Heat-Killed Bifidobacterium breve B-3 Enhances Muscle Functions: Possible Involvement of Increases in Muscle Mass and Mitochondrial Biogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Samples
2.2. Animal Experiments
2.3. Western Blotting
2.4. Quantitative Real-Time PCR (qPCR) Analysis
2.5. Histological Analysis
2.6. Grip Test
2.7. Statistical Analysis
2.8. Data Availability
3. Results
3.1. The Effects of B-3 Administration on Body and Tissue Weight in Rats
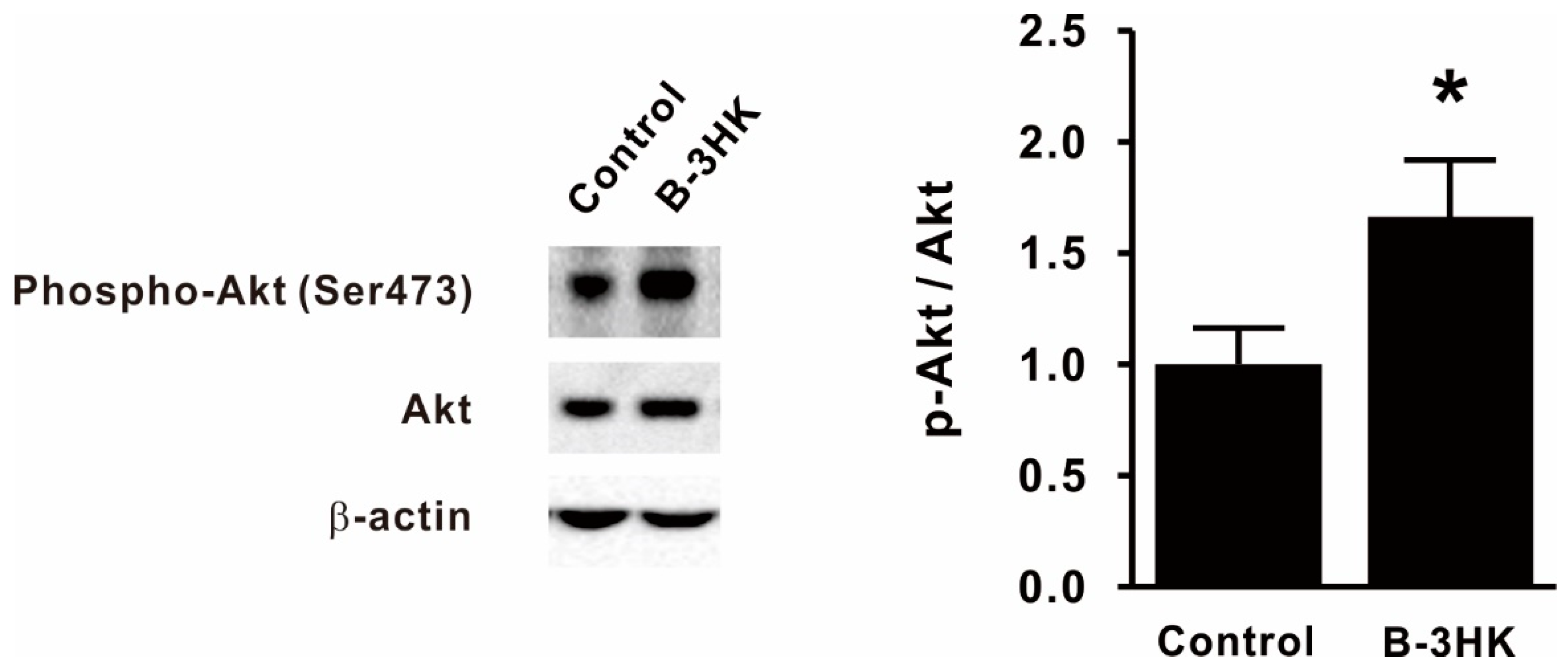
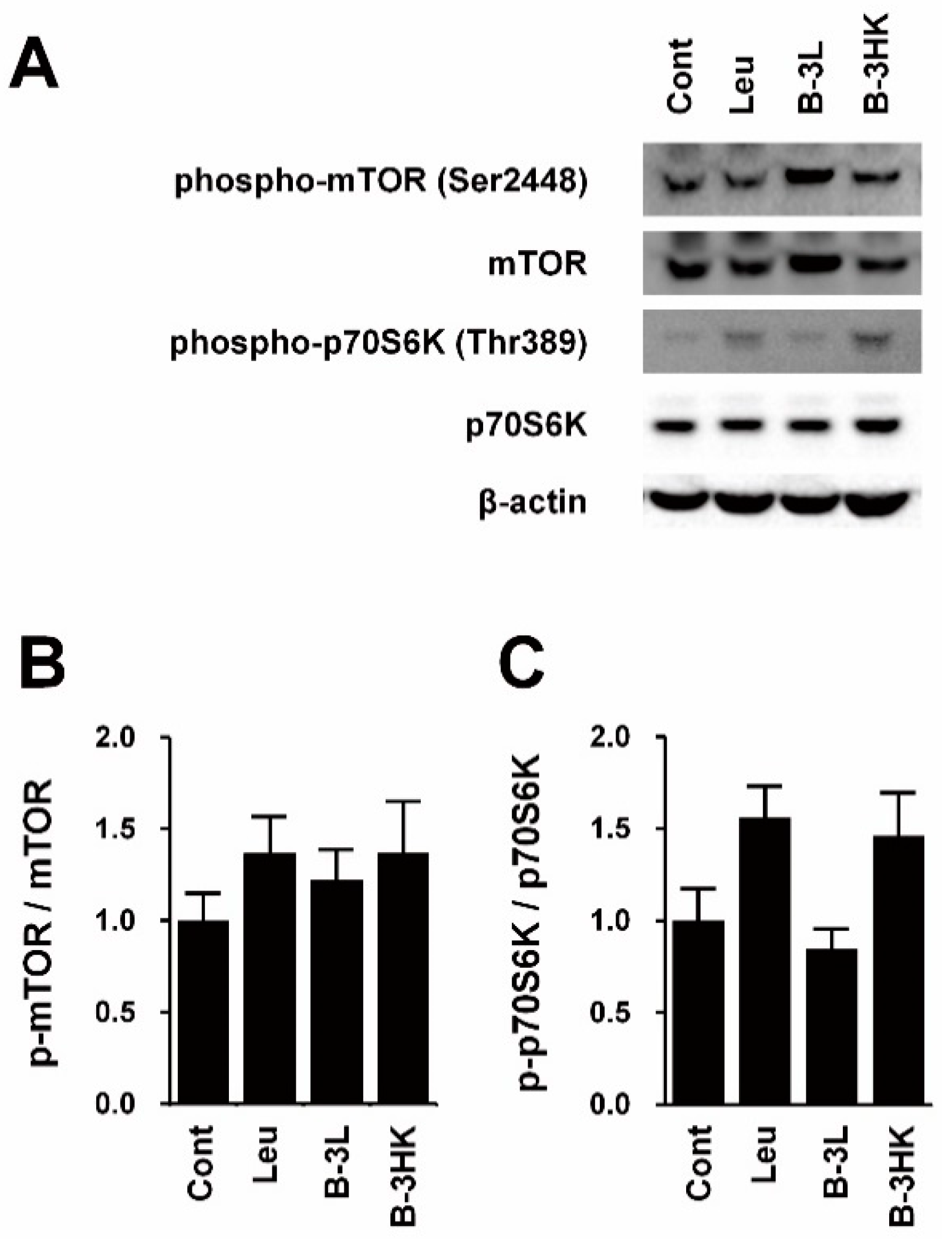
3.2. B-3HK Promoted Phosphorylation of Akt in the Rat Soleus
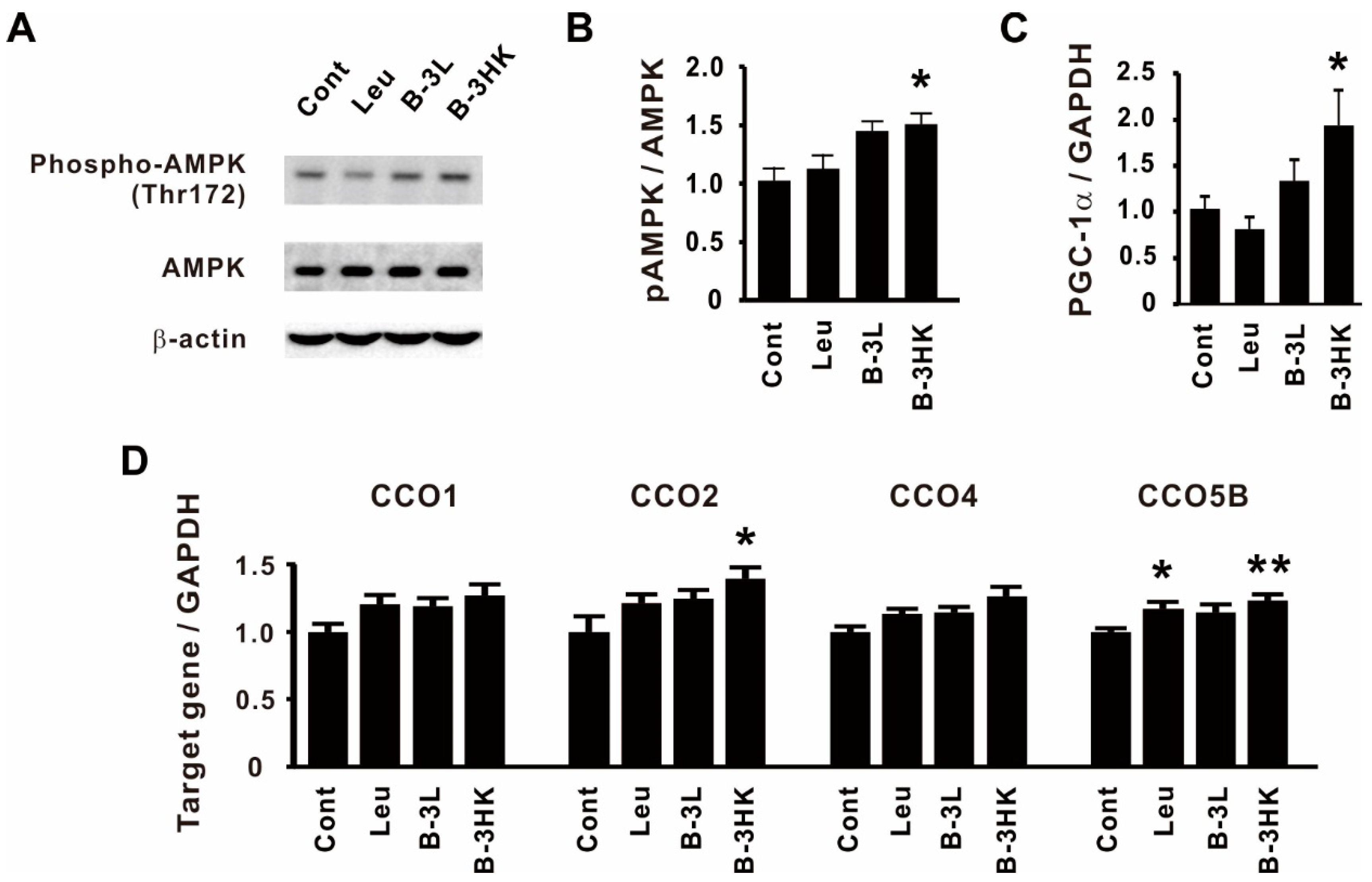
3.3. The Effects of B-3 on the AMPK-PGC-1α-Mitochondrial Biogenesis Pathway in the Rat Soleus
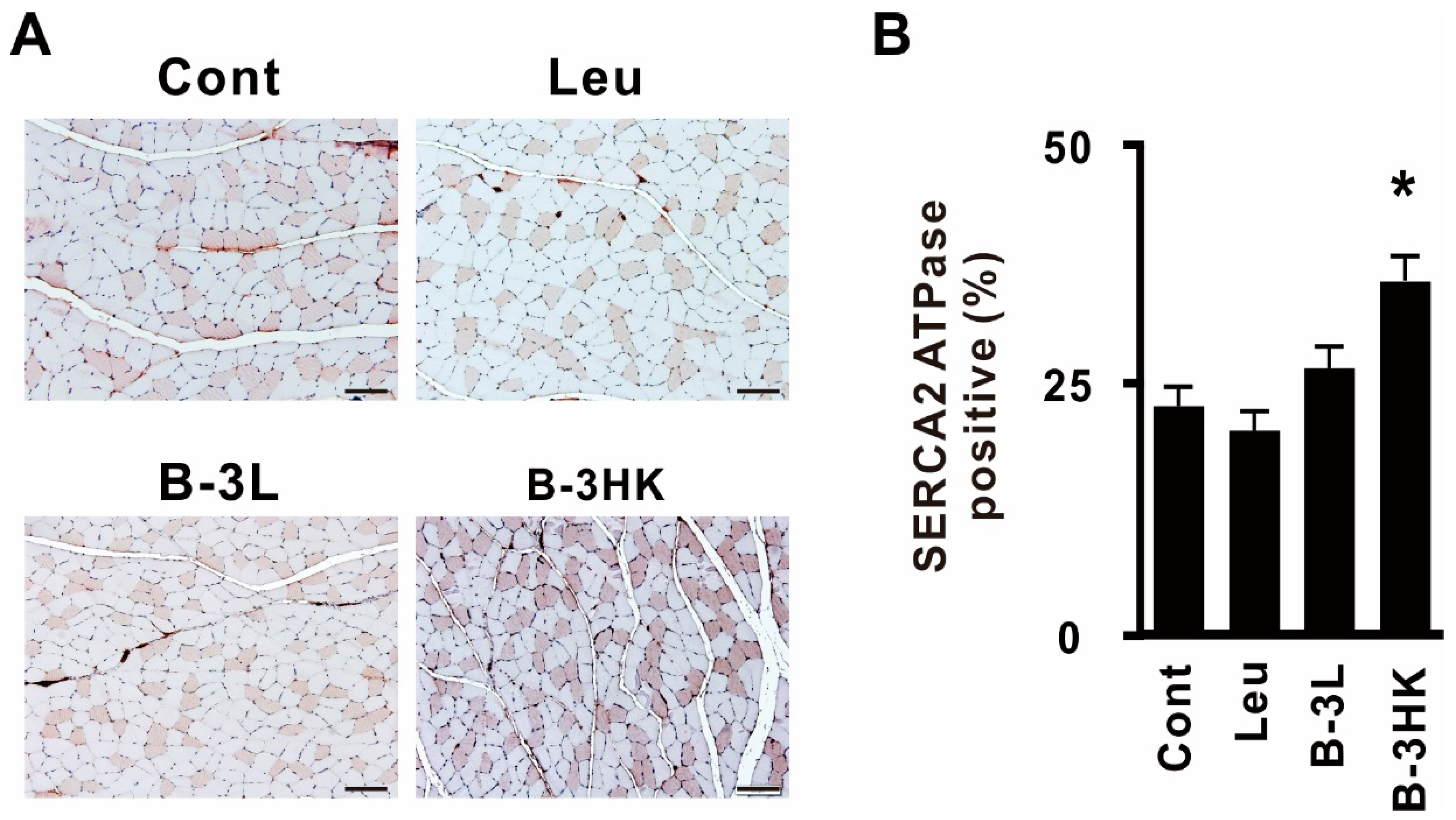
3.4. B-3HK Promoted the Distribution of Oxidative Fibers in the Gastrocnemius in Rats
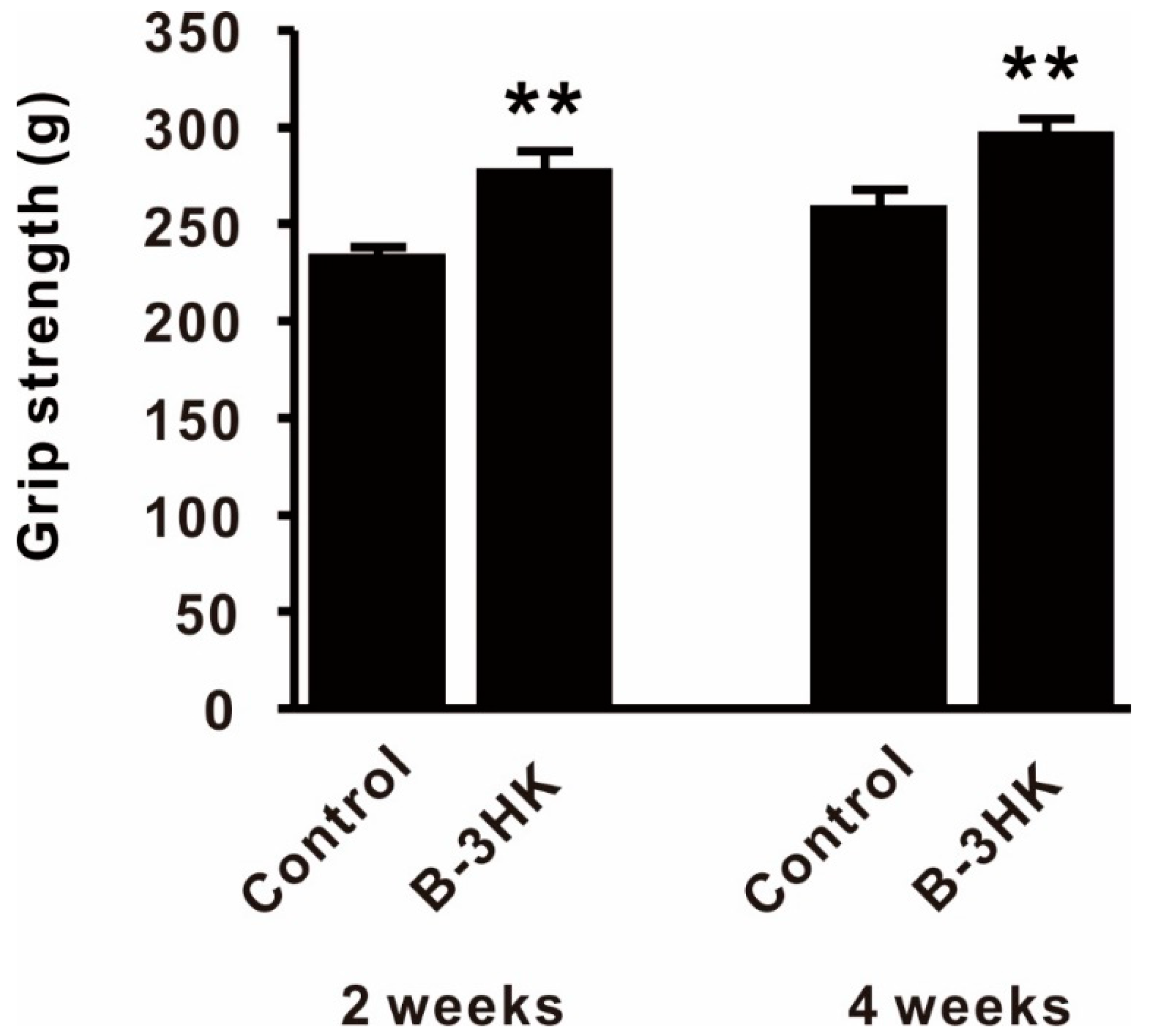
3.5. B-3HK-Enhanced Fitness Performance in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kondo, S.; Xiao, J.; Satoh, T.; Odamaki, T.; Takahashi, S.; Sugahara, H.; Yaeshima, T.; Iwatsuki, K.; Kamei, A.; Abe, K. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci. Biotechnol. Biochem. 2010, 74, 1656–1661. [Google Scholar] [CrossRef]
