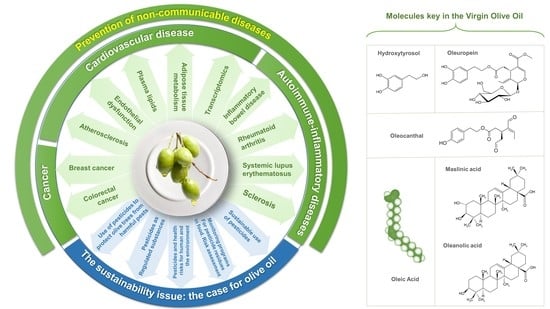
Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018
Abstract
1. Introduction
2. Future Challenges to Public Health and the Role of Virgin Olive Oil
3. Extra-Virgin Olive Oils and Prevention of NCDs
3.1. Extra-Virgin Olive Oil and Cancer
3.1.1. Breast Cancer
3.1.2. Colorectal Cancer
3.2. Extra-Virgin Olive Oil and Cardiovascular Disease
Modulation of Classic Cardiovascular Risk Factors
4. Extra-Virgin Olive Oil and Health: Molecular Mechanisms
4.1. Cancer
4.2. Cardiovascular Disease
4.2.1. Extra Virgin Olive Oil (EVOO) and Atherosclerosis
4.2.2. EVOO and Endothelial Dysfunction
4.2.3. EVOO and Transcriptomics
4.2.4. EVOO and Plasma Lipids
4.2.5. EVOO and Adipose Tissue Metabolism
4.3. Autoimmune Diseases/Immune-Inflammatory Diseases
4.3.1. Inflammatory Bowel Disease (IBD)
4.3.2. Rheumatoid Arthritis (RA)
4.3.3. Systemic Lupus Erythematosus (SLE)
4.3.4. Sclerosis
5. The Sustainability Issue: The Case for Olive Oil
5.1. Use of Pesticides to Protect Olive Trees from Harmful Pests
5.2. Pesticides as Regulated Substances
5.3. Pesticides and Health Risks for Humans and the Environment
5.4. Monitoring Programs for Pesticide Residues in Food: Risk Assessment
5.5. Sustainable Use of Pesticides
6. Other Health Properties of EVOO
7. Conclusions
- The MedDiet is an excellent model of healthy eating. Olive oil, particularly extra-virgin olive oil, is the main and most characteristic component of the MedDiet. Indeed, without the use of olive oil, applying the label of “MedDiet” to another dietary pattern would represent a fairly inconsistent definition.
- One of the greatest public health challenges worldwide is the obesity pandemic. There are sufficient studies reporting that the use of virgin olive oil as the only culinary fat, ingested in a moderate and continuous way, was associated with a reduced body mass index. Long-term randomized trials are warranted to confirm this observation.
- Hypertension is the major risk factor for cardiovascular disease worldwide. The available randomized trials indicate that virgin olive oils reduce blood pressure and thus, the global cardiovascular burden of disease and its associated pharmaceutical costs.
- Virgin olive oils have anti-atherosclerotic potential, favoring endothelial function and preserving blood pressure, maintaining lipoprotein functionality, exerting anti-inflammatory and antioxidant effects, and modulating gene expression in several tissues to maintain proper homeostasis.
- Nevertheless, the type of olive oil should be considered when providing recommendations to the population because additional benefits can be conferred when the phenolic content of olive oil is high.
- Epidemiological studies are concordant in supporting that a diet where virgin olive oils are the foremost source of fat is associated with chemoprevention. Animal studies are suggestive of a preventive effect of olive polyphenols and many in vitro studies are clarifying their mechanisms of action. However, the relevance of such data is often weakened by the use of non-physiological concentrations and doses. Human studies on chemoprevention with a single nutrient are nearly impossible to carry out. However, the advice to use olive oil as the principal source of visible fat to lower cancer risk rests on solid accumulated observations.
- The Mediterranean diet—as an overall dietary pattern—has been shown to be associated with reduced risk of postmenopausal breast cancer in both observational human studies and a randomized trial. Moreover, observational studies suggest that specifically virgin olive oils may play a role in the prevention of postmenopausal breast cancer.
- The Mediterranean diet has also been suggested to reduce the risk of colorectal cancer; nevertheless, the evidence is limited to observational data in humans and to date, there is no large body of evidence on the specific protective role of virgin olive oils on colorectal cancer prevention.
- Although virgin olive oils have the potential to reduce the risk of some types of cancer (primary prevention), we have no strong clinical evidence to support that they can affect the long-term progression of pre-malignant or cancerous lesions after diagnosis (treatment).
- Experimental studies have confirmed significant anti-inflammatory and immunomodulatory effects of dietary virgin olive oils and its bioactive components supplementation in preclinical models of autoimmune diseases, such as inflammatory bowel disease, rheumatoid arthritis, systemic lupus erythematosus and sclerosis. Thus, the consumption of virgin olive oil and its minor constituents may acquire a great importance in nutritional therapy, especially in immunocompromised patients, and could be an alternative approach for the prevention and management of different immune-inflammatory diseases. However, future clinical studies are necessary to mechanistically define the effective doses in humans and the dose-dependence of their effects.
- Chemical pollution is one of the main determinants of morbidity and mortality in the world and represents an increasingly important threat for humans and the environment. However, only regulated chemicals (i.e., pesticides, food additives and veterinary medicinal products) have been subject to pre-market evaluation by means of rigorous toxicological testing in Western countries since the past two decades. In contrast, this is not the case for food contaminants and we still need to implement ways to reduce or prevent food contamination. While there is no current evidence that chemical pollution represents a health concern regarding olive oil, we need to shift towards a sustainable production of olive oil in order to reduce the potential chemical burden associated with its production.
- The use of pesticides is necessary to combat pests and diseases in olive groves in order to increase olive oil production in terms of quantity and quality. However, an inappropriate use of pesticides may pose health risks to humans, non-target species, and the environment. Although theoretically, pesticide residues may remain in olive oil, the European Union Coordinated Programme for pesticide residues in food (for the year 2015) showed that only approximately 0.1% of the olive oil samples analyzed exceeded the MRL currently adopted. This indicates that the consumption of olive oil is unlikely to pose health concern to consumers. An integrated pest management approach is being implemented in the European Union to reduce the use of pesticides and foster a sustainable use of these substances without impairing crop production. This approach is essential and will optimize food production while minimizing risks to humans and the environment, thus contributing to achieving the integral concept of healthy food.
- Sustainable food production is indispensable and unavoidable to feed the growing population. Better agronomic practices are urgently needed to guarantee nutritious food that is accessible to everyone and respectful of the environment. This also applies to olive oil.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Trichopoulou, A.; Martinez-Gonzalez, M.A.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and Potential Health Benefits of the Mediterranean Diet: Views from Experts Around the World. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Estruch, R.; Corella, D.; Fito, M.; Ros, E.; PREDIMED INVESTIGATORS. Benefits of the Mediterranean Diet: Insights from the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Hershey, M.S.; Zazpe, I.; Trichopoulou, A. Transferability of the Mediterranean Diet to Non-Mediterranean Countries. what is and what is Not the Mediterranean Diet. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean Diet and Multiple Health Outcomes: An Umbrella Review of Meta-Analyses of Observational Studies and Randomised Trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Román-Viñas, B.; Sanchez-Villegas, A.; Guasch-Ferré, M.; Corella, D.; La Vecchia, C. Benefits of the Mediterranean diet: Epidemiological and molecular aspects. Mol. Aspects. Med. 2019, 67, 1–55. [Google Scholar] [CrossRef]
- Carlos, S.; De La Fuente-Arrillaga, C.; Bes-Rastrollo, M.; Razquin, C.; Rico-Campa, A.; Martinez-Gonzalez, M.A.; Ruiz-Canela, M. Mediterranean Diet and Health Outcomes in the SUN Cohort. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Alvarez-Alvarez, I.; Zazpe, I.; Perez de Rojas, J.; Bes-Rastrollo, M.; Ruiz-Canela, M.; Fernandez-Montero, A.; Hidalgo-Santamaria, M.; Martinez-Gonzalez, M.A. Mediterranean Diet, Physical Activity and their Combined Effect on all-Cause Mortality: The Seguimiento Universidad De Navarra (SUN) Cohort. Prev. Med. 2018, 106, 45–52. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean Diet, Traditional Risk Factors, and the Rate of Cardiovascular Complications After Myocardial Infarction: Final Report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ros, E.; Covas, M.I.; Fiol, M.; Warnberg, J.; Aros, F.; Ruiz-Gutierrez, V.; Lamuela-Raventos, R.M.; et al. Cohort Profile: Design and Methods of the PREDIMED Study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Missbach, B.; Konig, J.; Hoffmann, G. Adherence to a Mediterranean Diet and Risk of Diabetes: A Systematic Review and Meta-Analysis. Public Health Nutr. 2015, 18, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, RM.; Bhutta, ZA.; Carter, A.; Casey, DC.; Charlson, FJ.; Chen, AZ.; Coates, M.M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A Comparative Risk Assessment of Burden of Disease and Injury Attributable to 67 Risk Factors and Risk Factor Clusters in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Danaei, G.; Finucane, M.M.; Lin, J.K.; Singh, G.M.; Paciorek, C.J.; Cowan, M.J.; Farzadfar, F.; Stevens, G.A.; Lim, S.S.; Riley, L.M.; et al. National, Regional, and Global Trends in Systolic Blood Pressure since 1980: Systematic Analysis of Health Examination Surveys and Epidemiological Studies with 786 Country-Years and 5.4 Million Participants. Lancet 2011, 377, 568–577. [Google Scholar] [CrossRef]
