Glycemic Index and Insulinemic Index of Foods: An Interlaboratory Study Using the ISO 2010 Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
2.2. Data Analysis
3. Results
3.1. Study Population
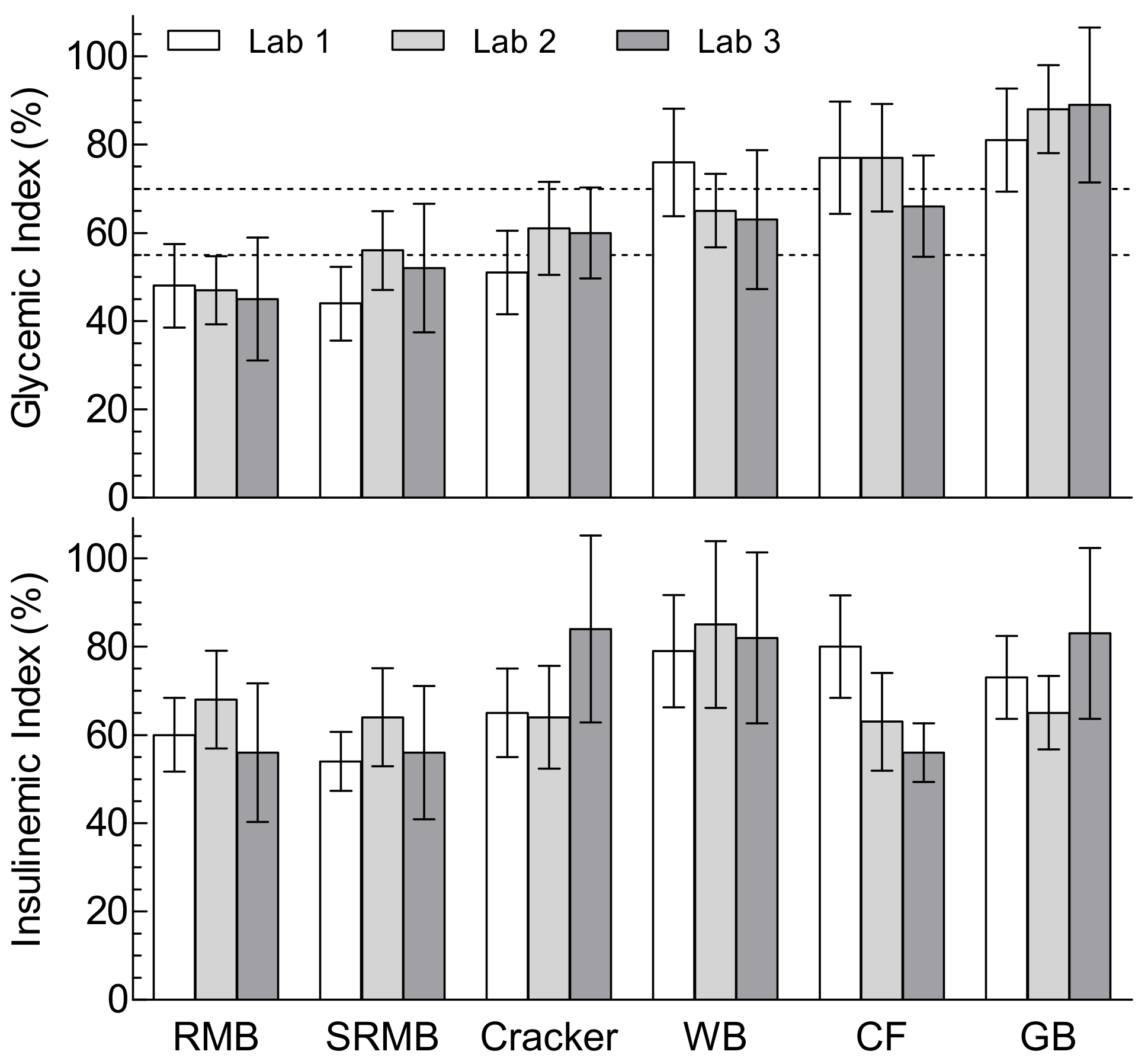
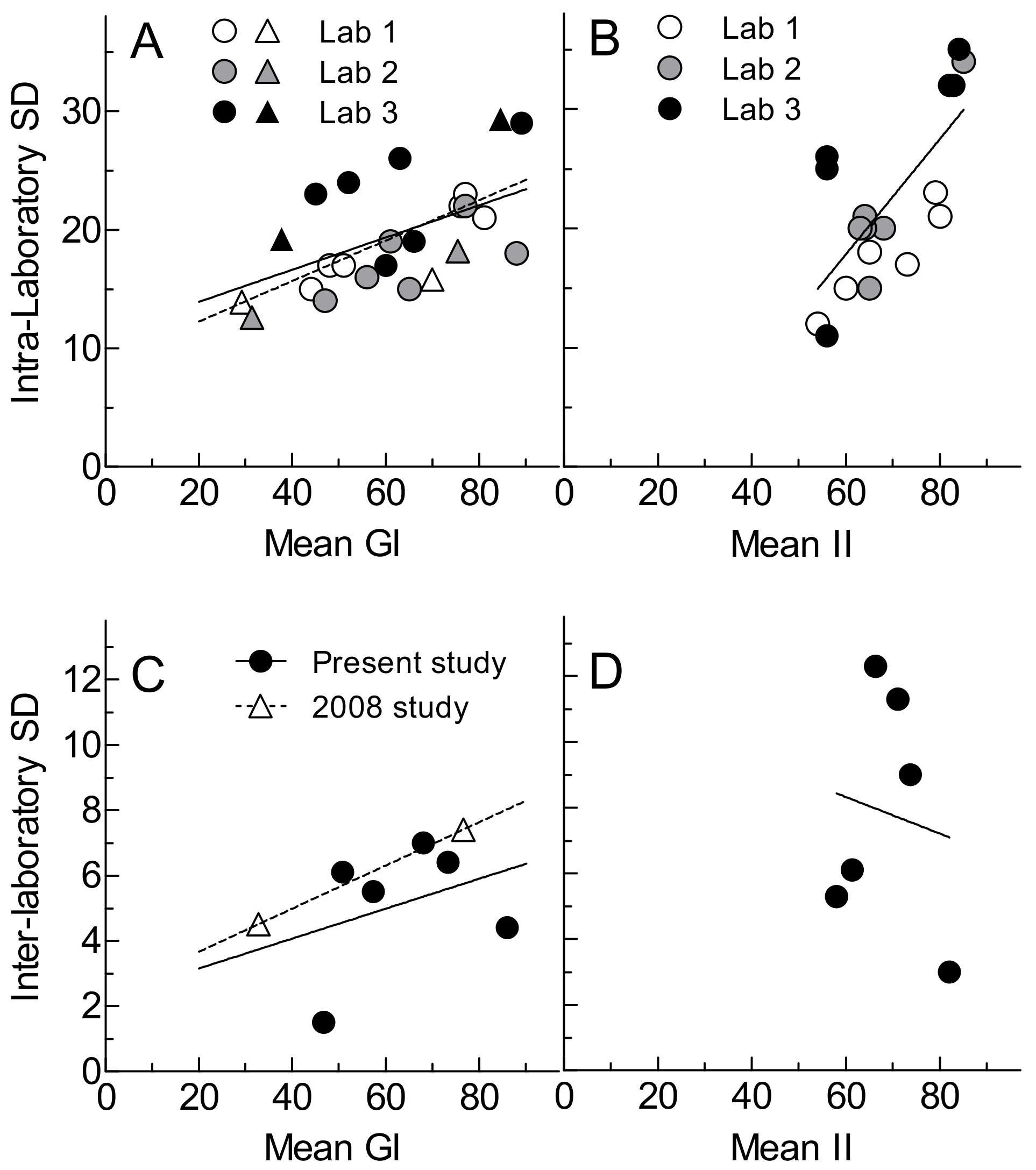
3.2. Glycemic Index and Glycemic Response Parameters
3.3. Insulinemic Index and Insulin Response Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An international scientific consensus summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary glycemic index and load and the risk of type 2 diabetes: A systematic review and updated meta-analysis of prospective cohort studies. Nutrients 2019, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary glycemic index and load and risk of type 2 diabetes: Assessment of causal relations. Nutrients 2019, 11, 1436. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.M.; Wolever, T.M.S. Perceived barriers to application of glycemic index: Valid concerns or lost in translation? Nutrients 2011, 3, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Report. Carbohydrates in Human Nutrition; FAO Food and Nutrition; Paper 66; FAO: Rome, Italy, 1988. [Google Scholar]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Organization for Standardization. ISO 26642:2010: Food Products—Determination of the Glycaemic Index (GI) and Recommendation for Food Classification; ISO: Geneva, Switzerland, 2010. [Google Scholar]
- Wolever, T.M.S.; Vorster, H.H.; Björk, I.; Brand-Miller, J.; Brighenti, F.; Mann, J.I.; Ramdath, D.D.; Granfeldt, Y.; Holt, S.; Perry, T.L.; et al. Determination of the glycaemic index of foods: Interlaboratory study. Eur. J. Clin. Nutr. 2003, 57, 475–482. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Brand-Miller, J.C.; Abernethy, J.; Astrup, A.; Atkinson, F.; Axelsen, M.; Björck, I.; Brighenti, F.; Brown, R.; Brynes, A.; et al. Measuring the glycemic index of foods: Interlaboratory study. Am. J. Clin. Nutr. 2008, 87, S247–S257. [Google Scholar] [CrossRef]
- Després, J.-P.; Lemieux, I.; Prud’homme, D. Treatment of obesity: Need to focus on high risk abdominally obese patients. Br. Med. J. 2001, 322, 716–720. [Google Scholar] [CrossRef]
