The Role of Water Homeostasis in Muscle Function and Frailty: A Review
Abstract
1. Introduction
2. Water Structure, Properties and Biological Functions
- (1)
- A metabolic function. Water is the medium in which all biochemical metabolism reactions occur. Water acts as a solvent and as a reactive in different metabolic reactions, mediates the recognition of molecules, acts as a communication channel between the inside and outside of proteins and increases the mobility or flexibility of enzymes, facilitating the enzymatic attack necessary for reactions to occur [6,7]. Thus, for example, the fact that each gram of muscle glycogen is stored with 2.7 g of water allows glycogen to be easily attacked by hydrolytic enzymes that quickly release glucose, the fuel for exercising muscles [1,8]. Apart from facilitating the enzymatic function, water also allows nervous transmission of electric current [5].
- (2)
- A transport function. Circulating blood is the transport system that enables substances (nutrients, hormones, oxygen, metabolites, etc) to be exchanged between different organs and systems in body, while blood filtration by the kidneys eliminates the waste products of metabolism through the urine [1,9].
- (3)
- A temperature control function. Water maintains a constant body temperature regardless of the ambient temperature and metabolic activity for several reasons: it has a high capacity to store energy in hydrogen bonds in such a way as to cushion temperature changes, it has high thermal conductivity which ensures rapid distribution and transfer of heat to the skin, and it requires a great deal of energy to be evaporated. By absorbing heat, distributing it among the liquid compartments of the body, and removing it through the skin through the evaporation of sweat, water keeps the body temperature within a very narrow range.
- (4)
- A structural function. Water bound to cytoplasmic proteins determines cell volume, which, in turn, influences physiological mechanisms such as cellular performance and the regulation of cell proliferation or apoptotic cell death [10,11,12]. Water also determines plasma volume and perfusion of tissues.
- (5)
- A mechanical function. Water acts as a lubricant in the mouth (through saliva), eyes (through tears) and joints (through synovial fluid), protects and promotes mucous membrane cleansing, and prevents injuries and fractures by adding flexibility and elasticity to tissues [9].
3. Water in the Body (Body Composition)
4. Water Homeostasis
4.1. Water Inputs and Outputs
4.2. Water Balance Control Mechanisms
5. Osmolarity and Tonicity
6. Transmembrane Water Transport: Aquaporins
7. Dehydration in the Elderly
8. Hyperosmotic Stress in the Elderly
- (1)
- An important inflammatory response. The increased synthesis and secretion of different cytokines (Tumor Necrosis Factor-α, Interleukin-1β, Interleukin-6, Interleukin-8, Interleukin-18) may contribute to the development of chronic inflammatory diseases such as arthritis, inflammatory bowel disease and liver fibrosis [65,66]. Recent evidence also suggests that chronic inflammation may play an important role in carcinogenesis [67] and ageing [68,69].
- (2)
- (3)
- Diabetes, insulin resistance, and metabolic disorders. It has been observed that high levels of fasting AVP is a risk factor for type 2 diabetes and is associated with metabolic syndrome components (obesity, insulin resistance and hypertension). The explanation lies in the effect of AVP on adrenocorticotropic hormone (ACTH) and cortisol release [71]. Some studies have shown that high water intake decreases plasma osmolarity and AVP plasma levels and leads to greater glycaemic control, weight loss and reduced cardiovascular risk [72].
- (4)
- (5)
- Kidney disorders. High AVP levels have been associated with an increased risk of chronic kidney disease in two population-based cohorts in Sweden [72], although mechanisms and causes are unknown. Dehydration also promotes renal lithiasis.
- (6)
- An increased risk of mortality [73].