- Alonso, A.; Ruiz-Gutierrez, V.; Martinez-Gonzalez, M.A. Monounsaturated Fatty Acids, Olive Oil and Blood Pressure: Epidemiological, Clinical and Experimental Evidence. Public Health Nutr. 2006, 9, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Sacks, F.M.; Carey, V.J.; Obarzanek, E.; Swain, J.F.; Miller, E.R., 3rd; Conlin, P.R.; Erlinger, T.P.; Rosner, B.A.; Laranjo, N.M.; et al. Effects of Protein, Monounsaturated Fat, and Carbohydrate Intake on Blood Pressure and Serum Lipids: Results of the OmniHeart Randomized Trial. JAMA 2005, 294, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Stamler, J.; Brown, I.J.; Ueshima, H.; Nakagawa, H.; Sakurai, M.; Chan, Q.; Appel, L.J.; Okayama, A.; Okuda, N.; et al. Relationship of Dietary Monounsaturated Fatty Acids to Blood Pressure: The International Study of Macro/Micronutrients and Blood Pressure. J. Hypertens. 2013, 31, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Domenech, M.; Roman, P.; Lapetra, J.; Garcia de la Corte, F.J.; Sala-Vila, A.; de la Torre, R.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Lamuela-Raventos, R.M.; et al. Mediterranean Diet Reduces 24-Hour Ambulatory Blood Pressure, Blood Glucose, and Lipids: One-Year Randomized, Clinical Trial. Hypertension 2014, 64, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Zamora, Z.; Martínez-Galiano, J.M.; Gaforio, J.J.; Delgado-Rodríguez, M. Effects of Olive Oil on Blood Pressure: A Systematic Review and Meta-Analysis. Grasas Y Aceites 2018, 69, e272. [Google Scholar] [CrossRef]
- Eguaras, S.; Bes-Rastrollo, M.; Ruiz-Canela, M.; Carlos, S.; de la Rosa, P.; Martinez-Gonzalez, M.A. May the Mediterranean Diet Attenuate the Risk of Type 2 Diabetes Associated with Obesity: The Seguimiento Universidad De Navarra (SUN) Cohort. Br. J. Nutr. 2017, 117, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, Regional, and Global Trends in Body-Mass Index since 1980: Systematic Analysis of Health Examination Surveys and Epidemiological Studies with 960 Country-Years and 9.1 Million Participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Arnold, M.; Pandeya, N.; Byrnes, G.; Renehan, P.A.G.; Stevens, G.A.; Ezzati, P.M.; Ferlay, J.; Miranda, J.J.; Romieu, I.; Dikshit, R.; et al. Global Burden of Cancer Attributable to High Body-Mass Index in 2012: A Population-Based Study. Lancet Oncol. 2015, 16, 36–46. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; Norat, T.; Janszky, I.; Romundstad, P.; Tonstad, S.; Vatten, L.J. Body Mass Index, Abdominal Fatness, and Heart Failure Incidence and Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Circulation 2016, 133, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Romaguera, D.; Norat, T.; Vergnaud, A.C.; Mouw, T.; May, A.M.; Agudo, A.; Buckland, G.; Slimani, N.; Rinaldi, S.; Couto, E.; et al. Mediterranean Dietary Patterns and Prospective Weight Change in Participants of the EPIC-PANACEA Project. Am. J. Clin. Nutr. 2010, 92, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Naska, A.; Orfanos, P.; Trichopoulos, D. Mediterranean Diet in Relation to Body Mass Index and Waist-to-Hip Ratio: The Greek European Prospective Investigation into Cancer and Nutrition Study. Am. J. Clin. Nutr. 2005, 82, 935–940. [Google Scholar] [CrossRef]
- Beunza, J.J.; Toledo, E.; Hu, F.B.; Bes-Rastrollo, M.; Serrano-Martinez, M.; Sanchez-Villegas, A.; Martinez, J.A.; Martinez-Gonzalez, M.A. Adherence to the Mediterranean Diet, Long-Term Weight Change, and Incident Overweight Or Obesity: The Seguimiento Universidad De Navarra (SUN) Cohort. Am. J. Clin. Nutr. 2010, 92, 1484–1493. [Google Scholar] [CrossRef]
- Buckland, G.; Bach, A.; Serra-Majem, L. Obesity and the Mediterranean Diet: A Systematic Review of Observational and Intervention Studies. Obes. Rev. 2008, 9, 582–593. [Google Scholar] [CrossRef]
- Esposito, K.; Kastorini, C.M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean Diet and Weight Loss: Meta-Analysis of Randomized Controlled Trials. Metab. Syndr. Relat. Disord. 2011, 9, 1–12. [Google Scholar] [CrossRef]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Fito, M.; Chiva-Blanch, G.; Fiol, M.; Gomez-Gracia, E.; Aros, F.; Lapetra, J.; et al. Effect of a High-Fat Mediterranean Diet on Bodyweight and Waist Circumference: A Prespecified Secondary Outcomes Analysis of the PREDIMED Randomised Controlled Trial. Lancet Diabetes Endocrinol. 2016, 4, 666–676. [Google Scholar] [CrossRef]
- Bes-Rastrollo, M.; Sanchez-Villegas, A.; de la Fuente, C.; de Irala, J.; Martinez, J.A.; Martinez-Gonzalez, M.A. Olive Oil Consumption and Weight Change: The SUN Prospective Cohort Study. Lipids 2006, 41, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Soriguer, F.; Almaraz, M.; Ruiz-de-Adana, M.S.; Esteva, I.; Linares, F.; Almeida, J.; Morcillo, S.; Garcia-Escobar, E.; Olveira, G.; Rojo-Martinez, G. Incidence of Obesity is Lower in Persons Who Consume Olive Oil. Eur. J. Clin. Nutr. 2009, 63, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Zamora, Z.; Martínez-Galiano, J.M.; Gaforio, J.J.; Delgado-Rodríguez, M.; Aceite De Oliva, Y. Peso Corporal. Revisión Sistemática Y Metaanálisis De Ensayos Controlados Aleatorizados. Rev. Esp. Salud Publ. 2018, 92, e1–e15. [Google Scholar]
- World Health Organization. Globocan. Cancer Fact Sheets: Breast Cancer; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Danona, L.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Trichopoulou, A.; Lagiou, P.; Kuper, H.; Trichopoulos, D. Cancer and Mediterranean Dietary Traditions. Cancer Epidemiol. Biomarkers Prev. 2000, 9, 869–873. [Google Scholar] [PubMed]
- Van den Brandt, P.A.; Schulpen, M. Mediterranean Diet Adherence and Risk of Postmenopausal Breast Cancer: Results of a Cohort Study and Meta-Analysis. Int. J. Cancer 2017, 140, 2220–2231. [Google Scholar] [CrossRef]
- World Cancer Research Fund & American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Breast Cancer Risk; World Cancer Research Fund & American Institute for Cancer Research: London, UK, 2017; Revised 2018. [Google Scholar]
- Buckland, G.; Travier, N.; Cottet, V.; Gonzalez, C.A.; Lujan-Barroso, L.; Agudo, A.; Trichopoulou, A.; Lagiou, P.; Trichopoulos, D.; Peeters, P.H.; et al. Adherence to the Mediterranean Diet and Risk of Breast Cancer in the European Prospective Investigation into Cancer and Nutrition Cohort Study. Int. J. Cancer 2013, 132, 2918–2927. [Google Scholar] [CrossRef]
- Buckland, G.; Mayen, A.L.; Agudo, A.; Travier, N.; Navarro, C.; Huerta, J.M.; Chirlaque, M.D.; Barricarte, A.; Ardanaz, E.; Moreno-Iribas, C.; et al. Olive Oil Intake and Mortality within the Spanish Population (EPIC-Spain). Am. J. Clin. Nutr. 2012, 96, 142–149. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive Oil Intake is Inversely Related to Cancer Prevalence: A Systematic Review and a Meta-Analysis of 13,800 Patients and 23,340 Controls in 19 Observational Studies. Lipids Health. Dis. 2011, 10, 127. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Retraction and Republication: Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2018, 378, 2441–2442. [Google Scholar] [CrossRef]
- Toledo, E.; Salas-Salvado, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fito, M.; Hu, F.B.; Aros, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Machowetz, A.; Poulsen, H.E.; Gruendel, S.; Weimann, A.; Fito, M.; Marrugat, J.; de la Torre, R.; Salonen, J.T.; Nyyssonen, K.; Mursu, J.; et al. Effect of Olive Oils on Biomarkers of Oxidative DNA Stress in Northern and Southern Europeans. FASEB J. 2007, 21, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Salvini, S.; Sera, F.; Caruso, D.; Giovannelli, L.; Visioli, F.; Saieva, C.; Masala, G.; Ceroti, M.; Giovacchini, V.; Pitozzi, V.; et al. Daily Consumption of a High-Phenol Extra-Virgin Olive Oil Reduces Oxidative DNA Damage in Postmenopausal Women. Br. J. Nutr. 2006, 95, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Tunstall-Pedoe, H.; Kuulasmaa, K.; Mahonen, M.; Tolonen, H.; Ruokokoski, E.; Amouyel, P. Contribution of Trends in Survival and Coronary-Event Rates to Changes in Coronary Heart Disease Mortality: 10-Year Results from 37 WHO MONICA Project Populations. Monitoring Trends and Determinants in Cardiovascular Disease. Lancet 1999, 353, 1547–1557. [Google Scholar] [CrossRef]