- Minami, J.; Iwabuchi, N.; Tanaka, M.; Yamauchi, K.; Xiao, J.Z.; Abe, F.; Sakane, N. Effects of Bifidobacterium breve B-3 on body fat reductions in pre-obese adults: A randomized, double-blind, placebo-controlled trial. Biosci. Microbiota Food Health 2018, 37, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Kurose, Y.; Minami, J.; Sen, A.; Iwabuchi, N.; Abe, F.; Xiao, J.; Suzuki, T. Bioactive factors secreted by Bifidobacterium breve B-3 enhance barrier function in human intestinal Caco-2 cells. Benef. Microbes 2019, 10, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediat. Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef] [PubMed]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef]
- Wosinska, L.; Cotter, P.D.; O’Sullivan, O.; Guinane, C. The Potential Impact of Probiotics on the Gut Microbiome of Athletes. Nutrients 2019, 11, 2270. [Google Scholar] [CrossRef]
- Maruta, H.; Yoshimura, Y.; Araki, A.; Kimoto, M.; Takahashi, Y.; Yamashita, H. Activation of AMP-Activated Protein Kinase and Stimulation of Energy Metabolism by Acetic Acid in L6 Myotube Cells. PLoS ONE 2016, 11, e0158055. [Google Scholar] [CrossRef]
- Pan, J.H.; Kim, J.H.; Kim, H.M.; Lee, E.S.; Shin, D.H.; Kim, S.; Shin, M.; Kim, S.H.; Lee, J.H.; Kim, Y.J. Acetic acid enhances endurance capacity of exercise-trained mice by increasing skeletal muscle oxidative properties. Biosci. Biotechnol. Biochem. 2015, 79, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed]