9. Cell Volume Role in Cell Functions
10. Water’s Role in Metabolic Muscle Function
11. Water’s Role in Mechanical Muscle Function
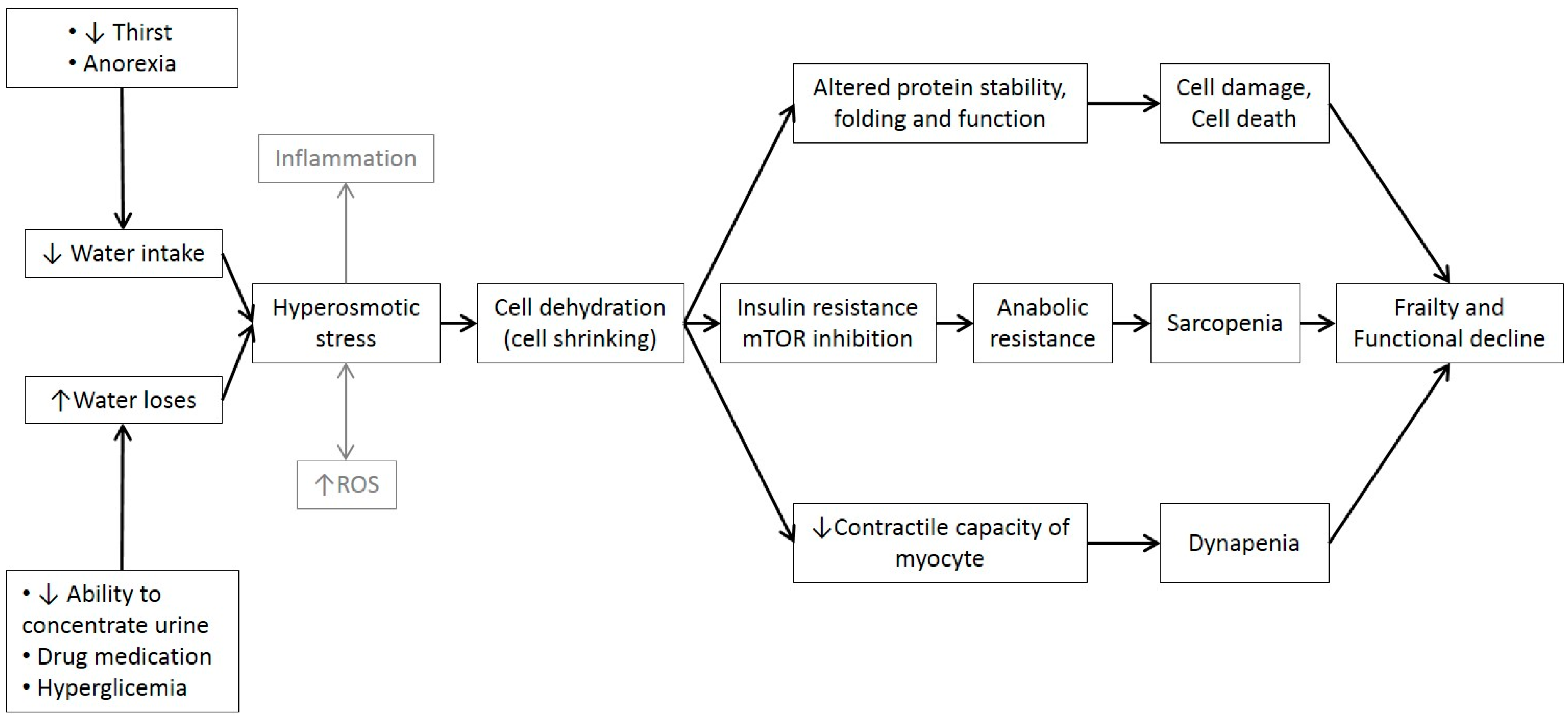
12. Cell Hydration, Functional Capacity and Frailty
13. Final Remarks
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Carbajal, A.; González, M. Propiedades y funciones biológicas del agua. In Agua Para la Salud, Pasado, Presente y Futuro; Vaquero, M.P., Toxqui, L., Eds.; CSIC: Madrid, Spain, 2012; pp. 33–45. ISBN 978-84-00-09572-7. [Google Scholar]
- Sharp, K.A.; Vanderkooi, J.A. Water in the half shell: Structure of water, focusing on angular structure and solvation. Acc. Chem. Res. 2010, 43, 231–239. [Google Scholar] [CrossRef]
- Chaplin, M.F. Water’s Hydrogen Bond Strength. In Water and Life: The Unique Properties of H2O; Lynden-Bell, R.M., Morris, S.C., Barrow, J.D., Finney, J.L., Harper, C., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 69–85. [Google Scholar]
- Ball, P. Water as an active constituent in cell biology. Chem Rev. 2008, 108, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, D.; Underwood, R. Unraveling water’s entropic mysteries: A unified view of nonpolar, polar, and ionic hydration. Acc. Chem. Res. 2008, 41, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Jéquier, E.; Constant, F. Water as an essential nutrient: The physiological basis of hydration. Eur. J. Clin. Nutr. 2010, 64, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Halling, P.J. What can we learn by studying enzymes in non-aqueous media? Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2004, 359, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Elías, V.E.; Ortega, J.F.; Nelson, R.K.; Mora-Rodriguez, R. Relationship between muscle water and glycogen recovery after prolonged exercise in the heat in humans. Eur. J. Appl. Physiol. 2015, 115, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, A.C.; Campbell, S.M. Hydration: Fluids for Life; ILSI North America: Washington, DC, USA, 2004. [Google Scholar]
- Lang, F. Mechanisms and significance of cell volume regulation. J. Am. Coll. Nutr. 2007, 26 (Suppl. S5), 613S–623S. [Google Scholar] [CrossRef] [PubMed]
- Rand, R.P. Probing the role of water in protein conformation and function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Häussinger, D. The role of cellular hydration in the regulation of cell function. Biochem. J. 1996, 313, 697–710. [Google Scholar] [CrossRef]
- European Food Safety Authority. Panel on dietetic products nutrition and allergies (NDA). Scientific opinion on dietary reference values for water. EFSA J. 2010, 8, 1459–1507. [Google Scholar]
- Davidhizar, R.; Dunn, C.L.; Hart, A.N. A review of the literature on how important water is to the world’s elderly population. Int. Nurs. Rev. 2004, 51, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Gautam Bhave, G.; Neilson, E.G. Body fluid dynamics: Back to the future. J. Am. Soc. Nephrol. 2011, 22, 2166–2181. [Google Scholar] [CrossRef] [PubMed]
- Valtin, H.; Schafer, J.A. Renal Function: Mechanisms Preserving Fluid and Solute Balance in Health, 3rd ed.; Little, Brown, and Co.: Boston, MA, USA, 1995. [Google Scholar]