- Degano, I.R.; Elosua, R.; Marrugat, J. Epidemiology of Acute Coronary Syndromes in Spain: Estimation of the Number of Cases and Trends from 2005 to 2049. Rev. Esp. Cardiol. 2013, 66, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Aravanis, C.; Corcondilas, A.; Dontas, A.S.; Lekos, D.; Keys, A. Coronary Heart Disease in Seven Countries. IX. the Greek Islands of Crete and Corfu. Circulation 1970, 41, I88–I100. [Google Scholar] [CrossRef]
- McGovern, P.G.; Pankow, J.S.; Shahar, E.; Doliszny, K.M.; Folsom, A.R.; Blackburn, H.; Luepker, R.V. Recent Trends in Acute Coronary Heart Disease—Mortality, Morbidity, Medical Care, and Risk Factors. the Minnesota Heart Survey Investigators. N. Engl. J. Med. 1996, 334, 884–890. [Google Scholar] [CrossRef]
- Bendinelli, B.; Masala, G.; Saieva, C.; Salvini, S.; Calonico, C.; Sacerdote, C.; Agnoli, C.; Grioni, S.; Frasca, G.; Mattiello, A.; et al. Fruit, Vegetables, and Olive Oil and Risk of Coronary Heart Disease in Italian Women: The EPICOR Study. Am. J. Clin. Nutr. 2011, 93, 275–283. [Google Scholar] [CrossRef]
- Samieri, C.; Feart, C.; Proust-Lima, C.; Peuchant, E.; Tzourio, C.; Stapf, C.; Berr, C.; Barberger-Gateau, P. Olive Oil Consumption, Plasma Oleic Acid, and Stroke Incidence: The Three-City Study. Neurology 2011, 77, 418–425. [Google Scholar] [CrossRef]
- Guasch-Ferre, M.; Hu, F.B.; Martinez-Gonzalez, M.A.; Fito, M.; Bullo, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gomez-Gracia, E.; et al. Olive Oil Intake and Risk of Cardiovascular Disease and Mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated Fatty Acids, Olive Oil and Health Status: A Systematic Review and Meta-Analysis of Cohort Studies. Lipids Health. Dis. 2014, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Weinbrenner, T.; Fito, M.; de la Torre, R.; Saez, G.T.; Rijken, P.; Tormos, C.; Coolen, S.; Albaladejo, M.F.; Abanades, S.; Schroder, H.; et al. Olive Oils High in Phenolic Compounds Modulate Oxidative/Antioxidative Status in Men. J. Nutr. 2004, 134, 2314–2321. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.I.; de la Torre, K.; Farre-Albaladejo, M.; Kaikkonen, J.; Fito, M.; Lopez-Sabater, C.; Pujadas-Bastardes, M.A.; Joglar, J.; Weinbrenner, T.; Lamuela-Raventos, R.M.; et al. Postprandial LDL Phenolic Content and LDL Oxidation are Modulated by Olive Oil Phenolic Compounds in Humans. Free Radic. Biol. Med. 2006, 40, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Covas, M.I.; Fiol, M.; Gomez-Gracia, E.; Lopez-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-Style Diet on Cardiovascular Risk Factors: A Randomized Trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Casillas, R.; Bullo, M.; Castaner, O.; Ros, E.; Saez, G.T.; Toledo, E.; Estruch, R.; Ruiz-Gutierrez, V.; Fito, M.; et al. A Mediterranean Diet Supplemented with Extra Virgin Olive Oil or Nuts Improves Endothelial Markers Involved in Blood Pressure Control in Hypertensive Women. Eur. J. Nutr. 2017, 56, 89–97. [Google Scholar] [CrossRef]
- Toledo, E.; Hu, F.B.; Estruch, R.; Buil-Cosiales, P.; Corella, D.; Salas-Salvado, J.; Covas, M.I.; Aros, F.; Gomez-Gracia, E.; Fiol, M.; et al. Effect of the Mediterranean Diet on Blood Pressure in the PREDIMED Trial: Results from a Randomized Controlled Trial. BMC Med. 2013, 11, 207. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight Loss with a Low-Carbohydrate, Mediterranean, Or Low-Fat Diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef]
- Schwarzfuchs, D.; Golan, R.; Shai, I. Four-Year Follow-Up After Two-Year Dietary Interventions. N. Engl. J. Med. 2012, 367, 1373–1374. [Google Scholar] [CrossRef]
- Hohmann, C.D.; Cramer, H.; Michalsen, A.; Kessler, C.; Steckhan, N.; Choi, K.; Dobos, G. Effects of High Phenolic Olive Oil on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Phytomedicine 2015, 22, 631–640. [Google Scholar] [CrossRef]
- Fito, M.; Cladellas, M.; de la Torre, R.; Marti, J.; Alcantara, M.; Pujadas-Bastardes, M.; Marrugat, J.; Bruguera, J.; Lopez-Sabater, M.C.; Vila, J.; et al. Antioxidant Effect of Virgin Olive Oil in Patients with Stable Coronary Heart Disease: A Randomized, Crossover, Controlled, Clinical Trial. Atherosclerosis 2005, 181, 149–158. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL-Cholesterol Concentrations (ID 1639), Maintenance of Normal Blood Pressure (ID 3781), “anti-Inflammatory Properties” (ID 1882), “contributes to the Upper Respiratory Tract Health” (ID 3468), “can Help to Maintain a Normal Function of Gastrointestinal Tract” (3779), and “contributes to Body Defences Against External Agents” (ID 3467) Pursuant to Article 13(1) of Regulation (EC) no 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar]
- EFSA Panel on Dietetic Products Nutrition and Allergy (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive Oil and Protection of LDL Particles from Oxidative Damage. EFSA J. 2011, 9, 2033. [Google Scholar]
- Holvoet, P.; Mertens, A.; Verhamme, P.; Bogaerts, K.; Beyens, G.; Verhaeghe, R.; Collen, D.; Muls, E.; Van de Werf, F. Circulating Oxidized LDL is a Useful Marker for Identifying Patients with Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Baumert, J.; Khuseyinova, N.; Loewel, H.; Koenig, W. Plasma Oxidized Low-Density Lipoprotein, a Strong Predictor for Acute Coronary Heart Disease Events in Apparently Healthy, Middle-Aged Men from the General Population. Circulation 2005, 112, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Spence, J.D.; Giovannucci, E.L.; Kim, Y.I.; Josse, R.; Vieth, R.; Blanco Mejia, S.; Viguiliouk, E.; Nishi, S.; Sahye-Pudaruth, S.; et al. Supplemental Vitamins and Minerals for CVD Prevention and Treatment. J. Am. Coll Cardiol. 2018, 71, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Svegliati Baroni, S.; Amelio, M.; Fiorito, A.; Gaddi, A.; Littarru, G.; Battino, M. Monounsaturated Diet Lowers LDL Oxidisability in Type IIb and Type IV Dyslipidemia without Affecting Coenzyme Q10 and Vitamin E Contents. Biofactors 1999, 9, 325–330. [Google Scholar] [CrossRef]
- Hernaez, A.; Fernandez-Castillejo, S.; Farras, M.; Catalan, U.; Subirana, I.; Montes, R.; Sola, R.; Munoz-Aguayo, D.; Gelabert-Gorgues, A.; Diaz-Gil, O.; et al. Olive Oil Polyphenols Enhance High-Density Lipoprotein Function in Humans: A Randomized Controlled Trial. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2115–2119. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and Atherosclerotic Cardiovascular Disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef]
- Hernaez, A.; Castaner, O.; Elosua, R.; Pinto, X.; Estruch, R.; Salas-Salvado, J.; Corella, D.; Aros, F.; Serra-Majem, L.; Fiol, M.; et al. Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals: A Randomized Controlled Trial. Circulation 2017, 135, 633–643. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Krause, M.; Schmucker, C.; Hoffmann, G.; Rücker, G.; Meerpohl, J.J. Impact of different types of olive oil on cardiovascular risk factors: A systematic review and network meta-analysis. Nutr. Metabol. Cardiovasc. Dis. 2019. in Press. [Google Scholar] [CrossRef] [PubMed]
- Ruano, J.; Lopez-Miranda, J.; de la Torre, R.; Delgado-Lista, J.; Fernandez, J.; Caballero, J.; Covas, M.I.; Jimenez, Y.; Perez-Martinez, P.; Marin, C.; et al. Intake of Phenol-Rich Virgin Olive Oil Improves the Postprandial Prothrombotic Profile in Hypercholesterolemic Patients. Am. J. Clin. Nutr. 2007, 86, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de la Puebla, R.A.; Perez-Martinez, P.; Carmona, J.; Lopez-Miranda Carmen Marin, J.; Paniagua, J.A.; Fuentes, F.; Perez-Jimenez, F. Factor VII Polymorphisms Influence the Plasma Response to Diets with Different Fat Content, in a Healthy Caucasian Population. Mol. Nutr. Food Res. 2007, 51, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Garcia-Rios, A.; Perez-Martinez, P.; Lopez-Miranda, J.; Perez-Jimenez, F. Olive Oil and Haemostasis: Platelet Function, Thrombogenesis and Fibrinolysis. Curr. Pharm. Des. 2011, 17, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Capurso, C.; Massaro, M.; Scoditti, E.; Vendemiale, G.; Capurso, A. Vascular Effects of the Mediterranean Diet Part I: Anti-Hypertensive and Anti-Thrombotic Effects. Vascul. Pharmacol. 2014, 63, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Caruso, D.; Grande, S.; Bosisio, R.; Villa, M.; Galli, G.; Sirtori, C.; Galli, C. Virgin Olive Oil Study (VOLOS): Vasoprotective potential of extra virgin olive oil in mildly dyslipidemic patients. Eur. J. Nutr. 2005, 44, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Franco, M.; Toledo, E.; Luchsinger, J.; Willett, W.C.; Hu, F.B.; Martinez-Gonzalez, M.A. Olive oil and prevention of chronic diseases: Summary of an International conference. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, Y.; López, S.; Bermúdez, B.; Abia, R.; Muriana, F. Extra-Virgin Vs. Refined Olive Oil on Postprandial Hemostatic Markers in Healthy Subjects. J. Thromb. Haemost. 2006, 4, 1421–1422. [Google Scholar] [CrossRef] [PubMed]
- Oosthuizen, W.; Vorster, H.H.; Jerling, J.C.; Barnard, H.C.; Smuts, C.M.; Silvis, N.; Kruger, A.; Venter, C.S. Both Fish Oil and Olive Oil Lowered Plasma Fibrinogen in Women with High Baseline Fibrinogen Levels. Thromb. Haemost. 1994, 72, 557–562. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diabetes-Fact Sheet. 2017. Available online: http://Www.Who.Int/Mediacentre/Factsheets/fs312/En (accessed on 23 May 2019).
