Racial Variations in Appetite-Related Hormones, Appetite, and Laboratory-Based Energy Intake from the E-MECHANIC Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcome Variables
2.2.1. Anthropometry and Body Composition
2.2.2. Laboratory Food Intake Tests
2.3. Statistical Analysis
3. Results
3.1. Participants
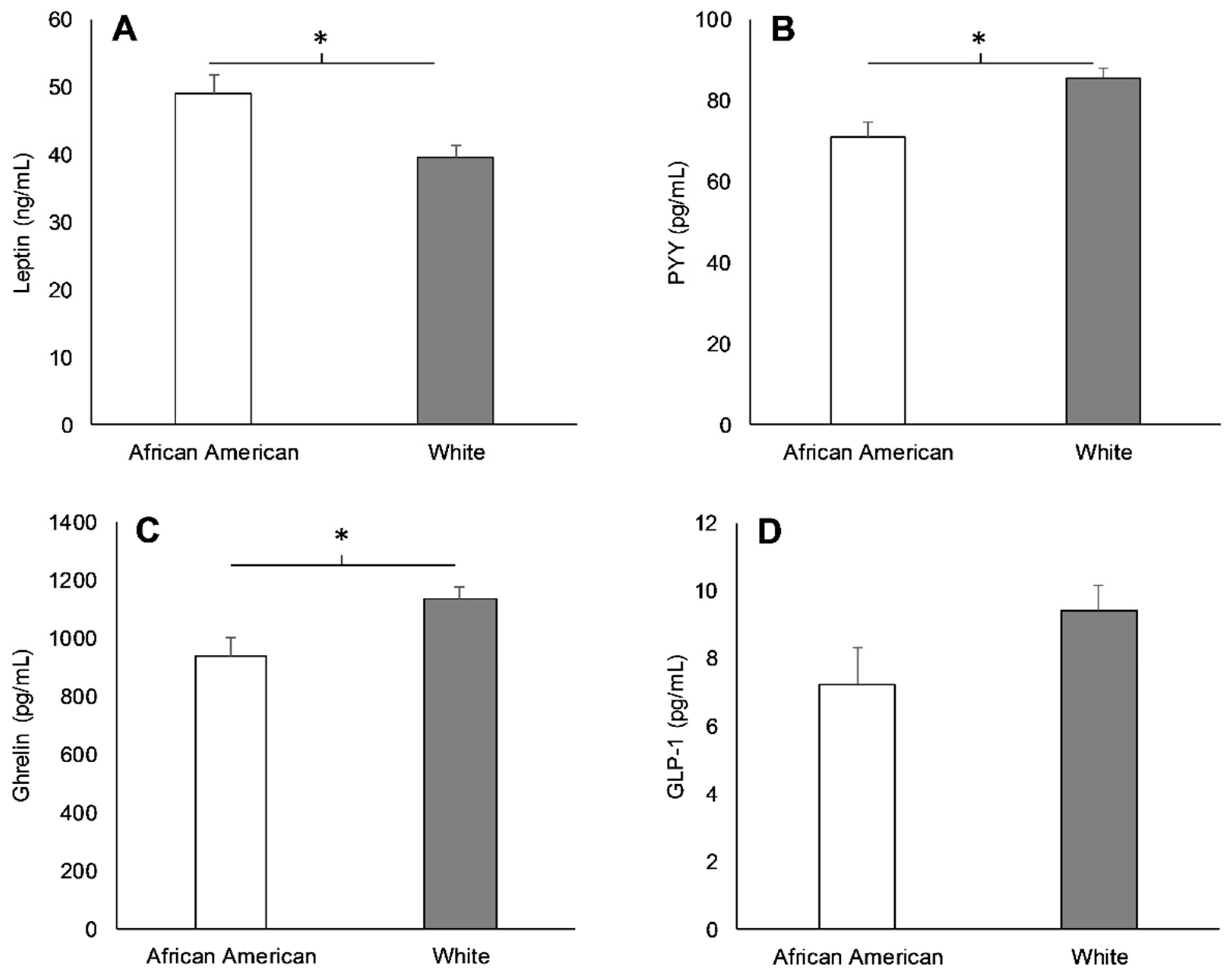
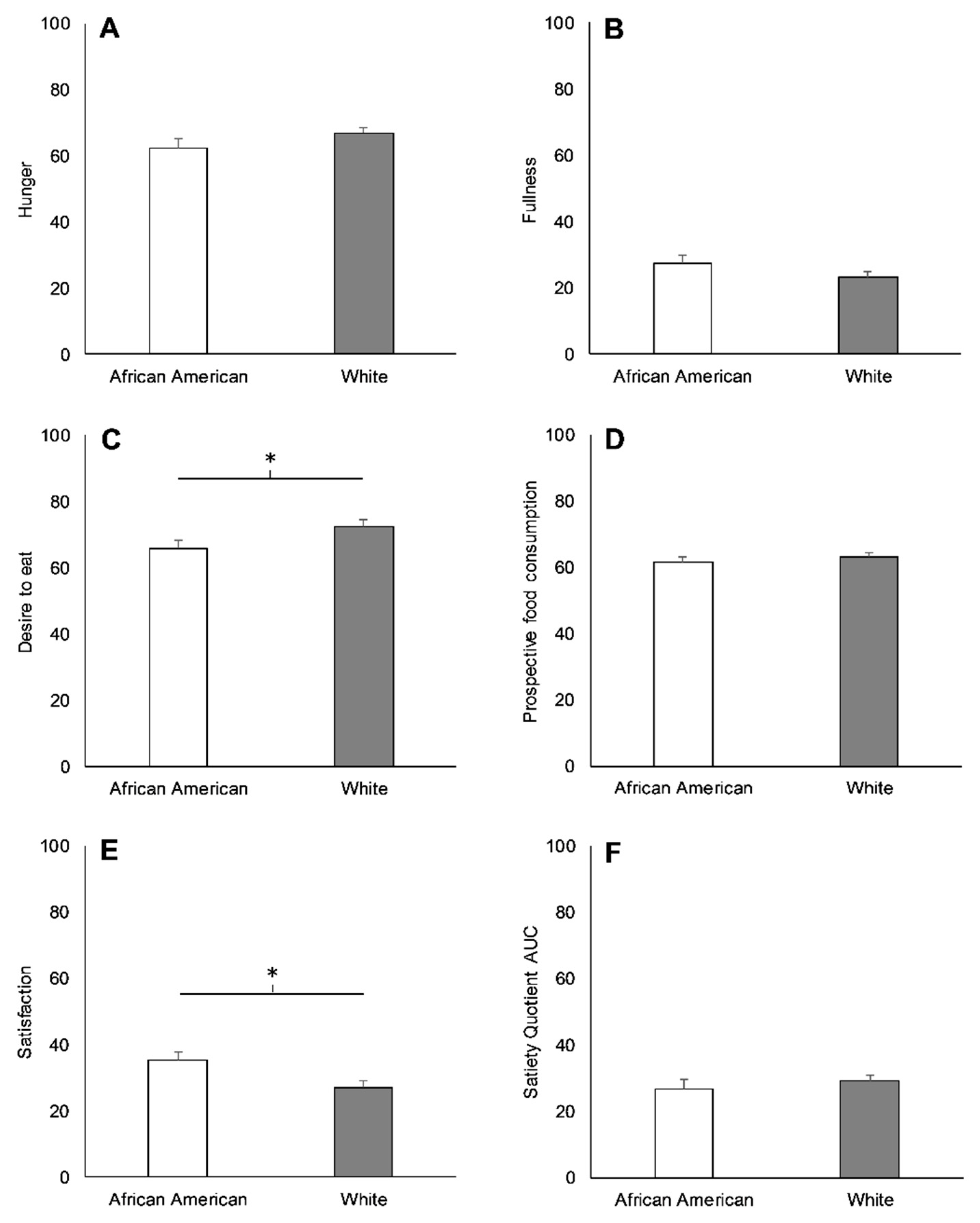
3.2. Racial Differences in Appetite-Related Hormones, Appetite, and Food Intake at Baseline
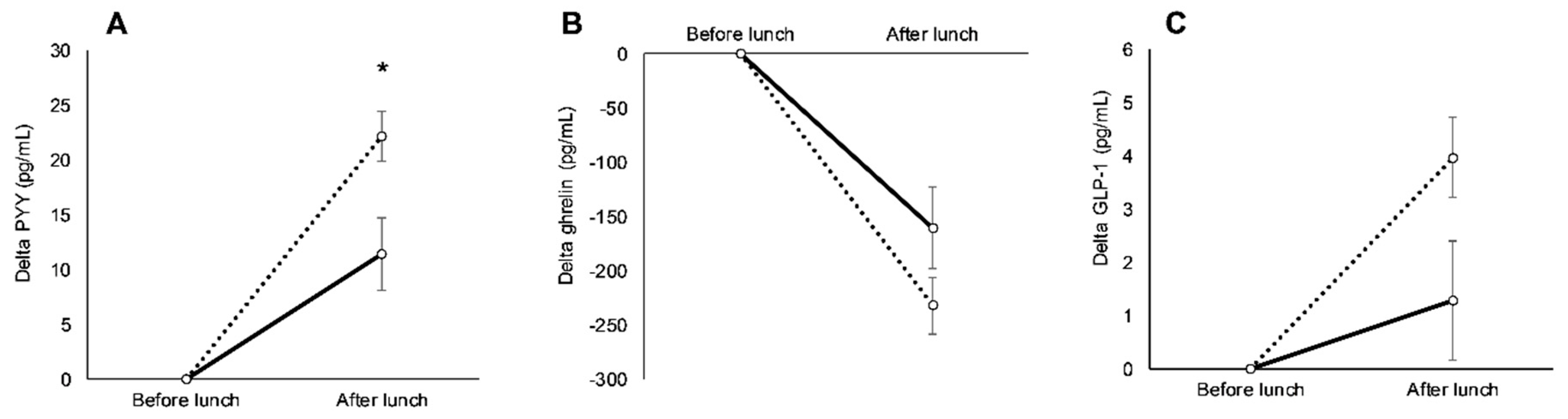
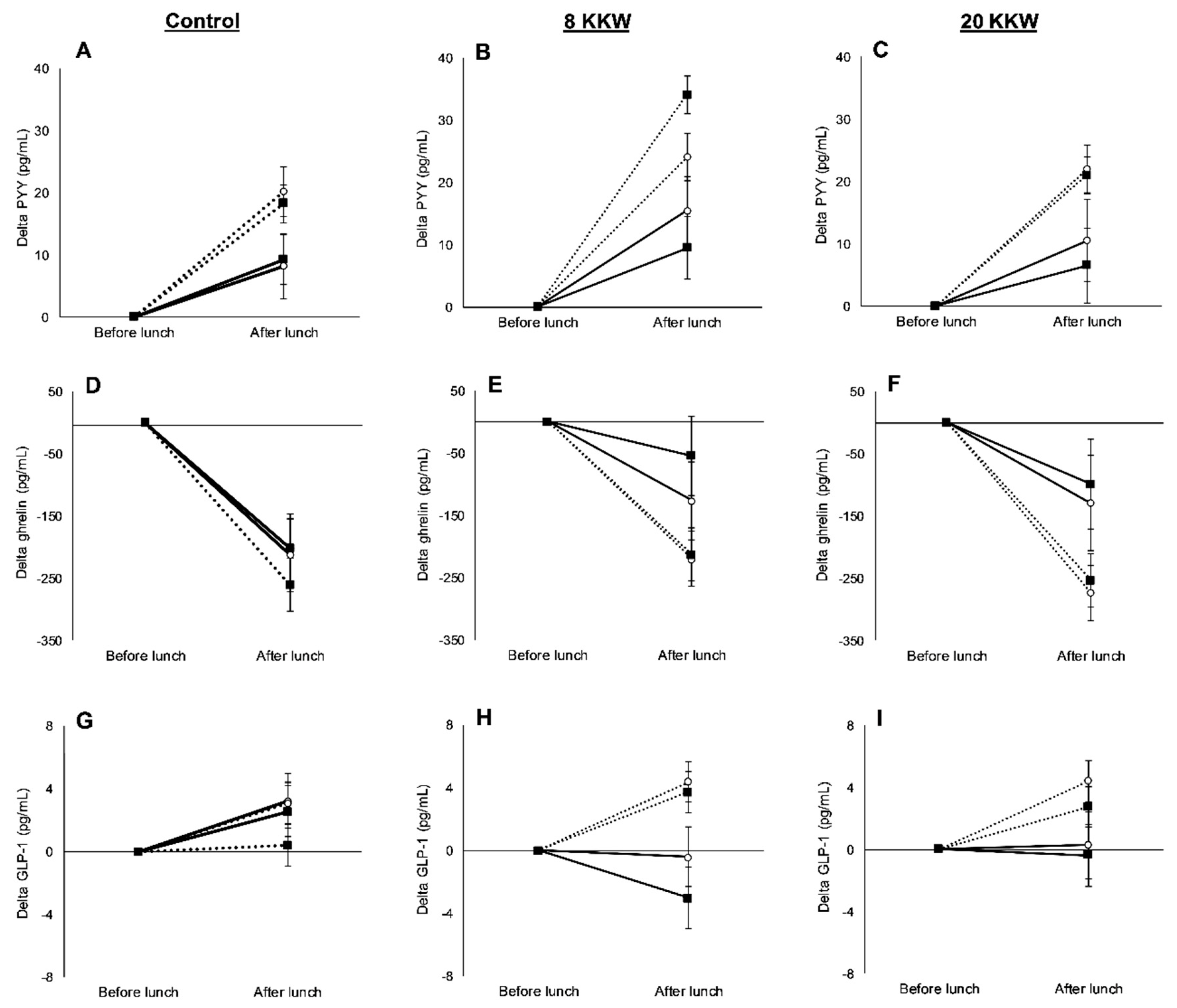
3.3. Racial Variations in Appetite-Related Hormones, Appetite, and Food Intake in Response to Exercise
3.4. Racial Variations in Anthropometry and Body Composition in Response to Exercise
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cossrow, N.; Falkner, B. Race/ethnic issues in obesity and obesity-related comorbidities. J. Clin. Endocrinol. Metab. 2004, 89, 2590–2594. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA J. Am. Med. Assoc. 