- European Food Safety Authority. Guidance on the scientific requirements for health claims related to appetite ratings, weight management, and blood glucose concentrations. EFSA J. 2012, 10, 2604. [Google Scholar]
- Health Canada. Draft Guidance Document on Food Health Claims Related to the Reduction in Post-Prandial Glycaemic Response. June 2013. Available online: http://www.hc-sc.gc.ca/fn-an/consult/glyc-postprandial/index-eng.php (accessed on 1 October 2013).
- Marcovina, S.; Bowsher, R.R.; Miller, W.G.; Staten, M.; Myers, G.; Caudill, S.P.; Campbell, S.E.; Steffes, M.W.; Insulin Standardization Workgroup. Standardization of insulin immunoassays: Report of the American Diabetes Association Workgroup. Clin. Chem. 2007, 53, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Manley, S.E.; Stratton, I.M.; Clark, P.M.; Luzio, S.D. Comparison of 11 human insulin assays: Implications for clinical investigation and research. Clin. Chem. 2007, 53, 922–932. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011, 34 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Kingman, S.M.; Hudson, G.J.; Cummings, J.H. Measurement of resistant starch in vitro and in vivo. Br. J. Nutr. 1996, 75, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Matthan, N.R.; Ausman, L.M.; Meng, H.; Tighiouart, H.; Lichtenstein, A.H. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. Am. J. Clin. Nutr. 2016, 104, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.S.; Jenkins, A.L.; Vuksan, V.; Campbell, J. The glycaemic index values of foods containing fructose are affected by metabolic differences between subjects. Eur. J. Clin. Nutr. 2009, 63, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Wolever TMs Giddens, J.L.; Sievenpiper, J.L. Effect of ethnicity on glycaemic index: A systematic review and meta-analysis. Nutr. Diabetes 2015, 5, e170. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values, 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Lan-Pidhainy, X.; Wolever, T.M. Are the glycemic and insulinemic index values of carbohydrate foods similar in healthy control, hyperinsulinemic and type 2 diabetic patients? Eur. J. Clin. Nutr. 2011, 65, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Holt, S.H.A.; Brand Miller, J.C.; Petocz, P. Interrelationships among postprandial satiety, glucose and insulin responses and changes in subsequent food intake. Eur. J. Clin. Nutr. 1996, 50, 788–797. [Google Scholar]
- Brand Miller, J.; Pang, E.; Broomhead, L. The glycaemic index of foods containing sugars: Comparison of foods with naturally-occurring v. added sugars. Br. J. Nutr. 1995, 73, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; de Jong, V.; Atkinson, F.; Petocz, P.; Brand-Miller, J.C. Food insulin index: Physiologic basis for predicting insulin demand evoked by composite meals. Am. J. Clin. Nutr. 2011, 93, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.J.; Bao, J.; Petocz, P.; Colagiuri, S.; Brand-Miller, J.C. Validation of the food insulin index in lean, young, healthy individuals, and type 2 diabetes in the context of mixed meals: An acute randomized crossover trial. Am. J. Clin. Nutr. 2015, 102, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.G.; Thienpont, L.M.; Van Uytfanghe, K.; Clark, P.M.; Lindstedt, P.; Nilsson, G.; Steffes, M.W. Insulin Standardization Work Group. Toward standardization of insulin immunoassays. Clin. Chem. 2009, 55, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Clausen, J.O.; Borch-Johnsen, K.; Ibsen, H.; Bergman, R.N.; Hougaard, P.; Winther, K.; Pedersen, O. Insulin sensitivity index, acute insulin response, and glucose effectiveness in a population-based sample of 380 young healthy Caucasians: Analysis of the impact of gender, body fat, physical fitness and life-style factors. J. Clin. Investig. 1996, 98, 1195–1209. [Google Scholar] [CrossRef]
- Lan-Pidhainy, X.; Wolever, T.M.S. The hypoglycemic effect of fat and protein is not attenuated by insulin resistance. Am. J. Clin. Nutr. 2010, 91, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Meynier, A.; Goux, A.; Atkinson, F.; Brack, O.; Vinoy, S. Postprandial glycaemic response: How is it influenced by characteristics of cereal products? Br. J. Nutr. 2015, 113, 1931–1939. [Google Scholar] [CrossRef]