- Mudge, G.; Weiner, I. Agents affecting volume and composition of body fluids. In Goodman and Gilman’s The Pharmacological Basis of Therapeutics; Goodman Gilman, A., Rall, T.W., Nies, A.S., Taylor, P., Eds.; Pergamon Press: Elmsford, NY, USA, 1990; pp. 682–707. [Google Scholar]
- Vivanti, A.P. Origins for the estimations of water requirements in adults. Eur. J. Clin. Nutr. 2012, 66, 1282. [Google Scholar] [CrossRef] [PubMed]
- Kalman, D.; Lepeley, A. A review of hydration. Strength Cond. J. 2010, 32, 56–63. [Google Scholar] [CrossRef]
- Liska, D.; Mah, E.; Brisbois, T.; Barrios, P.L.; Baker, L.B.; Spriet, L.L. Narrative review of hydration and selected health outcomes in the general population. Nutrients 2019, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Thornton, S.N. Thirst and hydration: Physiology and consequences of dysfunction. Physiol. Behav. 2010, 100, 15–21. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Dietary reference values for nutrients: Summary report. EFSA Support. Publ. 2017, 14, e15121E. [Google Scholar] [CrossRef]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Hooper, L.; Bunn, D.; Jimoh, F.O.; Fairweather-Tait, S.J. Water-loss dehydration and aging. Mech. Ageing Dev. 2014, 136, 50–58. [Google Scholar] [CrossRef]
- Fronius, M.; Clauss, W.G.; Althaus, M. Why do we have to move fluid to be able to breathe? Front. Physiol. 2012, 3, 146. [Google Scholar] [CrossRef]
- Kleiner, S.M. Water: An essential but overlooked nutrient. J. Am. Diet. Assoc. 1999, 99, 200–206. [Google Scholar] [CrossRef]
- Kondo, N.; Taylor, N.; Shibasaki, M.; Aoki, K.; Muhamed, A.M. Thermoregulatory adaption in humans and its modifying factors. Glob. Environ. Res. 2009, 13, 35–41. [Google Scholar]
- Barrett, K.E.; Barman, S.M.; Boitano, S.; Brooks, H. Hypothalamic regulation of hormonal functions. In Ganong’s Review of Medical Physiology, 23th ed.; McGraw-Hill Medic: New York, NY, USA, 2010; pp. 273–288. [Google Scholar]
- Treschan, T.A.; Peters, J. The vasopressin system: Physiology and clinical strategies. Anesthesiology 2006, 105, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F.; Mythen, M.; Montgomery, H. The sensitivity of the human thirst response to changes in plasma osmolality: A systematic review. Perioper. Med. 2018, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Berl, T.; Anderson, R.J. Osmotic and nonosmotic control of vasopressin release. Am. J. Physiol. 1979, 236, F321–F332. [Google Scholar] [CrossRef] [PubMed]
- Bichet, D.G. Vasopressin and the regulation of thirst. Ann. Nutr. Metab. 2018, 72, 3–7. [Google Scholar] [CrossRef]
- Leib, D.E.; Zimmerman, C.A.; Knight, Z.A. Thirst. Curr. Biol. 2016, 26, R1260–R1265. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.G. Vasopressin and disorders of water balance: The physiology and pathophysiology of vasopressin. Ann. Clin. Biochem. 2007, 44, 417–431. [Google Scholar] [CrossRef]
- McKinley, M.J.; Johnson, A.K. The physiological regulation of thirst and fluid intake. News Physiol. Sci. 2004, 19, 1–6. [Google Scholar] [CrossRef]
