Discharging Women with Advanced Ovarian Cancer on Home Parenteral Nutrition: Making and Implementing the Decision
Abstract
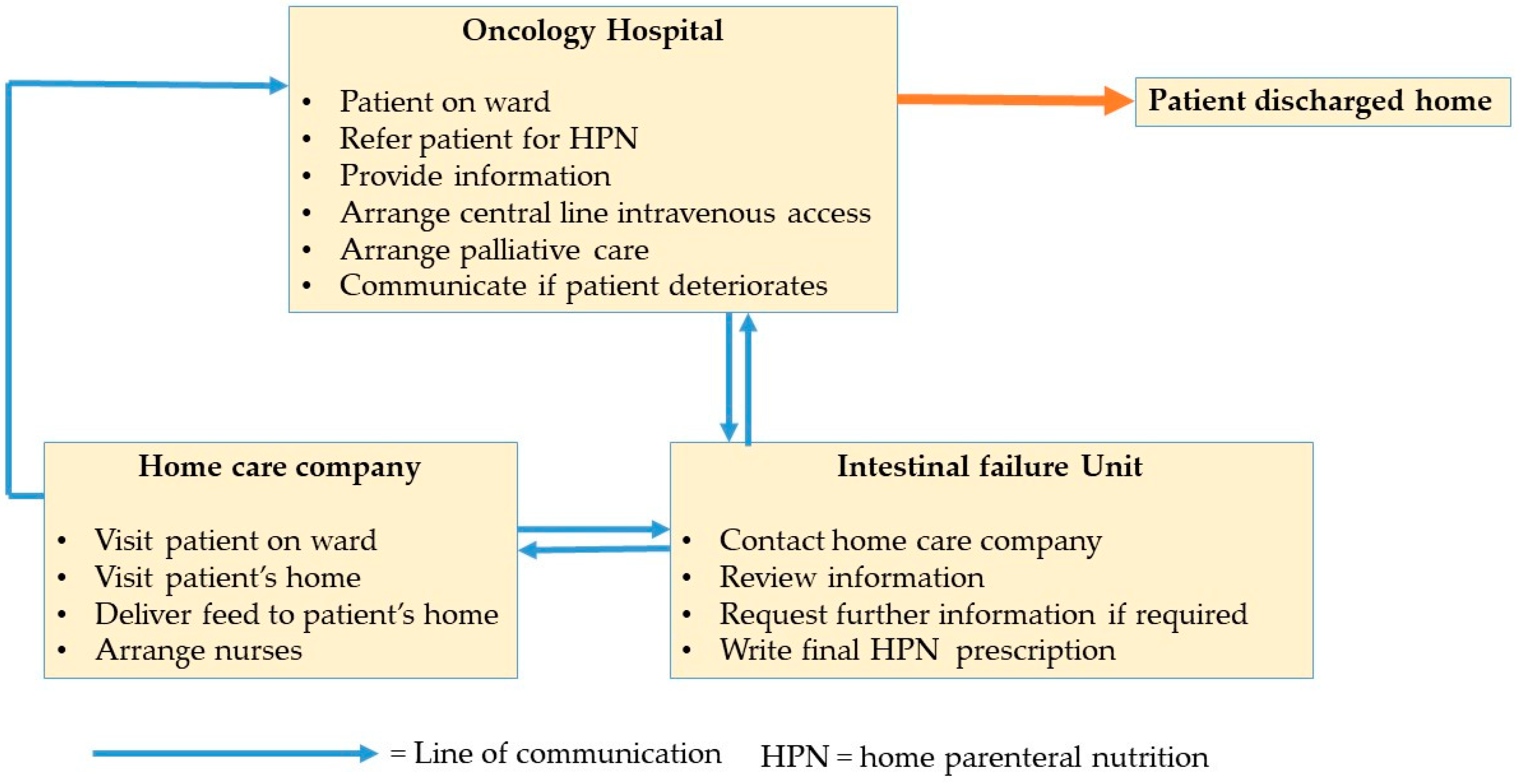
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Context
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Decision to Start Home Parenteral Nutrition
3.2. Barriers to Timely Discharge
3.3. Facilitators
3.4. Patient Perceptions of the Process
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Culine, S.; Chambrier, C.; Tadmouri, A.; Senesse, P.; Seys, P.; Radji, A.; Rotarski, M.; Balian, A.; Dufour, P. Home parenteral nutrition improves quality of life and nutritional status in patients with cancer: A French observational multicentre study. Support Care Cancer 2014, 22, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Sowerbutts, A.M.; Lal, S.; Sremanakova, J.; Clamp, A.; Todd, C.; Jayson, G.C.; Teubner, A.; Raftery, A.M.; Sutton, E.J.; Hardy, L.; et al. Home parenteral nutrition for people with inoperable malignant bowel obstruction. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Naghibi, M. Artificial nutrition support in the UK 2005–2015. In Adult Home Parenteral Nutrition & Home Intravenous Fluids; On Behalf of the BANS Committee; BANS Report 2016; BANS Committee: UK, 2017. [Google Scholar]
- Bozzetti, F.; Arends, J.; Lundholm, K.; Micklewright, A.; Zurcher, G.; Muscaritoli, M. ESPEN Guidelines on Parenteral Nutrition: Non-surgical oncology. Clin. Nutr. 2009, 28, 445–454. [Google Scholar] [CrossRef]
- Chermesh, I.; Mashiach, T.; Amit, A.; Haim, N.; Papier, I.; Efergan, R.; Lachter, J.; Eliakim, R. Home parenteral nutrition (HTPN) for incurable patients with cancer with gastrointestinal obstruction: Do the benefits outweigh the risks? Med. Oncol. 2011, 28, 83–88. [Google Scholar] [CrossRef]
- Orrevall, Y.; Tishelman, C.; Herrington, M.K.; Permert, J. The path from oral nutrition to home parenteral nutrition: A qualitative interview study of the experiences of advanced cancer patients and their families. Clin. Nutr. 2004, 23, 1280–1287. [Google Scholar] [CrossRef]
- Venkatasalu, M.R.; Clarke, A.; Atkinson, J. Being a conduit’between hospital and home: stakeholders views and perceptions of a nurse-led Palliative Care Discharge Facilitator Service in an acute hospital setting. J. Clin. Nurs. 2015, 24, 1676–1685. [Google Scholar] [CrossRef]
- Sowerbutts, A.M.; Lal, S.; Clamp, A.; Jayson, G.C.; Todd, C.; Raftery, A.-M.; Hardy, L.; Sutton, E.; Sremanakova, J.; Burden, S. 42 Bittersweet life in the face of loss: The experience of parenteral nutrition for women with ovarian cancer and their relatives. BMJ Support Palliat. Care 2018, 8, 375–376. [Google Scholar] [CrossRef]
- Sowerbutts, A.M.; Lal, S.; Sremanakova, J.; Clamp, A.; Jayson, G.C.; Teubner, A.; Hardy, L.; Todd, C.; Raftery, A.-M.; Sutton, E.; et al. Palliative home parenteral nutrition in patients with ovarian cancer and malignant bowel obstruction: experiences of women and family caregivers. BMC Palliat. Care 2019, 18, 120. [Google Scholar] [CrossRef]