- Wolever, T.M.S. Is glycaemic index (GI) a valid measure of carbohydrate quality? Eur. J. Clin. Nutr. 2013, 67, 522–531. [Google Scholar] [CrossRef]
| Rotary-Molded Biscuit | Sandwiched Rotary-Molded Biscuit | Cracker | White Bread | Corn Flakes | Ginger-Bread | |
|---|---|---|---|---|---|---|
| Portion weight | 67.4 | 63.8 | 75.9 | 94.8 | 53.5 | 68.2 |
| Moisture | 1.6 | 1.2 | 1.0 | 29.3 | 1.9 | 11.9 |
| Protein | 4.7 | 4.4 | 5.7 | 9.0 | 4.0 | 2.0 |
| Fat | 11.5 | 11.1 | 17.1 | 4.4 | 0.4 | 1.2 |
| Total sugars | 21.2 | 20.5 | 5.8 | 7.0 | 5.0 | 30.2 |
| Monosaccharides | 3.0 | 2.5 | 0.7 | 1.9 | 1.6 | 25.4 |
| Disaccharides | 18.2 | 18.1 | 5.1 | 5.1 | 3.4 | 4.8 |
| Available starch | 25.4 | 26.0 | 40.0 | 38.9 | 40.8 | 17.7 |
| Slowly digestible starch | 11.0 | 8.2 | 4.5 | 0.3 | 1.6 | 0.1 |
| Available carbohydrate 1 | 50.0 | 50.0 | 50.0 | 50.1 | 50.0 | 50.0 |
| Dietary fiber | 3.3 | 2.0 | 1.6 | 1.9 | 1.2 | 1.7 |
| Lab 1 (n = 15) | Lab 2 (n = 16) | Lab 3 (n = 16) | |
|---|---|---|---|
| Males/Females (n) | 8/7 | 7/9 | 3/13 |
| Age (y) | 24.5 ± 4.3 | 24.9 ± 4.9 | 27.6 ± 3.9 |
| BMI (kg/m²) | 22.6 ± 1.7 | 23.0 ± 1.8 | 22.0 ± 1.9 |
| HOMA-IR | 0.90 ± 0.16 | 0.87 ± 0.32 | 0.94 ± 0.30 |
| Fasting glucose (mmol/L) | 4.57 ± 0.35 a | 4.08 ± 0.35 b | 4.67 ± 0.31 a |
| Reference CV (glucose) 1 | 14.5 ± 6.0 b | 23.0 ± 11.0 a,b | 28.0 ± 14.2 a |
| Intra-individual CV (glucose) 2 | 18.0 | 24.7 | 29.7 |
| Inter-individual CV (glucose) 2 | 28.1 | 19.0 | 23.9 |
| Fasting insulin (mIU/L) | 4.40 ± 0.63 | 4.81 ± 1.74 | 4.54 ± 1.43 |
| Reference CV (insulin) 1 | 17.1 ± 9.9 | 26.3 ± 14.4 | 24.5 ± 14.3 |
| Intra-individual CV (insulin) 2 | 22.2 | 43.8 | 27.0 |
| Inter-individual CV (insulin) 2 | 40.8 | 57.8 | 40.5 |
| Glycemic Index (%) | |||||
| Food | Lab 1 (n = 15) | Lab 2 (n = 15) | Lab 3 (n = 13) | Overall (n = 43) | SD (CV) of Lab Means |
| RM Biscuit | 48 ± 17 | 47 ± 14 | 45 ± 23 1 | 47 ± 18 1,e | 1.6 (3.3) |
| SRM Biscuit | 44 ± 15 1 | 56 ± 16 2 | 52 ± 24 1 | 50 ± 19 4,d,e | 5.8 (11.4) |