- Silverthorn, D.U. Isosmotic is not always isotonic: The five-minute version. Adv. Physiol. Educ. 2016, 40, 499–500. [Google Scholar] [CrossRef]
- Vujovic, P.; Chirillo, M.; Silverthorn, D.U. Learning (by) osmosis: An approach to teaching osmolarity and tonicity. Adv. Physiol. Educ. 2018, 42, 626–635. [Google Scholar] [CrossRef]
- Pitonzo, D.; Skach, W.R. Molecular mechanisms of aquaporin biogenesis by the endoplasmic reticulum Sec61 translocon. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Day, R.E.; Kitchen, P.; Owen, D.S.; Bland, C.; Marshall, L.; Conner, A.C.; Bill, R.M.; Conner, M.T. Human aquaporins: Regulators of transcellular water flow. Biochim. Biophys. Acta 2014, 1840, 1492–1506. [Google Scholar] [CrossRef] [PubMed]
- Galán-Cobo, A.; Ramírez-Lorca, R.; Echevarría, M. Role of aquaporins in cell proliferation: What else beyond water permeability? Channels 2016, 10, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sater, K.A. Physiology of the Aquaporins. Am. J. Biomed. Sci. 2018, 10, 167–183. [Google Scholar] [CrossRef]
- Osorio, G.; Bernabéua, J.; Echevarría, M.; Conejo-Mir, J. Acuaporinas: Moléculas revelación en cosmética y oncología cutánea. Piel 2009, 24, 193–200. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012, 11, 535–544. [Google Scholar] [CrossRef]
- Tamma, G.; Valenti, G.; Grossini, E.; Donnini, S.; Marino, A.; Marinelli, R.A.; Calamita, G. Aquaporin membrane channels in oxidative stress, cell signalling, and ageing: Recent advances and research trends. Oxid. Med. Cell. Longev. 2018, 2018, 1501847. [Google Scholar] [CrossRef]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef]
- Medraño-Fernandez, I.; Bestetti, S.; Bertolotti, M.; Bienert, G.P.; Bottino, C.; Laforenza, U.; Rubartelli, A.; Sitia, R. Stress regulates aquaporin-8 permeability to impact cell growth and survival. Antioxid. Redox Signal. 2016, 24, 1031–1044. [Google Scholar] [CrossRef]
- Lutoslawska, G. Aquaporins in physiology and pathology. Sport Sci. 2014, 4, 185–194. [Google Scholar]
- Wakayama, Y. Aquaporin expression in normal and pathological skeletal muscles: A brief review with focus on AQP4. J. Biomed. Biotechnol. 2010, 2010, 731569. [Google Scholar] [CrossRef] [PubMed]
- Oplatka, A. The role of water in the mechanism of muscular contraction. FEBS Lett. 1994, 355, 1–3. [Google Scholar] [CrossRef]
- Yoo, H.; Nagornyak, E.; Das, R.; Wexler, A.D.; Pollack, H.G. Contraction-induced changes in hydrogen bonding of muscle hydration water. J. Phys. Chem. Lett. 2014, 5, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J. Hydration, morbidity, and mortality in vulnerable populations. Nutr. Rev. 2012, 70 (Suppl. S2), S152–S155. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, A.M.; Watson, P.; Neal, K.R.; Ljungqvist, O.; Maughan, R.J.; Sahota, O.; Lobo, D.N. Hydration and outcome in older patients admitted to hospital (The HOOP prospective cohort study). Age Ageing 2015, 44, 943–947. [Google Scholar] [CrossRef]
- Wotton, K.; Crannitch, K.; Munt, R. Prevalence, risk factors and strategies to prevent dehydration in older adults. Contemp. Nurse 2008, 31, 44–56. [Google Scholar] [CrossRef]