2010, 288, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Slack, T.; Myers, C.A.; Martin, C.K.; Heymsfield, S.B. The geographic concentration of US adult obesity prevalence and associated social, economic, and environmental factors. Obesity 2014, 22, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Mokdad, A.H.; Ford, E.S.; Greenlund, K.J.; Croft, J.B. State of disparities in cardiovascular health in the United States. Circulation 2005, 111, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, W.H.; Fonong, T.; Toth, M.J.; Ades, P.A.; Calles-Escandon, J.; Walston, J.D.; Poehlman, E.T. Total daily energy expenditure in free-living older African-Americans and Caucasians. Am. J. Physiol. Metab. 1998, 274, E96–E101. [Google Scholar] [CrossRef] [PubMed]
- Weinsier, R.L.; Hunter, G.R.; Zuckerman, P.A.; Redden, D.T.; Darnell, B.E.; Larson, D.E.; Newcomer, B.R.; Goran, M.I. Energy expenditure and free-living physical activity in black and white women: Comparison before and after weight loss. Am. J. Clin. Nutr. 2000, 71, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Brownley, K.A.; Light, K.C.; Grewen, K.M.; Bragdon, E.E.; Hinderliter, A.L.; West, S.G. Postprandial ghrelin is elevated in black compared with white women. J. Clin. Endocrinol. Metab. 2004, 89, 4457–4463. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Hickner, R.C.; Tanenberg, R.J.; Barakat, H. Peptide-YY levels after a fat load in black and white women. Obes. Res. 2005, 13, 2055–2057. [Google Scholar] [CrossRef] [PubMed]
- Brownley, K.A.; Heymen, S.; Hinderliter, A.L.; Galanko, J.; MacIntosh, B. Low-glycemic load decreases postprandial insulin and glucose and increases postprandial ghrelin in white but not black women. J. Nutr. 2012, 142, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Araneta, M.R.G.; Barrett-Connor, E. Adiponectin and ghrelin levels and body size in normoglycemic Filipino, African-American, and white women. Obesity 2007, 15, 2454–2462. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Brownley, K.A.; Heymen, S.; Hinderliter, A.L.; MacIntosh, B. Effect of glycemic load on peptide-yy levels in a biracial sample of obese and normal weight women. Obesity 2010, 18, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Delany, J.P.; Jakicic, J.M.; Lowery, J.B.; Hames, K.C.; Kelley, D.E.; Goodpaster, B.H. African American women exhibit similar adherence to intervention but lose less weight due to lower energy requirements. Int. J. Obes. 2014, 38, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- King, N.A.; Hopkins, M.; Caudwell, P.; Stubbs, R.J.; Blundell, J.E. Individual variability following 12 weeks of supervised exercise: Identification and characterization of compensation for exercise-induced weight loss. Int. J. Obes. 2008, 32, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Dorling, J.; Broom, D.R.; Burns, S.F.; Clayton, D.J.; Deighton, K.; James, L.J.; King, J.A.; Miyashita, M.; Thackray, A.E.; Batterham, R.L.; et al. Acute and chronic effects of exercise on appetite, energy intake, and appetite-related hormones: The modulating effect of adiposity, sex, and habitual physical activity. Nutrients 2018, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.A.; Johnson, W.D.; Earnest, C.P.; Rood, J.C.; Tudor-Locke, C.; Johannsen, N.M.; Cocreham, S.; Harris, M.; Church, T.S.; Martin, C.K. Examination of mechanisms (E-MECHANIC) of exercise-induced weight compensation: Study protocol for a randomized controlled trial. Trials 2014, 2014, 212. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.K.; Coulon, S.M.; Markward, N.; Greenway, F.L.; Anton, S.D. Association between energy intake and viewing television, distractibility, and memory for advertisements. Am. J. Clin. Nutr. 2009, 89, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, J.K.C.; Montville, J.B.; Omolewa-tomobi, G.; Heendeniya, K.Y.; Martin, C.L.; Steinfeldt, L.C.; Anand, J.; Adler, M.E.; Lacomb, R.P.; Moshfegh, A.J. The USDA Food and Nutrient Database for Dietary Studies, 5.0. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/fndds/fndds5_doc.pdf (accessed on 20 August 2012).