| Cracker | 51 ± 17 1 | 61 ± 19 1 | 60 ± 17 1 | 57 ± 18 3,c,d | 5.5 (9.5) |
| White bread | 76 ± 22 | 65 ± 15 1 | 63 ± 26 1 | 68 ± 22 2,b,c | 6.7 (9.8) |
| Corn flakes | 77 ± 23 | 77 ± 22 1 | 66 ± 19 1 | 74 ± 21 2,a,b | 6.4 (8.7) |
| Ginger-bread | 81 ± 21 | 88 ± 18 | 89 ± 29 | 86 ± 23 a | 4.8 (5.6) |
| Mean of Means | 63 ± 17 | 66 ± 15 | 63 ± 15 | 0.202 * | 5.1 ± 1.9 |
| Mean of SDs | [19 ± 3] | [17 ± 3] | [23 ± 4] | (8.1 ± 3.0) | |
| Insulinemic Index (%) | |||||
| Food | Lab 1 (n = 15) | Lab 2 (n = 15) | Lab 3 (n = 13) | Overall (n = 43) | SD (CV) of Lab Means |
| RM Biscuit | 60 ± 15 | 68 ± 20 | 56 ± 26 1 | 62 ± 20 1 | 5.8 (9.4) |
| SRM Biscuit | 54 ± 12 1 | 64 ± 20 1 | 56 ± 25 1 | 58 ± 19 3 | 5.5 (9.6) |
| Cracker | 65 ± 18 1 | 64 ± 21 1 | 84 ± 35 1 | 71 ± 26 3 | 11.0 (15.5) |
| White bread | 79 ± 23 | 85 ± 34 | 82 ± 32 | 82 ± 29 | 3.3 (4.0) |
| Corn flakes | 80 ± 21 a | 63 ± 20 1,a,b | 56 ± 11 1,b | 67 ± 21 2 | 12.5 (18.7) |
| Ginger-bread | 73 ± 17 | 65 ± 15 1 | 83 ± 32 | 73 ± 23 1 | 9.0 (12.2) |
| Mean of Means | 69 ± 11 | 68 ± 8 | 70 ± 15 | 0.014 * | 7.8 ± 3.5 |
| Mean of SDs | [18 ± 4] | [22 ± 6] | [27 ± 9] | (11.6 ± 5.2) | |
| Glucose iAUC (mmol × min/L) | |||||
| Food | Lab 1 (n = 15) | Lab 2 (n = 15) | Lab 3 (n = 13) | Overall (n = 43) | SD (CV) of Lab Means |
| RM Biscuit | 102 ± 53 | 105 ± 44 | 127 ± 63 | 111 ± 8 e | 13.5 (12.1) |
| SRM Biscuit | 103 ± 80 | 130 ± 37 | 148 ± 67 | 126 ± 10 d,e | 22.1 (17.4) |
| Cracker | 119 ± 73 | 141 ± 42 | 170 ± 62 | 141 ± 10 d,f | 24.5 (17.1) |
| White bread | 155 ± 63 | 150 ± 43 | 182 ± 72 | 159 ± 9 c,f | 12.2 (7.6) |
| Corn flakes | 160 ± 63 | 174 ± 50 | 188 ± 84 | 172 ± 10 b,c | 12.9 (7.5) |
| Ginger-bread | 164 ± 55 | 198 ± 59 | 230 ± 70 | 195 ± 10 b | 31.6 (16.1) |
| Glucose | 207 ± 62 | 218 ± 59 | 268 ± 75 | 230 ± 10 a | 27.7 (12.0) |
| Mean of means | 144 ± 38 b | 159 ± 40 a,b | 187 ± 48 a | 0.5829 * | 20.6 ± 7.8 |
| Mean of SDs | [64 ± 10 a] | [48 ± 9 b] | [70 ± 8 a] | (12.8 ± 4.2) | |
| Insulin iAUC (nmol × min/L) | |||||
| Food | Lab 1 (n = 15) | Lab 2 (n = 15) | Lab 3 (n = 13) | Overall (n = 43) | SD (CV) of Lab Means |