- Sowerbutts, A.; Lal, S.; Sremanakova, J.; Clamp, A.; Jayson, G.; Teubner, A.; Hardy, L.; Todd, C.; Raftery, A.-M.; Sutton, E. Living life in the face of loss: Parenteral nutrition in ovarian cancer patients in bowel obstruction. Clin. Nutr. 2018, 37, S37. [Google Scholar] [CrossRef]
- Bond, A.; Teubner, A.; Taylor, M.; Gillespie, L.; Farrer, K.; Abraham, A.; Wilbraham, L.; Clamp, A.; Jayson, G.; Lal, S. A remote discharge pathway for patients requiring palliative home parenteral nutrition. Clin. Nutr. 2018, 37, S75. [Google Scholar] [CrossRef]
- Van Manen, M. Researching Lived Experiences; State University of New York Press: Albany, NY, USA, 1990. [Google Scholar]
- Birt, L.; Scott, S.; Cavers, D.; Campbell, C.; Walter, F. Member checking: A tool to enhance trustworthiness or merely a nod to validation? Qual. Health Res. 2016, 26, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.; Gafni, A.; Whelan, T. Decision-making in the physician–patient encounter: Revisiting the shared treatment decision-making model. Soc. Sci. Med. 1999, 49, 651–661. [Google Scholar] [CrossRef]
- Pollard, S.; Bansback, N.; Bryan, S. Physician attitudes toward shared decision making: A systematic review. Patient Educ. Couns. 2015, 98, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Ciambrone, D. Treatment decision-making among older women with breast cancer. J. Women Aging 2006, 18, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Levinson, W.; Kao, A.; Kuby, A.; Thisted, R.A. Not all patients want to participate in decision making: A national study of public preferences. J. Gen. Intern. Med. 2005, 20, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Ziebland, S.; Chapple, A.; Evans, J. Barriers to shared decisions in the most serious of cancers: A qualitative study of patients with pancreatic cancer treated in the UK. Health Expect. 2015, 18, 3302–3312. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.; Whelan, T.; Gafni, A.; Reyno, L.; Redko, C. Doing nothing is no choice: Lay constructions of treatment decision-making among women with early-stage breast cancer. Sociol. Health Illn. 1998, 20, 71–95. [Google Scholar] [CrossRef]
- Finucane, T.E. How gravely ill becomes dying: A key to end-of-life care. JAMA 1999, 282, 1670–1672. [Google Scholar] [CrossRef]
- Nancarrow, S.A.; Booth, A.; Ariss, S.; Smith, T.; Enderby, P.; Roots, A. Ten principles of good interdisciplinary team work. Hum. Resour. Health 2013, 11, 19. [Google Scholar] [CrossRef]
- Lewis, P.J.; Tully, M.P. Uncomfortable prescribing decisions in hospitals: The impact of teamwork. J. R. Soc. Med. 2009, 102, 481–488. [Google Scholar] [CrossRef]