- Sakai, F.; Hosoya, T.; Ono-Ohmachi, A.; Ukibe, K.; Ogawa, A.; Moriya, T.; Kadooka, Y.; Shiozaki, T.; Nakagawa, H.; Nakayama, Y.; et al. Lactobacillus gasseri SBT2055 induces TGF-β expression in dendritic cells and activates TLR2 signal to produce IgA in the small intestine. PLoS ONE 2014, 9, e105370. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Tomosada, Y.; Villena, J.; Chiba, E.; Shimazu, T.; Aso, H.; Iwabuchi, N.; Xiao, J.Z.; Saito, T.; Kitazawa, H. Bifidobacterium breve MCC-117 Induces Tolerance in Porcine Intestinal Epithelial Cells: Study of the Mechanisms Involved in the Immunoregulatory Effect. Biosci. Microbiota Food Health 2014, 33, 1–10. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Yoon, M.S. mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Arai, S.; Iwabuchi, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Hachimura, S. Orally administered heat-killed Lactobacillus paracasei MCC1849 enhances antigen-specific IgA secretion and induces follicular helper T cells in mice. PLoS ONE 2018, 13, e0199018. [Google Scholar] [CrossRef]
- Tanaka, M.; Yoshino, Y.; Takeda, S.; Toda, K.; Shimoda, H.; Tsuruma, K.; Shimazawa, M.; Hara, H. Fermented Rice Germ Extract Alleviates Morphological and Functional Damage to Murine Gastrocnemius Muscle by Inactivation of AMP-Activated Protein Kinase. J. Med. Food 2017, 20, 969–980. [Google Scholar] [CrossRef]
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Mounier, R.; Lantier, L.; Leclerc, J.; Sotiropoulos, A.; Pende, M.; Daegelen, D.; Sakamoto, K.; Foretz, M.; Viollet, B. Important role for AMPKalpha1 in limiting skeletal muscle cell hypertrophy. FASEB J. 2009, 23, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–890S. [Google Scholar] [CrossRef]
- Diaz, F. Cytochrome c oxidase deficiency: Patients and animal models. Biochim. Biophys. Acta 2010, 1802, 100–110. [Google Scholar] [CrossRef]
- Pacelli, C.; Latorre, D.; Cocco, T.; Capuano, F.; Kukat, C.; Seibel, P.; Villani, G. Tight control of mitochondrial membrane potential by cytochrome c oxidase. Mitochondrion 2011, 11, 334–341. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.; Wu, W.; Hou, L.; Chen, H.; Zuo, B.; Xiong, Y.; Yang, J. Skeletal Muscle-Specific Overexpression of PGC-1α Induces Fiber-Type Conversion through Enhanced Mitochondrial Respiration and Fatty Acid Oxidation in Mice and Pigs. Int. J. Biol. Sci. 2017, 13, 1152–1162. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Zurlo, F.; Nemeth, P.M.; Choksi, R.M.; Sesodia, S.; Ravussin, E. Whole-body energy metabolism and skeletal muscle biochemical characteristics. Metabolism 1994, 43, 481–486. [Google Scholar] [CrossRef]