- Panagiotakos, D.B.; Tzima, N.; Pitsavos, C.; Chrysohoou, C.; Zampelas, A.; Toussoulis, D.; Stefanadis, C. The Association between Adherence to the Mediterranean Diet and Fasting Indices of Glucose Homoeostasis: The ATTICA Study. J. Am. Coll. Nutr. 2007, 26, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvado, J.; Bullo, M.; Estruch, R.; Ros, E.; Covas, M.I.; Ibarrola-Jurado, N.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; et al. Prevention of Diabetes with Mediterranean Diets: A Subgroup Analysis of a Randomized Trial. Ann. Intern. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Koloverou, E.; Esposito, K.; Giugliano, D.; Panagiotakos, D. The Effect of Mediterranean Diet on the Development of Type 2 Diabetes Mellitus: A Meta-Analysis of 10 Prospective Studies and 136,846 Participants. Metabolism 2014, 63, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A Journey into a Mediterranean Diet and Type 2 Diabetes: A Systematic Review with Meta-Analyses. BMJ Open 2015, 5, e008222. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Chiodini, P.; Maiorino, M.I.; Bellastella, G.; Panagiotakos, D.; Giugliano, D. Which Diet for Prevention of Type 2 Diabetes? A Meta-Analysis of Prospective Studies. Endocrine 2014, 47, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Loffredo, L.; Del Ben, M.; Angelico, F.; Nocella, C.; Petruccioli, A.; Bartimoccia, S.; Monticolo, R.; Cava, E.; Visioli, F. Extra virgin olive oil improves post-prandial glycemic and lipid profile in patients with impaired fasting glucose. Clinical Nutrition 2017, 36, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Soriguer, F.; Esteva, I.; Rojo-Martinez, G.; Ruiz de Adana, M.S.; Dobarganes, M.C.; Garcia-Almeida, J.M.; Tinahones, F.; Beltran, M.; Gonzalez-Romero, S.; Olveira, G.; et al. Oleic Acid from Cooking Oils is Associated with Lower Insulin Resistance in the General Population (Pizarra Study). Eur. J. Endocrinol. 2004, 150, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Filesi, C.; Vari, R.; Scazzocchio, B.; Filardi, T.; Fogliano, V.; D’Archivio, M.; Giovannini, C.; Lenzi, A.; Morano, S.; et al. Consumption of Extra-Virgin Olive Oil Rich in Phenolic Compounds Improves Metabolic Control in Patients with Type 2 Diabetes Mellitus: A Possible Involvement of Reduced Levels of Circulating Visfatin. J. Endocrinol. Invest. 2016, 39, 1295–1301. [Google Scholar] [CrossRef]
- Basterra-Gortari, FJ.; Ruiz-Canela, M.; Martínez-González, MA.; Babio, N.; Sorlí, JV.; Fito, M.; Ros, E.; Gómez-Gracia, E.; Fiol, M.; Lapetra, J.; et al. Effects of a Mediterranean Eating Plan on the Need for Glucose-Lowering Medications in Participants With Type 2 Diabetes: A Subgroup Analysis of the PREDIMED Trial. Diabetes Care. 2019. [Google Scholar] [CrossRef]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef]
- Yazulla, S. Neurochemistry. is GABA the Neurotransmitter for some Photoreceptors? Nature 1986, 320, 685–686. [Google Scholar] [CrossRef]
- Iliakis, G.; Kurtzman, S. Mechanism of Radiosensitization by Halogenated Pyrimidines: Bromodeoxyuridine and Beta-Arabinofuranosyladenine Affect Similar Subsets of Radiation-Induced Potentially Lethal Lesions in Plateau-Phase Chinese Hamster Ovary Cells. Radiat. Res. 1991, 127, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Femia, A.P.; Dolara, P.; Servili, M.; Esposto, S.; Taticchi, A.; Urbani, S.; Giannini, A.; Salvadori, M.; Caderni, G. No Effects of Olive Oils with Different Phenolic Content Compared to Corn Oil on 1,2-Dimethylhydrazine-Induced Colon Carcinogenesis in Rats. Eur. J. Nutr. 2008, 47, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Deiana, M.; Incani, A.; Vauzour, D.; Dessi, M.A.; Spencer, J.P. Hydroxytyrosol Inhibits the Proliferation of Human Colon Adenocarcinoma Cells through Inhibition of ERK1/2 and Cyclin D1. Mol. Nutr. Food Res. 2009, 53, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, B.; Mavilia, C.; Fabbri, S.; Romani, A.; Ieri, F.; Tanini, A.; Tonelli, F.; Brandi, M.L. In Vitro Effects of Extracts of Extra Virgin Olive Oil on Human Colon Cancer Cells. Nutr. Cancer 2014, 66, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Hashim, Y.Z.; Worthington, J.; Allsopp, P.; Ternan, N.G.; Brown, E.M.; McCann, M.J.; Rowland, I.R.; Esposito, S.; Servili, M.; Gill, C.I. Virgin Olive Oil Phenolics Extract Inhibit Invasion of HT115 Human Colon Cancer Cells in Vitro and in Vivo. Food Funct. 2014, 5, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Coccia, A.; Bastianelli, D.; Mosca, L.; Monticolo, R.; Panuccio, I.; Carbone, A.; Calogero, A.; Lendaro, E. Extra Virgin Olive Oil Phenols Suppress Migration and Invasion of T24 Human Bladder Cancer Cells through Modulation of Matrix Metalloproteinase-2. Nutr. Cancer 2014, 66, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Coccia, A.; Mosca, L.; Puca, R.; Mangino, G.; Rossi, A.; Lendaro, E. Extra-Virgin Olive Oil Phenols Block Cell Cycle Progression and Modulate Chemotherapeutic Toxicity in Bladder Cancer Cells. Oncol. Rep. 2016, 36, 3095–3104. [Google Scholar] [CrossRef]
- Khanal, P.; Oh, W.K.; Yun, H.J.; Namgoong, G.M.; Ahn, S.G.; Kwon, S.M.; Choi, H.K.; Choi, H.S. P-HPEA-EDA, a Phenolic Compound of Virgin Olive Oil, Activates AMP-Activated Protein Kinase to Inhibit Carcinogenesis. Carcinogenesis 2011, 32, 545–553. [Google Scholar] [CrossRef]
- Fogli, S.; Arena, C.; Carpi, S.; Polini, B.; Bertini, S.; Digiacomo, M.; Gado, F.; Saba, A.; Saccomanni, G.; Breschi, M.C.; et al. Cytotoxic Activity of Oleocanthal Isolated from Virgin Olive Oil on Human Melanoma Cells. Nutr. Cancer 2016, 68, 873–877. [Google Scholar] [CrossRef]
- Cusimano, A.; Balasus, D.; Azzolina, A.; Augello, G.; Emma, M.R.; Di Sano, C.; Gramignoli, R.; Strom, S.C.; McCubrey, J.A.; Montalto, G.; et al. Oleocanthal Exerts Antitumor Effects on Human Liver and Colon Cancer Cells through ROS Generation. Int. J. Oncol. 2017, 51, 533–544. [Google Scholar] [CrossRef]
- Ayoub, N.M.; Siddique, A.B.; Ebrahim, H.Y.; Mohyeldin, M.M.; El Sayed, K.A. The Olive Oil Phenolic (-)-Oleocanthal Modulates Estrogen Receptor Expression in Luminal Breast Cancer in Vitro and in Vivo and Synergizes with Tamoxifen Treatment. Eur. J. Pharmacol. 2017, 810, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.M.; Chai, E.Q.; Cai, H.Y.; Miao, G.Y.; Ma, W. Oleuropein Induces Apoptosis Via Activation of Caspases and Suppression of Phosphatidylinositol 3-Kinase/Protein Kinase B Pathway in HepG2 Human Hepatoma Cell Line. Mol. Med. Rep. 2015, 11, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Rosignoli, P.; Fuccelli, R.; Sepporta, M.V.; Fabiani, R. In Vitro Chemo-Preventive Activities of Hydroxytyrosol: The Main Phenolic Compound Present in Extra-Virgin Olive Oil. Food Funct. 2016, 7, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Toteda, G.; Lupinacci, S.; Vizza, D.; Bonofiglio, R.; Perri, E.; Bonofiglio, M.; Lofaro, D.; La Russa, A.; Leone, F.; Gigliotti, P.; et al. High Doses of Hydroxytyrosol Induce Apoptosis in Papillary and Follicular Thyroid Cancer Cells. J. Endocrinol. Invest. 2017, 40, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Lopez de Las Hazas, M.C.; Pinol, C.; Macia, A.; Motilva, M.J. Hydroxytyrosol and the Colonic Metabolites Derived from Virgin Olive Oil Intake Induce Cell Cycle Arrest and Apoptosis in Colon Cancer Cells. J. Agric. Food Chem. 2017, 65, 6467–6476. [Google Scholar] [CrossRef] [PubMed]
- Corominas-Faja, B.; Cuyàs, E.; Lozano-Sánchez, J.; Cufí, S.; Verdura, S.; Fernández-Arroyo, S.; Borrás-Linares, I.; Martin-Castillo, B.; Martin, Á.G.; Lupu, R.; et al. Extra-virgin olive oil contains a metabolo-epigenetic inhibitor of cancer stem cells. Carcinogenesis 2018, 39, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Verdura, S.; Cuyàs, E.; Lozano-Sánchez, J.; Bastidas-Velez, C.; Llorach-Parés, L.; Fernández-Arroyo, S.; Hernández-Aguilera, A.; Joven, J.; Nonell-Canals, A.; Bosch-Barrera, J. An olive oil phenolic is a new chemotype of mutant isocitrate dehydrogenase 1 (IDH1) inhibitors. Carcinogenesis 2019, 40, 27–40. [Google Scholar] [CrossRef]