- Mentes, J. Oral hydration in older adults: Greater awareness is needed in preventing, recognizing, and treating dehydration. Am. J. Nurs. 2006, 106, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L.E.; Hodak, S.P.; Verbalis, J.G. Age-associated abnormalities of water homeostasis. Endocrinol. Metab. Clin. North. Am. 2013, 42, 349–370. [Google Scholar] [CrossRef]
- Picetti, D.; Foster, S.; Pangle, A.K.; Schrader, A.; George, M.; Wei, J.Y.; Azhar, G. Hydration health literacy in the elderly. Nutr. Healthy Aging 2017, 4, 227–237. [Google Scholar] [CrossRef]
- Stookey, J.D.; Pieper, C.F.; Cohen, H.J. Is the prevalence of dehydration among community-dwelling older adults really low? Informing current debate over the fluid recommendation for adults aged 70 + years. Public Health Nutr. 2005, 8, 1275–1285. [Google Scholar] [CrossRef]
- Rolls, B.J.; Phillips, P.A.M.B. Aging and disturbances of thirst and fluid balance. Nutr. Rev. 1990, 48, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Tamma, G.; Goswami, N.; Reichmuth, J.; De Santo, N.G.; Valenti, G. Aquaporins, vasopressin, and aging: Current perspectives. Endocrinology 2015, 156, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Stöllberger, C.; Lutz, W.; Finsterer, J. Heat-related side-effects of neurological and non-neurological medication may increase heatwave fatalities. Eur. J. Neurol. 2009, 16, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Fouillet, A.; Rey, G.; Laurent, F.; Pavillon, G.; Bellec, S.; Guihenneuc-Jouyaux, C.; Clavel, J.; Jougla, E.; Hémon, D. Excess mortality related to the August 2003 heat wave in France. Int. Arch. Occup. Environ. Health 2006, 80, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Burg, M.B.; Ferraris, J.D.; Dmitrieva, N.I. Cellular response to hyperosmotic stresses. Physiol. Rev. 2007, 87, 1441–1474. [Google Scholar] [CrossRef]
- Brocker, C.; Thompson, D.C.; Vasiliou, V. The role of hyperosmotic stress in inflammation and disease. Biomol. Concepts 2012, 3, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Küper, C.; Beck, F.X.; Neuhofer, W. Osmoadaptation of mammalian cells—An orchestrated network of protective genes. Curr. Genomics 2007, 8, 209–218. [Google Scholar] [CrossRef]
- Neuhofer, W. Role of NFAT5 in inflammatory disorders associated with Osmotic stress. Curr. Genom. 2010, 11, 584–590. [Google Scholar] [CrossRef][Green Version]
- Schwartz, L.; Guais, A.; Pooya, M.; Abolhassani, M. Is inflammation a consequence of extracellular hyperosmolarity? J. Inflamm. 2009, 6, 21. [Google Scholar] [CrossRef]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From inflammation to cancer. Ir. J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Source of Chronic Inflammation in Aging. Front. Cardiovasc. Med. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An update on inflamm-aging: Mechanisms, prevention, and treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef] [PubMed]
- Eisner, V.; Criollo, A.; Quiroga, C.; Olea-Azar, C.; Santibañez, J.F.; Troncoso, R.; Chiong, M.; Diaz-Araya, G.; Foncea, R.; Lavandero, S. Hyperosmotic stress-dependent NFkappaB activation is regulated by reactive oxygen species and IGF-1 in cultured cardiomyocytes. FEBS Lett. 2006, 580, 4495–4500. [Google Scholar] [CrossRef] [PubMed]