- Zurlo, F.; Larson, K.; Bogardus, C.; Ravussin, E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Investig. 1990, 86, 1423–1427. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Hsu, Y.J.; Li, H.; Kan, N.W.; Chen, Y.M.; Lin, J.S.; Hsu, T.K.; Tsai, T.Y.; Chiu, Y.S.; Huang, C.C. Effect of Lactobacillus Plantarum TWK10 on Improving Endurance Performance in Humans. Chin. J. Physiol. 2018, 61, 163–170. [Google Scholar] [CrossRef]
- Huang, W.; Lee, M.C.; Lee, C.; Ng, K.; Hsu, Y.; Tsai, T.Y.; Young, S.L.; Lin, J.S.; Huang, C.C. Effect of Lactobacillus plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans. Nutrients 2019, 11, 2836. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Villena, J.; Aso, H.; Kitazawa, H. Regulation of toll-like receptors-mediated inflammation by immunobiotics in bovine intestinal epitheliocytes: Role of signaling pathways and negative regulators. Front. Immunol. 2014, 5, 421. [Google Scholar] [CrossRef]
- Plantinga, T.S.; Van Maren, W.W.C.; Van Bergenhenegouwen, J.; Hameetman, M.; Nierkens, S.; Jacobs, C.; De Jong, D.J.; Joosten, L.A.B.; Van’t Land, B.; Garssen, J.; et al. Differential toll-like receptor recognition and induction of cytokine profile by Bifidobacterium breve and Lactobacillus strains of probiotics. Clin. Vaccine Immunol. 2011, 18, 621–628. [Google Scholar] [CrossRef]