- Cuyàs, E.; Castillo, D.; Llorach-Parés, L.; Lozano-Sánchez, J.; Verdura, S.; Nonell-Canals, A.; Brunet, J.; Bosch-Barrera, J.; Joven, J.; Valdés, R.; et al. Computational de-orphanization of the olive oil biophenol oleacein: Discovery of new metabolic and epigenetic targets. Food Chem. Toxicol. 2019, 131, 110529. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular Senescence in Aging and Age-Related Disease: From Mechanisms to Therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- Menicacci, B.; Cipriani, C.; Margheri, F.; Mocali, A.; Giovannelli, L. Modulation of the Senescence-Associated Inflammatory Phenotype in Human Fibroblasts by Olive Phenols. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Yang, C.C.; Shyur, L.F. Phytomedicine-Modulating Oxidative Stress and the Tumor Microenvironment for Cancer Therapy. Pharmacol. Res. 2016, 114, 128–143. [Google Scholar] [CrossRef] [PubMed]
- D’Ignazio, L.; Batie, M.; Rocha, S. Hypoxia and Inflammation in Cancer, Focus on HIF and NF-kappaB. Biomedicines 2017, 5. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra Virgin Olive Oil and Cardiovascular Diseases: Benefits for Human Health. Endocr Metab. Immune Disord. Drug Targets 2018, 18, 4–13. [Google Scholar] [CrossRef]
- Crespo, M.C.; Tomé-Carneiro, J.; Dávalos, A.; Visioli, F. Pharma-Nutritional Properties of Olive Oil Phenols. Transfer of New Findings to Human Nutrition. Foods 2018, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Buil-Cosiales, P.; Irimia, P.; Berrade, N.; Garcia-Arellano, A.; Riverol, M.; Murie-Fernández, M.; Martínez-Vila, E.; Martínez-González, M.A.; Serrano-Martínez, M. Carotid intima-media thickness is inversely associated with olive oil consumption. Atherosclerosis 2008, 196, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Sacanella, E.; Urpí-Sardà, M.; Chiva-Blanch, G.; Ros, E.; Martínez-González, M.A.; Covas, M.I.; Rosa, M.A.; Lamuela, R.; Salas-Salvadó, J.; et al. The effects of the mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS ONE 2014, 9, e100084. [Google Scholar] [CrossRef] [PubMed]
- Bullon, P.; Quiles, J.L.; Morillo, J.M.; Rubini, C.; Goteri, G.; Granados-Principal, S.; Battino, M.; Ramirez-Tortosa, M. Gingival Vascular Damage in Atherosclerotic Rabbits: Hydroxytyrosol and Squalene Benefits. Food Chem. Toxicol. 2009, 47, 2327–2331. [Google Scholar] [CrossRef]
- Rosenblat, M.; Volkova, N.; Coleman, R.; Almagor, Y.; Aviram, M. Antiatherogenicity of Extra Virgin Olive Oil and its Enrichment with Green Tea Polyphenols in the Atherosclerotic Apolipoprotein-E-Deficient Mice: Enhanced Macrophage Cholesterol Efflux. J. Nutr. Biochem. 2008, 19, 514–523. [Google Scholar] [CrossRef]
- Lou-Bonafonte, J.M.; Arnal, C.; Navarro, M.A.; Osada, J. Efficacy of Bioactive Compounds from Extra Virgin Olive Oil to Modulate Atherosclerosis Development. Mol. Nutr. Food Res. 2012, 56, 1043–1057. [Google Scholar] [CrossRef]
- Filipek, A.; Czerwinska, M.E.; Kiss, A.K.; Polanski, J.A.; Naruszewicz, M. Oleacein may Inhibit Destabilization of Carotid Plaques from Hypertensive Patients. Impact on High Mobility Group Protein-1. Phytomedicine 2017, 32, 68–73. [Google Scholar] [CrossRef]
- Torres-Pena, J.D.; Garcia-Rios, A.; Delgado-Casado, N.; Gomez-Luna, P.; Alcala-Diaz, J.F.; Yubero-Serrano, E.M.; Gomez-Delgado, F.; Leon-Acuna, A.; Lopez-Moreno, J.; Camargo, A.; et al. Mediterranean Diet Improves Endothelial Function in Patients with Diabetes and Prediabetes: A Report from the CORDIOPREV Study. Atherosclerosis 2018, 269, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.R.; Hodgson, J.M.; Woodman, R.; Bryan, J.; Wilson, C.; Murphy, K.J. A Mediterranean Diet Lowers Blood Pressure and Improves Endothelial Function: Results from the MedLey Randomized Intervention Trial. Am. J. Clin. Nutr. 2017, 105, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Villarejo, A.B.; Ramirez-Sanchez, M.; Segarra, A.B.; Martinez-Canamero, M.; Prieto, I. Influence of Extra Virgin Olive Oil on Blood Pressure and Kidney Angiotensinase Activities in Spontaneously Hypertensive Rats. Planta Med. 2015, 81, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, C.E.; Rosello-Catafau, J.; Pinto, X.; Mitjavila, M.T.; Moreno, J.J. Polyphenol Fraction of Extra Virgin Olive Oil Protects Against Endothelial Dysfunction Induced by High Glucose and Free Fatty Acids through Modulation of Nitric Oxide and Endothelin-1. Redox Biol. 2014, 2, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.I.; Kaliora, A.C.; Papalois, A.; Chiou, A.; Kalogeropoulos, N.; Agrogiannis, G.; Andrikopoulos, N.K. Serum Lipid Profile and Inflammatory Markers in the Aorta of Cholesterol-Fed Rats Supplemented with Extra Virgin Olive Oil, Sunflower Oils and Oil-Products. Int. J. Food Sci. Nutr. 2015, 66, 766–773. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, S.; Vacca, M.; Cariello, M.; Graziano, G.; D’Orazio, A.; Salvia, R.; Sasso, R.C.; Sabba, C.; Palasciano, G.; Moschetta, A. Genes and miRNA Expression Signatures in Peripheral Blood Mononuclear Cells in Healthy Subjects and Patients with Metabolic Syndrome After Acute Intake of Extra Virgin Olive Oil. Biochim. Biophys. Acta 2016, 1861, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Estruch, R.; Sacanella, E. The Protective Effects of Extra Virgin Olive Oil on Immune-Mediated Inflammatory Responses. Endocr Metab. Immune Disord. Drug Targets 2018, 18, 23–35. [Google Scholar] [CrossRef]
- Khaw, K.T.; Sharp, S.J.; Finikarides, L.; Afzal, I.; Lentjes, M.; Luben, R.; Forouhi, N.G. Randomised Trial of Coconut Oil, Olive Oil Or Butter on Blood Lipids and Other Cardiovascular Risk Factors in Healthy Men and Women. BMJ Open 2018, 8, e020167. [Google Scholar] [CrossRef]
- Anderson-Vasquez, H.E.; Perez-Martinez, P.; Ortega Fernandez, P.; Wanden-Berghe, C. Impact of the Consumption of a Rich Diet in Butter and it Replacement for a Rich Diet in Extra Virgin Olive Oil on Anthropometric, Metabolic and Lipid Profile in Postmenopausal Women. Nutr. Hosp. 2015, 31, 2561–2570. [Google Scholar]
- Maki, K.C.; Lawless, A.L.; Kelley, K.M.; Kaden, V.N.; Geiger, C.J.; Palacios, O.M.; Dicklin, M.R. Corn Oil Intake Favorably Impacts Lipoprotein Cholesterol, Apolipoprotein and Lipoprotein Particle Levels Compared with Extra-Virgin Olive Oil. Eur. J. Clin. Nutr. 2017, 71, 33–38. [Google Scholar] [CrossRef]
- Violi, F.; Loffredo, L.; Pignatelli, P.; Angelico, F.; Bartimoccia, S.; Nocella, C.; Cangemi, R.; Petruccioli, A.; Monticolo, R.; Pastori, D.; et al. Extra Virgin Olive Oil use is Associated with Improved Post-Prandial Blood Glucose and LDL Cholesterol in Healthy Subjects. Nutr. Diabetes 2015, 5, e172. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, A.; Castaner, O.; Goday, A.; Ros, E.; Pinto, X.; Estruch, R.; Salas-Salvado, J.; Corella, D.; Aros, F.; Serra-Majem, L.; et al. The Mediterranean Diet Decreases LDL Atherogenicity in High Cardiovascular Risk Individuals: A Randomized Controlled Trial. Mol. Nutr. Food Res. 2017, 61, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferre, M.; et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevencion Con Dieta Mediterranea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Wang, D.D.; Ruiz-Canela, M.; Clish, C.B.; Razquin, C.; Zheng, Y.; Guasch-Ferre, M.; Hruby, A.; Corella, D.; Gomez-Gracia, E.; et al. Plasma Lipidomic Profiles and Cardiovascular Events in a Randomized Intervention Trial with the Mediterranean Diet. Am. J. Clin. Nutr. 2017, 106, 973–983. [Google Scholar] [PubMed]
- Hao, J.; Shen, W.; Yu, G.; Jia, H.; Li, X.; Feng, Z.; Wang, Y.; Weber, P.; Wertz, K.; Sharman, E.; et al. Hydroxytyrosol Promotes Mitochondrial Biogenesis and Mitochondrial Function in 3T3-L1 Adipocytes. J. Nutr. Biochem. 2010, 21, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Giordano, E.; Davalos, A.; Visioli, F. Chronic Hydroxytyrosol Feeding Modulates Glutathione-Mediated Oxido-Reduction Pathways in Adipose Tissue: A Nutrigenomic Study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1144–1150. [Google Scholar] [CrossRef]
- Ricordi, C.; Garcia-Contreras, M.; Farnetti, S. Diet and Inflammation: Possible Effects on Immunity, Chronic Diseases, and Life Span. J. Am. Coll. Nutr. 2015, 34, 10–13. [Google Scholar] [CrossRef]
- Puertollano, M.A.; Puertollano, E.; Alvarez de Cienfuegos, G.; de Pablo Martinez, M.A. Olive Oil, Immune System and Infection. Nutricion Hospit. 2010, 25, 1–8. [Google Scholar]