- Ho, A.; Jameson, K.; Pavlish, C. An exploratory study of interprofessional collaboration in end-of-life decision-making beyond palliative care settings. J. Interprof. Care 2016, 30, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.M.; Devoe, M.; Kansagara, D.; Nicolaidis, C.; Englander, H. Did I do as best as the system would let me? Healthcare professional views on hospital to home care transitions. J. Gen. Intern. Med. 2012, 27, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Reeves, S.; Lewin, S. Interprofessional collaboration in the hospital: Strategies and meanings. J. Health Serv. Res. Policy 2004, 9, 218–225. [Google Scholar] [CrossRef] [PubMed]
- O’Daniel, M.; Rosenstein, A.H. Professional communication and team collaboration. In Patient Safety and Quality: An Evidence-Based Handbook for Nurses; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2008. [Google Scholar]
- NICE. Transition between inpatient hospital settings and community or care home settings for adults with social care needs. In National Institute for Clinical Excellence [online]; NICE: London, UK, 2015. [Google Scholar]
- Gonçalves-Bradley, D.C.; Lannin, N.A.; Clemson, L.M.; Cameron, I.D.; Shepperd, S. Discharge planning from hospital. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
| Relatives | Numbers |
| Husband | 8 |
| Daughter | 4 |
| Son | 1 |
| Total | 13 |
| Healthcare Professionals | Numbers |
| Oncologist | 4 |
| Gastroenterologist | 1 |
| Homecare Nurse | 5 |
| Dietitians | 9 |
| Dietetic Manager | 1 |
| Doctors (junior and senior) | 2 |
| Supportive and Palliative care nurses | 2 |
| Complex discharge nurse | 1 |
| Advanced nurse practitioner | 2 |
| Nutrition support nurse | 1 |
| Homecare nursing managers | 3 |
| Intestinal failure unit manager | 1 |
| Total | 32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowerbutts, A.M.; Lal, S.; Sremanakova, J.; Clamp, A.R.; Jayson, G.C.; Teubner, A.; Hardy, L.; Todd, C.; Raftery, A.-M.; Sutton, E.; et al. Discharging Women with Advanced Ovarian Cancer on Home Parenteral Nutrition: Making and Implementing the Decision. Nutrients 2020, 12, 166. https://doi.org/10.3390/nu12010166
Sowerbutts AM, Lal S, Sremanakova J, Clamp AR, Jayson GC, Teubner A, Hardy L, Todd C, Raftery A-M, Sutton E, et al. Discharging Women with Advanced Ovarian Cancer on Home Parenteral Nutrition: Making and Implementing the Decision. Nutrients. 2020; 12(1):166. https://doi.org/10.3390/nu12010166
Chicago/Turabian StyleSowerbutts, Anne Marie, Simon Lal, Jana Sremanakova, Andrew R. Clamp, Gordon C. Jayson, Antje Teubner, Lisa Hardy, Chris Todd, Anne-Marie Raftery, Eileen Sutton, and et al. 2020. "Discharging Women with Advanced Ovarian Cancer on Home Parenteral Nutrition: Making and Implementing the Decision" Nutrients 12, no. 1: 166. https://doi.org/10.3390/nu12010166
APA StyleSowerbutts, A. M., Lal, S., Sremanakova, J., Clamp, A. R., Jayson, G. C., Teubner, A., Hardy, L., Todd, C., Raftery, A.-M., Sutton, E., & Burden, S. (2020). Discharging Women with Advanced Ovarian Cancer on Home Parenteral Nutrition: Making and Implementing the Decision. Nutrients, 12(1), 166. https://doi.org/10.3390/nu12010166