- Donkor, O.N.; Ravikumar, M.; Proudfoot, O.; Day, S.L.; Apostolopoulos, V.; Paukovics, G.; Vasiljevic, T.; Nutt, S.L.; Gill, H. Cytokine profile and induction of T helper type 17 and regulatory T cells by human peripheral mononuclear cells after microbial exposure. Clin. Exp. Immunol. 2012, 167, 282–295. [Google Scholar] [CrossRef]
- Sugahara, H.; Yao, R.; Odamaki, T.; Xiao, J.Z. Differences between live and heat-killed bifidobacteria in the regulation of immune function and the intestinal environment. Benef. Microbes 2017, 8, 463–472. [Google Scholar] [CrossRef]
- Picca, A.; Fanelli, F.; Calvani, R.; Mulè, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediat. Inflamm. 2018, 2018, 7026198. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, F.; Mochizuki, S.; Sugahara, K. Differential dose response of mTOR signaling to oral administration of leucine in skeletal muscle and liver of rats. Biosci. Biotechnol. Biochem. 2013, 77, 839–842. [Google Scholar] [CrossRef] [PubMed]

| Control | Leucine | B-3L | B-3HK | ||
|---|---|---|---|---|---|
| Body weight (g) | initial | 311.2 ± 2.7 | 307.3 ± 3.5 | 302.1 ± 2.9 | 303.8 ± 3.6 |