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Green, S.M.; Delargy, H.J.; Joanes, D.; Blundell, J.E. A satiety quotient: A formulation to assess the satiating effect of food. Appetite 1997, 29, 291–304. [Google Scholar] [CrossRef]
- Karra, E.; Daly, O.G.O.; Choudhury, A.I.; Yousseif, A.; Millership, S.; Neary, M.T.; Scott, W.R.; Chandarana, K.; Manning, S.; Hess, M.E.; et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J. Clin. Investig. 2013, 123, 1–13. [Google Scholar] [CrossRef]
- Doucet, E.; St-Pierre, S.; Alméras, N.; Tremblay, A. Relation between appetite ratings before and after a standard meal and estimates of daily energy intake in obese and reduced obese individuals. Appetite 2003, 40, 137–143. [Google Scholar] [CrossRef]
- Cooper, J.A. Factors affecting circulating levels of peptide YY in humans: A comprehensive review. Nutr. Res. Rev. 2014, 27, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Heffron, H.; Kapoor, S.; Chivers, J.E.; Chandarana, K.; Herzog, H.; Le Roux, C.W.; Thomas, E.L.; Bell, J.D.; Withers, D.J. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006, 4, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; De Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; Van Der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Withers, D.J.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Inhibition of Food Intake in Obese Subjects by Peptide YY 3–36. N. Engl. J. Med. 2003, 349, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Caudwell, P.; Finlayson, G.; Webb, D.L.; Hellström, P.M.; Näslund, E.; Blundell, J.E. Comparison of postprandial profiles of ghrelin, active GLP-1, and total PYY to meals varying in fat and carbohydrate and their association with hunger and the phases of satiety. J. Clin. Endocrinol. Metab. 2013, 98, E847–E855. [Google Scholar] [CrossRef]
- Clayton, D.J.; Burrell, K.; Mynott, G.; Creese, M.; Skidmore, N.; Stensel, D.J.; James, L.J. Effect of 24-h severe energy restriction on appetite regulation and ad libitum energy intake in lean men and women1,2. Am. J. Clin. Nutr. 2016, 104, 1545–1553. [Google Scholar] [CrossRef]
- Lippl, F.; Erdmann, J.; Steiger, A.; Lichter, N.; Czogalla-Peter, C.; Bidlingmaier, M.; Tholl, S.; Schusdziarra, V. Low-dose ghrelin infusion—Evidence against a hormonal role in food intake. Regul. Pept. 2012, 174, 26–31. [Google Scholar] [CrossRef]
- Degen, L.; Oesch, S.; Casanova, M.; Graf, S.; Ketterer, S.; Drewe, J.; Beglinger, C. Effect of peptide YY3-36 on food intake in humans. Gastroenterology 2005, 129, 1430–1436. [Google Scholar] [CrossRef]
- Begg, D.P.; Woods, S.C. The endocrinology of food intake. Nat. Rev. Endocrinol. 2013, 9, 584–597. [Google Scholar] [CrossRef]
- Tai, K.; Visvanathan, R.; Hammond, A.J.; Wishart, J.M.; Horowitz, M.; Chapman, I.M. Fasting ghrelin is related to skeletal muscle mass in healthy adults. Eur. J. Nutr. 2009, 48, 176–183. [Google Scholar] [CrossRef]
- Lovejoy, J.C.; Smith, S.R.; Rood, J.C. Comparison of regional fat distribution and health risk factors in middle-aged white and African American women: The healthy transitions study. Obes. Res. 2001, 9, 10–16. [Google Scholar] [CrossRef]
- Cui, H.; López, M.; Rahmouni, K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017, 13, 338–351. [Google Scholar] [CrossRef]