- Montserrat-de la Paz, S.; Naranjo, M.C.; Lopez, S.; Abia, R.; Muriana, F.J.; Bermudez, B. Niacin and Olive Oil Promote Skewing to the M2 Phenotype in Bone Marrow-Derived Macrophages of Mice with Metabolic Syndrome. Food Funct. 2016, 7, 2233–2238. [Google Scholar] [CrossRef]
- Magdalon, J.; Vinolo, M.A.; Rodrigues, H.G.; Paschoal, V.A.; Torres, R.P.; Mancini-Filho, J.; Calder, P.C.; Hatanaka, E.; Curi, R. Oral Administration of Oleic Or Linoleic Acids Modulates the Production of Inflammatory Mediators by Rat Macrophages. Lipids 2012, 47, 803–812. [Google Scholar] [CrossRef]
- De La Puerta Vazquez, R.; Martinez-Dominguez, E.; Sanchez Perona, J.; Ruiz-Gutierrez, V. Effects of Different Dietary Oils on Inflammatory Mediator Generation and Fatty Acid Composition in Rat Neutrophils. Metabol. Clin. Exp. 2004, 53, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, E.; Levada-Pires, A.C.; Pithon-Curi, T.C.; Curi, R. Systematic Study on ROS Production Induced by Oleic, Linoleic, and Gamma-Linolenic Acids in Human and Rat Neutrophils. Free Radic. Biol. Med. 2006, 41, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Cury-Boaventura, M.F.; Gorjao, R.; de Lima, T.M.; Fiamoncini, J.; Torres, R.P.; Mancini-Filho, J.; Soriano, F.G.; Curi, R. Effect of Olive Oil-Based Emulsion on Human Lymphocyte and Neutrophil Death. JPEN 2008, 32, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Soto, M.; Sanchez-Hidalgo, M.; Cardeno, A.; Rosillo, M.A.; Sanchez-Fidalgo, S.; Utrilla, J.; Martin-Lacave, I.; Alarcon-de-la-Lastra, C. Dietary Extra Virgin Olive Oil Attenuates Kidney Injury in Pristane-Induced SLE Model Via Activation of HO-1/Nrf-2 Antioxidant Pathway and Suppression of JAK/STAT, NF-kappaB and MAPK Activation. J. Nutr. Biochem. 2016, 27, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Tontini, G.E.; Vecchi, M.; Pastorelli, L.; Neurath, M.F.; Neumann, H. Differential Diagnosis in Inflammatory Bowel Disease Colitis: State of the Art and Future Perspectives. World J. Gastroenterol. 2015, 21, 21–46. [Google Scholar] [CrossRef] [PubMed]
- Orel, R.; Kamhi Trop, T. Intestinal Microbiota, Probiotics and Prebiotics in Inflammatory Bowel Disease. World J. Gastroenterol. 2014, 20, 11505–11524. [Google Scholar] [CrossRef] [PubMed]
- Durchschein, F.; Petritsch, W.; Hammer, H.F. Diet Therapy for Inflammatory Bowel Diseases: The Established and the New. World J. Gastroenterol. 2016, 22, 2179–2194. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Wolf, A.; Parian, A.M. Nutritional Interventions in the Patient with Inflammatory Bowel Disease. Gastroenterol. Clin. North. Am. 2018, 47, 155–177. [Google Scholar] [CrossRef]
- Cabre, E.; Domenech, E. Impact of Environmental and Dietary Factors on the Course of Inflammatory Bowel Disease. World J. Gastroenterol. 2012, 18, 3814–3822. [Google Scholar] [CrossRef]
- Sanchez-Fidalgo, S.; Sanchez de Ibarguen, L.; Cardeno, A.; Alarcon de la Lastra, C. Influence of Extra Virgin Olive Oil Diet Enriched with Hydroxytyrosol in a Chronic DSS Colitis Model. Eur. J. Nutr. 2012, 51, 497–506. [Google Scholar] [CrossRef]
- Sanchez-Fidalgo, S.; Cardeno, A.; Sanchez-Hidalgo, M.; Aparicio-Soto, M.; Villegas, I.; Rosillo, M.A.; de la Lastra, C.A. Dietary Unsaponifiable Fraction from Extra Virgin Olive Oil Supplementation Attenuates Acute Ulcerative Colitis in Mice. Eur. J. Pharm. Sci. 2013, 48, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fidalgo, S.; Cardeno, A.; Sanchez-Hidalgo, M.; Aparicio-Soto, M.; de la Lastra, C.A. Dietary Extra Virgin Olive Oil Polyphenols Supplementation Modulates DSS-Induced Chronic Colitis in Mice. J. Nutr. Biochem. 2013, 24, 1401–1413. [Google Scholar] [CrossRef]
- Sanchez-Fidalgo, S.; Villegas, I.; Rosillo, M.A.; Aparicio-Soto, M.; de la Lastra, C.A. Dietary Squalene Supplementation Improves DSS-Induced Acute Colitis by Downregulating p38 MAPK and NFkB Signaling Pathways. Mol. Nutr. Food Res. 2015, 59, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Cardeno, A.; Magnusson, M.K.; Strid, H.; Alarcon de La Lastra, C.; Sanchez-Hidalgo, M.; Ohman, L. The Unsaponifiable Fraction of Extra Virgin Olive Oil Promotes Apoptosis and Attenuates Activation and Homing Properties of T Cells from Patients with Inflammatory Bowel Disease. Food Chem. 2014, 161, 353–360. [Google Scholar] [CrossRef]
- Sanchez-Fidalgo, S.; Villegas, I.; Cardeno, A.; Talero, E.; Sanchez-Hidalgo, M.; Motilva, V.; Alarcon de la Lastra, C. Extra-Virgin Olive Oil-Enriched Diet Modulates DSS-Colitis-Associated Colon Carcinogenesis in Mice. Clin. Nutr. 2010, 29, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.M. The Burden of Musculoskeletal Disease—A Global Perspective. Clin. Rheumatol. 2006, 25, 778–781. [Google Scholar] [CrossRef]
- Zvaifler, N.J. A Speculation on the Pathogenesis of Joint Inflammation in Rheumatoid Arthritis. Arthritis Rheum. 1965, 8, 289–293. [Google Scholar] [CrossRef]
- Boissier, M.C.; Semerano, L.; Challal, S.; Saidenberg-Kermanac’h, N.; Falgarone, G. Rheumatoid Arthritis: From Autoimmunity to Synovitis and Joint Destruction. J. Autoimmun. 2012, 39, 222–228. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Alarcon-de-la-Lastra, C.; Sanchez-Hidalgo, M. An Update on Dietary Phenolic Compounds in the Prevention and Management of Rheumatoid Arthritis. Food Funct. 2016, 7, 2943–2969. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Sanchez-Fidalgo, S.; Aparicio-Soto, M.; Villegas, I.; Alarcon-de-la-Lastra, C. Dietary Extra-Virgin Olive Oil Prevents Inflammatory Response and Cartilage Matrix Degradation in Murine Collagen-Induced Arthritis. Eur. J. Nutr. 2016, 55, 315–325. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Alcaraz, M.J.; Sanchez-Hidalgo, M.; Fernandez-Bolanos, J.G.; Alarcon-de-la-Lastra, C.; Ferrandiz, M.L. Anti-Inflammatory and Joint Protective Effects of Extra-Virgin Olive-Oil Polyphenol Extract in Experimental Arthritis. J. Nutr. Biochem. 2014, 25, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M.; Gomez, R.; Conde, J.; Lopez, V.; Gomez-Reino, J.J.; Lago, F.; Smith, A.B., 3rd; Gualillo, O. Further Evidence for the Anti-Inflammatory Activity of Oleocanthal: Inhibition of MIP-1alpha and IL-6 in J774 Macrophages and in ATDC5 Chondrocytes. Life Sci. 2012, 91, 1229–1235. [Google Scholar] [CrossRef]
- Iacono, A.; Gomez, R.; Sperry, J.; Conde, J.; Bianco, G.; Meli, R.; Gomez-Reino, J.J.; Smith, A.B., 3rd; Gualillo, O. Effect of Oleocanthal and its Derivatives on Inflammatory Response Induced by Lipopolysaccharide in a Murine Chondrocyte Cell Line. Arthritis Rheum. 2010, 62, 1675–1682. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Esposito, E.; Mazzon, E.; Paterniti, I.; Di Paola, R.; Morittu, V.M.; Procopio, A.; Britti, D.; Cuzzocrea, S. Oleuropein Aglycone, an Olive Oil Compound, Ameliorates Development of Arthritis Caused by Injection of Collagen Type II in Mice. J. Pharmacol. Exp. Ther. 2011, 339, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Castejon, M.L.; Rosillo, M.A.; Montoya, T.; Gonzalez-Benjumea, A.; Fernandez-Bolanos, J.G.; Alarcon-de-la-Lastra, C. Oleuropein Down-Regulated IL-1beta-Induced Inflammation and Oxidative Stress in Human Synovial Fibroblast Cell Line SW982. Food Funct. 2017, 8, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Sepodes, B.; Rocha, J.; Direito, R.; Fernandes, A.; Brites, D.; Freitas, M.; Fernandes, E.; Bronze, M.R.; Figueira, M.E. Protective Effects of Hydroxytyrosol-Supplemented Refined Olive Oil in Animal Models of Acute Inflammation and Rheumatoid Arthritis. J. Nutr. Biochem. 2015, 26, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Gonzalez-Benjumea, A.; Fernandez-Bolanos, J.G.; Lubberts, E.; Alarcon-de-la-Lastra, C. Preventive Effects of Dietary Hydroxytyrosol Acetate, an Extra Virgin Olive Oil Polyphenol in Murine Collagen-Induced Arthritis. Mol. Nutr. Food Res. 2015, 59, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Trujillo, M.; Pereira-Caro, G.; Madrona, A.; Cert, A.; Espartero, J.L. New Lipophilic Tyrosyl Esters. Comparative Antioxidant Evaluation with Hydroxytyrosyl Esters. J. Agric. Food Chem. 2008, 56, 10960–10966. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Pereira-Caro, G.; Saha, S.; Cert, R.; Redondo-Horcajo, M.; Bravo, L.; Kroon, P.A. Acetylation of Hydroxytyrosol Enhances its Transport Across Differentiated Caco-2 Cell Monolayers. Food Chem. 2011, 125, 865–872. [Google Scholar] [CrossRef]