| RM Biscuit | 6.04 ± 3.51 | 10.55 ± 4.75 | 9.58 ± 8.52 | 8.68 ± 6.03 b,c | 2.37 (27.2) |
| SRM Biscuit | 5.92 ± 4.15 | 11.03 ± 6.49 | 8.74 ± 4.57 | 8.45 ± 5.51 c | 2.56 (29.9) |
| Cracker | 6.91 ± 4.69 | 12.01 ± 9.75 | 12.98 ± 7.78 | 10.51 ± 8.06 b,c,d | 3.26 (30.7) |
| White bread | 7.45 ± 3.38 | 14.18 ± 8.43 | 11.45 ± 3.94 | 10.95 ± 6.37 d | 3.39 (30.7) |
| Corn flakes | 7.52 ± 2.88 | 11.36 ± 6.77 | 8.77 ± 3.18 | 9.18 ± 4.88 b,c,d | 1.96 (21.2) |
| Ginger-bread | 6.84 ± 2.71 | 11.65 ± 6.11 | 12.34 ± 7.08 | 10.26 ± 6.06 b,d | 3.00 (29.1) |
| Glucose | 9.64 ± 3.93 | 16.54 ± 9.53 | 14.84 ± 5.38 | 13.83 ± 7.51 a | 3.59 (26.3) |
| Mean of means | 7.19 ± 1.25 b | 12.47 ± 2.14 a | 11.24 ± 2.32 a | 0.5125 * | 2.93 ± 0.65 |
| Mean of SDs | [3.61 ± 0.70 b] | [7.41 ± 1.88 a] | [6.00 ± 2.05 a] | (28.3 ± 3.22) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolever, T.M.S.; Meynier, A.; Jenkins, A.L.; Brand-Miller, J.C.; Atkinson, F.S.; Gendre, D.; Leuillet, S.; Cazaubiel, M.; Housez, B.; Vinoy, S. Glycemic Index and Insulinemic Index of Foods: An Interlaboratory Study Using the ISO 2010 Method. Nutrients 2019, 11, 2218. https://doi.org/10.3390/nu11092218
Wolever TMS, Meynier A, Jenkins AL, Brand-Miller JC, Atkinson FS, Gendre D, Leuillet S, Cazaubiel M, Housez B, Vinoy S. Glycemic Index and Insulinemic Index of Foods: An Interlaboratory Study Using the ISO 2010 Method. Nutrients. 2019; 11(9):2218. https://doi.org/10.3390/nu11092218
Chicago/Turabian StyleWolever, Thomas M.S., Alexandra Meynier, Alexandra L. Jenkins, Jennie C. Brand-Miller, Fiona S. Atkinson, David Gendre, Sébastien Leuillet, Murielle Cazaubiel, Béatrice Housez, and Sophie Vinoy. 2019. "Glycemic Index and Insulinemic Index of Foods: An Interlaboratory Study Using the ISO 2010 Method" Nutrients 11, no. 9: 2218. https://doi.org/10.3390/nu11092218
APA StyleWolever, T. M. S., Meynier, A., Jenkins, A. L., Brand-Miller, J. C., Atkinson, F. S., Gendre, D., Leuillet, S., Cazaubiel, M., Housez, B., & Vinoy, S. (2019). Glycemic Index and Insulinemic Index of Foods: An Interlaboratory Study Using the ISO 2010 Method. Nutrients, 11(9), 2218. https://doi.org/10.3390/nu11092218