- Melander, O. Vasopressin, from regulator to disease predictor for diabetes and cardiometabolic risk. Ann. Nutr. Metab. 2016, 68 (Suppl. S2), 24–28. [Google Scholar] [CrossRef] [PubMed]
- Enhörning, S.; Melander, O. The vasopressin system in the risk of diabetes and cardiorenal disease, and hydration as a potential lifestyle intervention. Ann. Nutr. Metab. 2018, 72 (Suppl. S2), 21–27. [Google Scholar] [CrossRef] [PubMed]
- Tatlisu, M.A.; Kaya, A.; Keskin, M.; Uzman, O.; Borklu, E.B.; Cinier, G.; Hayiroglu, M.I.; Tatlisu, K.; Eren, M. Can we use plasma hyperosmolality as a predictor of mortality for ST-segment elevation myocardial infarction? Coron. Artery Dis. 2017, 28, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Pegoraro, A.F.; Mao, A.; Zhou, E.H.; Arany, P.R.; Han, Y.; Burnette, D.T.; Jensen, M.H.; Kasza, K.E.; Moore, J.R.; et al. Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc. Natl. Acad. Sci. USA 2017, 114, E8618–E8627. [Google Scholar] [CrossRef]
- Wang, Y.; Sukenik, S.; Davis, C.M.; Gruebele, M. Cell volume controls protein stability and compactness of the unfolded state. Phys. Chem. B 2018, 122, 11762–11770. [Google Scholar] [CrossRef]
- Lang, F.; Busch, G.L.; Ritter, M.; Volkl, H.; Waldegger, S.; Gulbins, E.; Haussinger, D. Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 1998, 78, 247–306. [Google Scholar] [CrossRef]
- Haussinger, D.; Roth, E.; Lang, F.; Gerok, W. Cellular hydration state: An important determinant of protein catabolism in health and disease. Lancet 1993, 341, 1330–1332. [Google Scholar] [CrossRef]
- Keller, U.; Szinnai, G.; Bilz, S.; Berneis, K. Effects of changes in hydration on protein, glucose and lipid metabolism in man: Impact on health. Eur. J. Clin. Nutr. 2003, 57 (Suppl. S2), s69–s74. [Google Scholar] [CrossRef]
- Camera, D.M.; West, D.W.; Burd, N.A.; Phillips, S.M.; Garnham, A.P.; Hawley, J.A.; Coffey, V.G. Low muscle glycogen concentration does not suppress the anabolic response to resistance exercise. J. Appl. Physiol. 2012, 113, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Plank, L.D.; Hill, G.L. Similarity of changes in body composition in intensive care patients following severe sepsis or major blunt injury. Ann. N.Y. Acad. Sci. 2000, 904, 592–602. [Google Scholar] [CrossRef]
- Malbrain, M.L.; Huygh, J.; Dabrowski, W.; De Waele, J.J.; Staelens, A.; Wauters, J. The use of bio-electrical impedance analysis (BIA) to guide fluid management, resuscitation and deresuscitation in critically ill patients: A bench-to-bedside review. Anaesthesiol. Intensive Ther. 2014, 46, 381–391. [Google Scholar] [CrossRef]
- Finn, P.J.; Plank, L.D.; Clark, M.A.; Connolly, A.B.; Hill, G.L. Progressive cellular dehydration and proteolysis in critically ill patients. Lancet 1996, 347, 654–656. [Google Scholar] [CrossRef]
- Nose, H.; Morimoto, T.; Ogura, K. Distribution of water losses among fluid compartments of tissues under thermal dehydration in the rat. Japanese J. Physiol. 1983, 33, 1019–1029. [Google Scholar] [CrossRef]