| 1 week | 361.1 ± 4.6 | 356.9 ± 5.5 | 358.4 ± 3.3 | 354.1 ± 5.1 | |
| 2 weeks | 408.0 ± 6.3 | 397.3 ± 9.5 | 400.5 ± 4.8 | 393.2 ± 7.5 | |
| 3 weeks | 430.7 ± 9.6 | 418.1 ± 9.7 | 425.9 ± 6.5 | 417.9 ± 9.0 | |
| 4 weeks | 433.2 ± 9.4 | 430.6 ± 10.7 | 430.1 ± 6.2 | 421.3 ± 9.4 | |
| Food intake (g) | initial | 31.8 ± 0.6 | 30.7 ± 0.8 | 30.5 ± 0.5 | 31.0 ± 0.9 |
| 1 week | 33.7 ± 1.1 | 31.6 ± 1.3 | 31.4 ± 0.7 | 29.8 ± 1.1 * | |
| 2 weeks | 31.2 ± 0.9 | 30.3 ± 1.0 | 30.7 ± 1.5 | 29.5 ± 1.1 | |
| 3 weeks | 30.8 ± 1.1 | 28.5 ± 1.2 | 30.7 ± 1.5 | 30.0 ± 1.3 | |
| 4 weeks | 33.5 ± 1.2 | 32.1 ± 1.4 | 31.8 ± 0.7 | 32.2 ± 1.1 | |
| Liver weight (g) | 12.0 ± 0.4 | 11.5 ± 0.4 | 11.7 ± 0.3 | 11.3 ± 0.4 | |
| Liver weight/body weight (mg/g) | 27.6 ± 0.8 | 26.5 ± 0.5 | 27.2 ± 0.5 | 26.8 ± 0.4 | |
| Soleus weight (mg) | 193.3 ± 5.8 | 203.5 ± 5.7 | 205.4 ± 5.7 | 209.7 ± 5.4 | |
| Soleus weight/body weight (mg/g) | 0.45 ± 0.01 | 0.47 ± 0.01 | 0.48 ± 0.01 | 0.50 ± 0.01 * | |
| Plantaris weight (mg) | 415.0 ± 21.6 | 431.4 ± 12.4 | 440.3 ± 12.4 | 456.4 ± 24.4 | |
| Plantaris weight/body weight (mg/g) | 0.96 ± 0.05 | 1.00 ± 0.02 | 1.03 ± 0.03 | 1.08 ± 0.05 | |
| Gastrocnemius weight (mg) | 2242.9 ± 50.3 | 2253.3 ± 48.1 | 2303.5 ± 44.8 | 2274.2 ± 53.4 | |
