org/10.3390/nu12010042
Posada-Quintero HF, Reljin N, Moutran A, Georgopalis D, Lee EC-H, Giersch GEW, Casa DJ, Chon KH. Mild Dehydration Identification Using Machine Learning to Assess Autonomic Responses to Cognitive Stress. Nutrients. 2020; 12(1):42. https://doi.org/10.3390/nu12010042
Chicago/Turabian StylePosada-Quintero, Hugo F., Natasa Reljin, Aurelie Moutran, Dimitrios Georgopalis, Elaine Choung-Hee Lee, Gabrielle E. W. Giersch, Douglas J. Casa, and Ki H. Chon. 2020. "Mild Dehydration Identification Using Machine Learning to Assess Autonomic Responses to Cognitive Stress" Nutrients 12, no. 1: 42. https://doi.org/10.3390/nu12010042
APA StylePosada-Quintero, H. F., Reljin, N., Moutran, A., Georgopalis, D., Lee, E. C.-H., Giersch, G. E. W., Casa, D. J., & Chon, K. H. (2020). Mild Dehydration Identification Using Machine Learning to Assess Autonomic Responses to Cognitive Stress. Nutrients, 12(1), 42. https://doi.org/10.3390/nu12010042