- Gosmanov, A.R.; Schneider, E.G.; Thomason, D.B. NKCC activity restores muscle water during hyperosmotic challenge independent of insulin, ERK, and p38 MAPK. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R655–R665. [Google Scholar] [CrossRef] [PubMed]
- Schliess, F.; Richter, L.; vom Dahl, S.; Häussinger, D. Cell hydration and mTOR-dependent signaling. Acta Physiol. 2006, 187, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.S. mTOR as a key regulator in maintaining skeletal muscle mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef]
- Pallafacchina, G.; Calabria, E.; Serrano, A.L.; Kalhovde, J.M.; Schiaffino, S. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc. Natl. Acad. Sci. USA 2002, 99, 9213–9218. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Léger, B.; Derave, W.; De Bock, K.; Hespel, P.; Russell, A.P. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008, 11, 163B–175B. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Barberi, L.; Bijlsma, A.Y.; Blaauw, B.; Dyar, K.A.; Milan, G.; Mammucari, C.; Meskers, C.G.M.; Pallafacchina, G.; Paoli, A.; et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 2013, 14, 303–323. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Traylor, D.A.; Weijs, P.J.M.; Phillips, S.M. Defining anabolic resistance: Implications for delivery of clinical care nutrition. Curr. Opin. Crit. Care 2018, 24, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Gorissen, S.H.; van Loon, L.J. Anabolic resistance of muscle protein synthesis with aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Peña-Oyarzun, D.; Troncoso, R.; Kretschmar, C.; Hernando, C.; Budini, M.; Morselli, E.; Sergio Lavandero, S.; Criollo, A. Hyperosmotic stress stimulates autophagy via polycystin-2. Oncotarget 2017, 8, 55984–55997. [Google Scholar]
- Chantranupong, L.; Sabatini, D.M. Cell biology: The TORC1 pathway to protein destruction. Nature 2016, 536, 155–156. [Google Scholar] [CrossRef][Green Version]
- Rasgado-Flores, H.; Theobald, J.; Ruiz, J.; Bitner, J.B.; Markowitz, S.; Zlatnick, D.; Yee, P.A.; Yee, P.P.; Lauren, T.; Gohar, K.; et al. Cell volume sensing and regulation in skeletal muscle cells: Lessons from an invertebrate. Adv. Exp. Med. Biol. 2004, 559, 263–292. [Google Scholar]
- Goertz, M.P.; Houston, J.E.; Zhu, X.Y. Hydrophilicity and the viscosity of interfacial water. Langmuir 2007, 23, 5491–5497. [Google Scholar] [CrossRef]
- Pollack, G.H. Cell electrical properties: Reconsidering the origin of the electrical potential. Cell. Biol. Int. 2015, 39, 237–242. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Lauretani, F.; Zamboni, V.; Bandinelli, S.; Bernabei, R.; Guralnik, J.M.; Ferrucci, L. Skeletal muscle and mortality results from the In CHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, ageing and body composition study cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Puig-Domingo, M.; Serra-Prat, M.; Merino, M.J.; Pubill, M.; Burdoy, E.; Papiol, M. Muscle strength in the Mataró ageing study participants and its relationship to successful ageing. Ageing Clin. Exp. Res. 2008, 20, 439–446. [Google Scholar] [CrossRef]