- Rubio, L.; Macia, A.; Valls, R.M.; Pedret, A.; Romero, M.P.; Sola, R.; Motilva, M.J. A New Hydroxytyrosol Metabolite Identified in Human Plasma: Hydroxytyrosol Acetate Sulphate. Food Chem. 2012, 134, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Castejon, M.L.; Montoya, T.; Gonzalez-Benjumea, A.; Fernandez-Bolanos, J.G.; Alarcon de la Lastra, C. Extra-Virgin Olive Oil Phenols Hydroxytyrosol and Hydroxytyrosol Acetate, Down-Regulate the Production of Mediators Involved in Joint Erosion in Human Synovial Cells. J. Funct. Foods 2017, 36, 27–33. [Google Scholar] [CrossRef]
- Petri, M.; Orbai, A.M.; Alarcon, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and Validation of the Systemic Lupus International Collaborating Clinics Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Yin, H.; Wang, L.; Gershwin, M.E.; Lu, Q. The Critical Role of Epigenetics in Systemic Lupus Erythematosus and Autoimmunity. J. Autoimmun. 2016, 74, 118–138. [Google Scholar] [CrossRef]
- Chun, H.Y.; Chung, J.W.; Kim, H.A.; Yun, J.M.; Jeon, J.Y.; Ye, Y.M.; Kim, S.H.; Park, H.S.; Suh, C.H. Cytokine IL-6 and IL-10 as Biomarkers in Systemic Lupus Erythematosus. J. Clin. Immunol. 2007, 27, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Paley, M.A.; Strand, V.; Kim, A.H. From Mechanism to Therapies in Systemic Lupus Erythematosus. Curr. Opin. Rheumatol. 2017, 29, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Su, D.L.; Lu, Z.M.; Shen, M.N.; Li, X.; Sun, L.Y. Roles of Pro- and Anti-Inflammatory Cytokines in the Pathogenesis of SLE. J. Biomed. Biotechnol. 2012, 2012, 347141. [Google Scholar] [CrossRef] [PubMed]
- Leng, R.X.; Pan, H.F.; Chen, G.M.; Wang, C.; Qin, W.Z.; Chen, L.L.; Tao, J.H.; Ye, D.Q. IL-23: A Promising Therapeutic Target for Systemic Lupus Erythematosus. Arch. Med. Res. 2010, 41, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.F.; Tao, J.H.; Ye, D.Q. Therapeutic Potential of IL-27 in Systemic Lupus Erythematosus. Expert Opin. Ther. Targets 2010, 14, 479–484. [Google Scholar] [CrossRef]
- Cai, Z.; Wong, C.K.; Dong, J.; Chu, M.; Jiao, D.; Kam, N.W.; Lam, C.W.; Tam, L.S. Remission of Systemic Lupus Erythematosus Disease Activity with Regulatory Cytokine Interleukin (IL)-35 in Murphy Roths Large (MRL)/Lpr Mice. Clin. Exp. Immunol. 2015, 181, 253–266. [Google Scholar] [CrossRef]
- Greco, C.M.; Nakajima, C.; Manzi, S. Updated Review of Complementary and Alternative Medicine Treatments for Systemic Lupus Erythematosus. Curr. Rheumatol. Rep. 2013, 15, 378. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Sanchez-Hidalgo, M.; Cárdeno, A.; Gonzalez-Benjumea, A.; Fernandez-Bolanos, J.G.; Alarcon de la Lastra, C. Dietary Hydroxytyrosol and Hydroxytyrosyl Acetate Supplementation Prevent Pristane-Induced Systemic Lupus Erythematous in Mice. J. Funct. Foods 2017, 29, 84–92. [Google Scholar] [CrossRef]
- McCombe, P.A.; Henderson, R.D. The Role of Immune and Inflammatory Mechanisms in ALS. Curr. Mol. Med. 2011, 11, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Olivan, S.; Martinez-Beamonte, R.; Calvo, A.C.; Surra, J.C.; Manzano, R.; Arnal, C.; Osta, R.; Osada, J. Extra Virgin Olive Oil Intake Delays the Development of Amyotrophic Lateral Sclerosis Associated with Reduced Reticulum Stress and Autophagy in Muscle of SOD1G93A Mice. J. Nutr. Biochem. 2014, 25, 885–892. [Google Scholar] [CrossRef] [PubMed]
- De Paola, M.; Sestito, S.E.; Mariani, A.; Memo, C.; Fanelli, R.; Freschi, M.; Bendotti, C.; Calabrese, V.; Peri, F. Synthetic and Natural Small Molecule TLR4 Antagonists Inhibit Motoneuron Death in Cultures from ALS Mouse Model. Pharmacol. Res. 2016, 103, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Milo, R.; Miller, A. Revised Diagnostic Criteria of Multiple Sclerosis. Autoimmun. Rev. 2014, 13, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Sospedra, M.; Martin, R. Immunology of Multiple Sclerosis. Annu. Rev. Immunol. 2005, 23, 683–747. [Google Scholar] [CrossRef]
- Steinman, L. Assessment of Animal Models for MS and Demyelinating Disease in the Design of Rational Therapy. Neuron 1999, 24, 511–514. [Google Scholar] [CrossRef]
- Martin, R.; Carvalho-Tavares, J.; Hernandez, M.; Arnes, M.; Ruiz-Gutierrez, V.; Nieto, M.L. Beneficial Actions of Oleanolic Acid in an Experimental Model of Multiple Sclerosis: A Potential Therapeutic Role. Biochem. Pharmacol. 2010, 79, 198–208. [Google Scholar] [CrossRef]
- Martin, R.; Hernandez, M.; Cordova, C.; Nieto, M.L. Natural Triterpenes Modulate Immune-Inflammatory Markers of Experimental Autoimmune Encephalomyelitis: Therapeutic Implications for Multiple Sclerosis. Br. J. Pharmacol. 2012, 166, 1708–1723. [Google Scholar] [CrossRef]
- Belanger, M.; Magistretti, P.J. The Role of Astroglia in Neuroprotection. Dialogues Clin. Neurosci. 2009, 11, 281–295. [Google Scholar]
- Liuzzi, G.M.; Latronico, T.; Brana, M.T.; Gramegna, P.; Coniglio, M.G.; Rossano, R.; Larocca, M.; Riccio, P. Structure-Dependent Inhibition of Gelatinases by Dietary Antioxidants in Rat Astrocytes and Sera of Multiple Sclerosis Patients. Neurochem. Res. 2011, 36, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R. The Eu Olive and Olive Oil Sector Main Features, Challenges and Prospects. PE 608.690. Briefing September 2017. European Parliamentary Research Service (EPRS). Members’ Research Service. Available online: https://www.oliveoiltimes.com/library/eu-sector-challenges.pdf (accessed on 17 September 2017).
- Feng, Y.; Zhang, A. A Floral Fragrance, Methyl Benzoate, is an Efficient Green Pesticide. Sci. Rep. 2017, 7, 42168. [Google Scholar] [CrossRef] [PubMed]
- Pretty, J.; Bharucha, Z. Integrated Pest Management for Sustainable Intensification of Agriculture in Asia and Africa. Insects 2015, 6, 152–182. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Short-Term Outlook for EU Agricultural Markets in 2017 and 2018; Directorate-General for Agriculture and Rural Development–Shortterm Outlook–N°17: Brussels, Belgium, 2017; p. 9. [Google Scholar]
- Gianessi, L.; Williams, A. Herbicide use in Spanish Olive Groves Conserves Soil and Water, International Pesticide Benefits case Study No. 38. Available online: https://Croplife.Org/Case-Study/Herbicide-use-in-Spanish-Olive-Groves-Conserves-Soil-and-Water/ (accessed on 30 August 2019).
- EEA (European Environment Agency). Pesticide Sales. Annual Indicator Report Series (AIRS) PO3.7, 2017. Available online: https://www.eea.europa.eu/airs/2018/environment-and-health/pesticides-sales. (accessed on 30 August 2019).
- Sporleder, M.; Lacey, L. Biopesticides. In: Insect Pests of Potato. In Global Perspectives on Biology and Managemen; Giordanengo, P., Vincent, C., Alyokhin, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 463–497. [Google Scholar]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public. Health. 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Kesavachandran, C.N.; Fareed, M.; Pathak, M.K.; Bihari, V.; Mathur, N.; Srivastava, A.K. Adverse Health Effects of Pesticides in Agrarian Populations of Developing Countries. Rev. Environ. Contam. Toxicol. 2009, 200, 33–52. [Google Scholar] [PubMed]
- Ntzani, E.; Chondrogiorgi, M.; Ntritsos, G.; Evangelou, E.; Tzoulaki, I. Literature Review on Epidemiological Studies Linking Exposure to Pesticides and Health Effects; EFSA Supporting Publication: Parma, Italy, 2013; Volume 497, p. 159. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/sp.efsa.2013.EN-497 (accessed on 30 August 2019).
- Miller, G.T. Sustaining the Earth: An integrated Approach, 6th ed.; Thompson Learning Inc.: Pacific Grove, CA, USA, 2004; pp. 211–216. [Google Scholar]
- Safa, M.; Watts, M. Energy Inputs in Pest Control using Pesticides in New Zealand. In Integrated Pest Management; Pimentel, D.P., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 99–126. [Google Scholar]
- Randall, C.; Hock, W.; Crow, E.; Hudak-Wise, C.; Kasai, J. Pesticides in the environment. In: Maryland Pesticide applicators core manual, National Association of State Departments of Agriculture Research Foundation Ed.; Arlington, VA, USA, 2008. pp. 105–122. Available online: https://mda.maryland.gov/plants-pests/Documents/MD%20Core%20Manual.pdf (accessed on 30 August 2019).