| Gastrocnemius weight/body weight (mg/g) | 5.19 ± 0.09 | 5.26 ± 0.13 | 5.37 ± 0.12 | 5.40 ± 0.06 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toda, K.; Yamauchi, Y.; Tanaka, A.; Kuhara, T.; Odamaki, T.; Yoshimoto, S.; Xiao, J.-z. Heat-Killed Bifidobacterium breve B-3 Enhances Muscle Functions: Possible Involvement of Increases in Muscle Mass and Mitochondrial Biogenesis. Nutrients 2020, 12, 219. https://doi.org/10.3390/nu12010219
Toda K, Yamauchi Y, Tanaka A, Kuhara T, Odamaki T, Yoshimoto S, Xiao J-z. Heat-Killed Bifidobacterium breve B-3 Enhances Muscle Functions: Possible Involvement of Increases in Muscle Mass and Mitochondrial Biogenesis. Nutrients. 2020; 12(1):219. https://doi.org/10.3390/nu12010219
Chicago/Turabian StyleToda, Kazuya, Yuki Yamauchi, Azusa Tanaka, Tetsuya Kuhara, Toshitaka Odamaki, Shin Yoshimoto, and Jin-zhong Xiao. 2020. "Heat-Killed Bifidobacterium breve B-3 Enhances Muscle Functions: Possible Involvement of Increases in Muscle Mass and Mitochondrial Biogenesis" Nutrients 12, no. 1: 219. https://doi.org/10.3390/nu12010219
APA StyleToda, K., Yamauchi, Y., Tanaka, A., Kuhara, T., Odamaki, T., Yoshimoto, S., & Xiao, J.-z. (2020). Heat-Killed Bifidobacterium breve B-3 Enhances Muscle Functions: Possible Involvement of Increases in Muscle Mass and Mitochondrial Biogenesis. Nutrients, 12(1), 219. https://doi.org/10.3390/nu12010219