- Manini, T.M.; Clark, B.C. Dynapenia and ageing: An update. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Fernhall, B.; Ploutz-Snyder, L.L. Adaptations in human neuromuscular function following prolonged unweighting: I. Skeletal muscle contractile properties and appliedischemia efficacy. J. Appl. Physiol. 2006, 101, 256–263. [Google Scholar] [PubMed]
- Delmonico, M.J.; Harris, T.B.; Lee, J.S.; Visser, M.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Newman, A.B. Alternative definitions of sarcopenia, lower extremity performance, andfunctional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007, 55, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Manini, T.M. Sarcopenia=/=dynapenia. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 829–834. [Google Scholar] [CrossRef]
- Goulet, E.D.B.; Mélançon, M.O.; Lafrenière, D.; Paquin, J.; Maltais, M.; Morais, J.A. Impact of mild hypohydration on muscle endurance, power, and strength in healthy, active older men. J. Strength Cond. Res. 2018, 32, 3405–3415. [Google Scholar] [CrossRef]
- Ritz, P. Body water spaces and cellular hydration during healthy aging. Ann. N.Y. Acad. Sci. 2000, 904, 474–483. [Google Scholar] [CrossRef]
- Yamada, Y.; Yoshida, T.; Yokoyama, K.; Watanabe, Y.; Miyake, M.; Yamagata, M.; Kimura, M.; Studyet, K.K. The extracellularto intracellular water ratio in upper legs is negatively associated with skeletal muscle strength and gait speed in older people. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 293–298. [Google Scholar]
- Serra-Prat, M.; Lorenzo, I.; Palomera, E.; Ramírez, S.; Yíbenes, J.C. Total Body water and intracellular water relationships with muscle strength, frailty and functional performance in an elderly population. J. Nutr. Health Aging 2019, 23, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Serra-Prat, M.; Lorenzo, I.; Palomera, E.; Yébenes, J.C.; Campins, L.; Cabré, M. Intracellular water content in lean mass is associated with muscle strength, functional capacity, and frailty in community-dwelling elderly individuals. A cross-sectional study. Nutrients 2019, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.; Malmstrom, T. Frailty, sarcopenia and hormones. Endocrinol. Metab. Clin. N. Am. 2013, 42, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo, I.; Serra-Prat, M.; Yébenes, J.C. The Role of Water Homeostasis in Muscle Function and Frailty: A Review. Nutrients 2019, 11, 1857. https://doi.org/10.3390/nu11081857
Lorenzo I, Serra-Prat M, Yébenes JC. The Role of Water Homeostasis in Muscle Function and Frailty: A Review. Nutrients. 2019; 11(8):1857. https://doi.org/10.3390/nu11081857
Chicago/Turabian StyleLorenzo, Isabel, Mateu Serra-Prat, and Juan Carlos Yébenes. 2019. "The Role of Water Homeostasis in Muscle Function and Frailty: A Review" Nutrients 11, no. 8: 1857. https://doi.org/10.3390/nu11081857
APA StyleLorenzo, I., Serra-Prat, M., & Yébenes, J. C. (2019). The Role of Water Homeostasis in Muscle Function and Frailty: A Review. Nutrients, 11(8), 1857. https://doi.org/10.3390/nu11081857