- Beketov, M.A.; Kefford, B.J.; Schafer, R.B.; Liess, M. Pesticides Reduce Regional Biodiversity of Stream Invertebrates. Proc. Natl. Acad. Sci. USA 2013, 110, 11039–11043. [Google Scholar] [CrossRef] [PubMed]
- Sadegh-Zadeh, F.; Abd-Wahid, S.; Jalili, B. Sorption, Degradation and Leaching of Pesticides in Soils Amended with Organic Matter: A Review. Adv. Environ. Tech. 2017, 2, 119–132. [Google Scholar]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of Pesticides use in Agriculture: Their Benefits and Hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Palmer, W.; Bromley, P.; Brandenburg, R. Wildlife & Pesticides-Peanuts; North Carolina Cooperative Extension Service: Raleigh, NC, USA, 2007. [Google Scholar]
- Cornell University. Pesticides in the Environment. In Pesticide Fact Sheets and Tutorial, Module, 6th ed.; Pesticide Safety Education Program: Urbana, IL, USA, 2007. [Google Scholar]
- Kohler, H.R.; Triebskorn, R. Wildlife Ecotoxicology of Pesticides: Can we Track Effects to the Population Level and Beyond? Science 2013, 341, 759–765. [Google Scholar] [CrossRef]
- Hanazato, T. Pesticide Effects on Freshwater Zooplankton: An Ecological Perspective. Environ. Pollut. 2001, 112, 1–10. [Google Scholar] [CrossRef]
- Helfrich, L.; Weigmann, D.; Hipkins, P.; Stinson, E. Pesticides and Aquatic Animals: A Guide to Reducing Impacts on Aquatic Systems; Virginia Cooperative Extension Publication: Blacksburg, VA, USA, 1996. [Google Scholar]
- EFSA (European Food Safety Authority). The 2015 Annual EU Monitoring Programme for Pesticide Residues in Food. EFSA J. 2017, 15, 4791. Available online: https://Efsa.Onlinelibrary.Wiley.Com/Doi/Epdf/10.2903/J.Efsa.2017.4791 (accessed on 22 May 2018).
- Beaufoy, G. The Environmental Impact of Olive Oil Production in the European Union: Practical Options for Improving the Environmental Impact. 2001. Available online: http://Ec.Europa.Eu/Environment/Agriculture/Pdf/Oliveoil.Pdf (accessed on 30 May 2018).
- Grovermann, C.; Schreinemachers, P.; Berger, T. Quantifying Pesticide Overuse from Farmer and Societal Points of View: An Application to Thailand. Crop. Prot 2013, 53, 161–168. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Foscolou, A.; Critselis, E.; Panagiotakos, D. Olive oil consumption and human health: A narrative review. Maturitas 2018, 118, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Bazal, P.; Gea, A.; de la Fuente-Arrillaga, C.; Barrio-López, M.T.; Martinez-González, M.A.; Ruiz-Canela, M. Olive oil intake and risk of atrial fibrillation in the SUN cohort. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 450–457. [Google Scholar] [CrossRef]
- Gavahian, M.; Khaneghah, A.; Lorenzo, J.M.; Munekata, P.; Garcia-Mantrana, I.; Collado, M.C.; Meléndez-Martínez, A.J.; Barba, F.J. Health benefits of olive oil and its components: Impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci. Technol. 2019, 88, 220–227. [Google Scholar] [CrossRef]
- Fernandes, J.; Fialho, M.; Santos, R.; Peixoto-Plácido, C.; Madeira, T.; Sousa-Santos, N.; Virgolino, A.; Santos, O.; Vaz Carneiro, A. Is olive oil good for you? A systematic review and meta-analysis on anti-inflammatory benefits from regular dietary intake. Nutrition 2019, 110559. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- López-Miranda, J.; Pérez-Jiménez, F.; Ros, E.; De Caterina, R.; Badimón, L.; Covas, M.I.; Escrich, E.; Ordovás, J.M.; Soriguer, F.; Abiá, R.; et al. Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.A.; Price, T.O.; Dominguez, L.J.; Motisi, A.; Saiano, F.; Niehoff, M.L.; Morley, J.E.; Banks, W.A.; Ercal, N.; Barbagallo, M. Extra virgin olive oil improves learning and memory in SAMP8 mice. J. Alzheimers Dis. 2012, 28, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M. The relevance of nutrition for the concept of cognitive frailty. Curr. Opin. Clin. Nut.r Metab. Care. 2017, 20, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Mediterranean and MIND Diets Containing Olive Biophenols Reduces the Prevalence of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 2797. [Google Scholar] [CrossRef] [PubMed]
| Experimental Models | Design | Effects | References |
|---|---|---|---|
| EVOO and atherosclerosis | |||
| High risk subjects from PREDIMED | Mediterranean diet with EVOO compared to low-fat diet | Low Intima-media thickness (IMT) | [118,119] |
| Apolipoprotein E-deficient mice | EVOO | Decreased atherosclerosis | [120,121] |
| Rabbit | Hydroxytyrosol or squalene | Decreased gingival vascular damage | [122] |
| Human plaques | Oleacin incubation | Reduced secretion of metalloproteinases | [123] |
| EVOO and endothelial dysfunction | |||
| Patients with prediabetes and diabetes participating in the CORDIOPREV clinical trial | Mediterranean diet with high EVOO compared to the same diet with low EVOO | Improved endothelial function | [57,124] |
| Healthy men and women | MedDiet rich in EVOO vs. regular diet | Lower systolic blood pressure and improved endothelial function | [125] |
| High risk subjects from PREDIMED | Mediterranean diet with EVOO compared to low-fat diet | Lower diastolic blood pressure | [59] |
| Hypertensive rats | Diet enriched in EVOO compared to chow diet | Decreased systolic blood pressure. Decreased oxide nitric (NO) and 8-isoprostane | [126] |
| ECV304 incubated with high glucose and fatty acids | Incubation with phenolic compounds | Increased endothelial NO synthase phosphorylation and NO levels. Decreased endothelin-1 | [127] |
| Wistar rats | EVOO with or without phenolic compounds compared to control animals | Decreased vascular endothelial adhesion molecule-1 and E-selectin | [128] |
| EVOO and transcriptomics | |||
| PBMC of healthy and metabolic syndrome subjects | Acute intake of high phenolic EVOO | Less deleterious inflammatory phenotype | [129] |
| EVOO and plasma lipids | |||
| Healthy subjects | 50 g daily of extra virgin coconut oil, EVOO or unsalted butter for 4 weeks | EVOO decreased total cholesterol/HDL-C ratio and non-HDL-C compared with butter. EVOO and coconut oil resulted in similar results for both parameters | [130] |
| Postmenopausal women | Butter or EVOO | EVOO decreased total/HDL-cholesterol and triglycerides/HDL-cholesterol | [131] |
| Healthy subjects (men and women) | 54 g of corn oil and EVOO for 21 days | EVOO increased non-HDL cholesterol compared to corn oil. No differences on HDL-cholesterol but APOA1 increased more with EVOO compared with corn oil | [132] |
| Healthy subjects | Post-prandial 2-h lipid profile of subjects consuming Mediterranean-type meal with 10 g of EVOO or corn oil | EVOO produced less increase of LDL-C and ox-LDL compared with the corn oil | [133] |
| High risk subjects from PREDIMED | Mediterranean diet with EVOO compared to low-fat diet | EVOO increased LDL resistance against oxidation and decreased degree of oxidized LDL | [134] |
| High risk subjects from PREDIMED | Mediterranean diet with EVOO compared to low-fat diet | EVOO increased cholesterol efflux capacity, decreased cholesteryl ester transfer protein activity and increased HDL ability to esterify cholesterol, paraoxonase-1 arylesterase activity, and HDL vasodilatory capacity resulting in a more functional HDL | [72] |
| Healthy European male volunteers | Subjects received 25 mL/d EVOO of high phenolic content compared to the same a phenolic–poor EVOO for 3 weeks | Increased cholesterol efflux capacity | [70] |
| Apolipoprotein E-deficient mice | EVOO at 7 µl/mouse/day for 2 months stimulated | EVOO increased cholesterol efflux rate from mouse peritoneal macrophages | [121] |
| High risk subjects from PREDIMED | Mediterranean diet with EVOO compared to low-fat diet | Decreased plasma ceramide levels | [136] |
| High risk subjects from PREDIMED | Mediterranean diet with EVOO compared to low-fat diet | Changed 20 lipid species | [137] |
| EVOO and adipose tissue metabolism | |||
| 3T3-L1 adipocytes | Hydroxytyrosol | Increased mitochondrial biogenesis | [138] |
| Mouse adipose tissue | Hydroxytyrosol | Increased glutathione-driven antioxidant enzymatic machinery | [139] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaforio, J.J.; Visioli, F.; Alarcón-de-la-Lastra, C.; Castañer, O.; Delgado-Rodríguez, M.; Fitó, M.; Hernández, A.F.; Huertas, J.R.; Martínez-González, M.A.; Menendez, J.A.; et al. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients 2019, 11, 2039. https://doi.org/10.3390/nu11092039
Gaforio JJ, Visioli F, Alarcón-de-la-Lastra C, Castañer O, Delgado-Rodríguez M, Fitó M, Hernández AF, Huertas JR, Martínez-González MA, Menendez JA, et al. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients. 2019; 11(9):2039. https://doi.org/10.3390/nu11092039
Chicago/Turabian StyleGaforio, José J., Francesco Visioli, Catalina Alarcón-de-la-Lastra, Olga Castañer, Miguel Delgado-Rodríguez, Monserrat Fitó, Antonio F. Hernández, Jesús R. Huertas, Miguel A. Martínez-González, Javier A. Menendez, and et al. 2019. "Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018" Nutrients 11, no. 9: 2039. https://doi.org/10.3390/nu11092039
APA StyleGaforio, J. J., Visioli, F., Alarcón-de-la-Lastra, C., Castañer, O., Delgado-Rodríguez, M., Fitó, M., Hernández, A. F., Huertas, J. R., Martínez-González, M. A., Menendez, J. A., Osada, J. d. l., Papadaki, A., Parrón, T., Pereira, J. E., Rosillo, M. A., Sánchez-Quesada, C., Schwingshackl, L., Toledo, E., & Tsatsakis, A. M. (2019). Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients, 11(9), 2039. https://doi.org/10.3390/nu11092039