- Scherer, T.; Buettner, C. Yin and Yang of hypothalamic insulin and leptin signaling in regulating white adipose tissue metabolism. Rev. Endocr. Metab. Disord. 2011, 12, 235–243. [Google Scholar] [CrossRef]
- Longo, K.A.; Charoenthongtrakul, S.; Giuliana, D.J.; Govek, E.K.; McDonagh, T.; Qi, Y.; DiStefano, P.S.; Geddes, B.J. Improved insulin sensitivity and metabolic flexibility in ghrelin receptor knockout mice. Regul. Pept. 2008, 150, 55–61. [Google Scholar] [CrossRef]
- Menke, A.; Casagrande, S.; Geiss, L.; Cowie, C.C. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA J. Am. Med. Assoc. 2015, 314, 1021–1029. [Google Scholar] [CrossRef]
- Chen, Y.C.; Edinburgh, R.M.; Hengist, A.; Smith, H.A.; Walhin, J.P.; Betts, J.A.; Thompson, D.; Gonzalez, J.T. Venous blood provides lower glucagon-like peptide-1 concentrations than arterialized blood in the postprandial but not the fasted state: Consequences of sampling methods. Exp. Physiol. 2018, 103, 1200–1205. [Google Scholar] [CrossRef]
- Shook, R.P.; Hand, G.A.; Wang, X.; Paluch, A.E.; Moran, R.; Hébert, J.R.; Swift, D.L.; Lavie, C.J.; Blair, S.N. Low fitness partially explains resting metabolic rate differences between African American and white women. Am. J. Med. 2014, 127, 436–442. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Most, J.; Redman, L.M.; Rood, J.; Ravussin, E. Energy expenditure and substrate oxidation in White and African American young adults without obesity. Eur. J. Clin. Nutr. 2018, 72, 920–922. [Google Scholar] [CrossRef]
- Park, S.; Blanck, H.M.; Sherry, B.; Brener, N.; O’Toole, T. Factors associated with sugar-sweetened beverage intake among United States high school students. J. Nutr. 2012, 142, 306–312. [Google Scholar] [CrossRef]
- Guelfi, K.J.; Donges, C.E.; Duffield, R. Beneficial effects of 12 weeks of aerobic compared with resistance exercise training on perceived appetite in previously sedentary overweight and obese men. Metabolism 2013, 62, 235–243. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef]
- Martin, C.K.; Johnson, W.D.; Myers, C.A.; Apolzan, J.W.; Earnest, C.P.; Thomas, D.M.; Rood, J.C.; Johannsen, N.M.; Tudor-Locke, C.; Harris, M.; et al. Effect of different doses of supervised exercise on food intake, metabolism, and non-exercise physical activity: The E-MECHANIC randomized controlled trial. Am. J. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Faerch, K.; Torekov, S.S.; Vistisen, D.; Johansen, N.B.; Witte, D.R.; Jonsson, A.; Pedersen, O.; Hansen, T.; Lauritzen, T.; Sandbaek, A.; et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: The ADDITION-PRO Study. Diabetes 2015, 64, 2513–2525. [Google Scholar] [CrossRef]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef]


| African American (AA) | White | p-Value | |
|---|---|---|---|
| Age (years) | 46.0 ± 10.3 | 50.4 ± 11.7 | 0.02 |
| Weight (kg) | 92.0 ± 13.5 | 87.1 ± 16.0 | 0.06 |
| Height (cm) | 165.3 ± 6.9 | 167.7 ± 8.5 | 0.08 |
| BMI (kg/m2) | 33.4 ± 4.8 | 30.6 ± 4.3 | <0.01 |
| Waist circumference (cm) | 98.7 ± 10.5 | 99.0 ± 13.3 | 0.87 |
| Fat-free mass (kg) | 48.5 ± 8.3 | 47.8 ± 10.6 | 0.69 |
| Fat mass (kg) | 40.4 ± 11.4 | 36.4 ± 8.9 | 0.01 |
| Fat (%) | 45.0 ± 8.3 | 43.2 ± 6.6 | 0.13 |
| VO2peak (L/min) | 1.9 ± 0.5 | 2.1 ± 0.6 | 0.08 |
| VO2peak (mL/kg/min) | 21.3 ± 5.0 | 24.3 ± 5.1 | <0.01 |
| RMR (kJ/day) | 6163 ± 1042 | 6422 ± 1381 | 0.23 |
| RMR # (kJ/day) | 6121 ± 791 | 6448 ± 791 | 0.01 |
| Glucose (mg/dL) | 91.5 ± 9.7 | 93.3 ± 6.5 | 0.15 |
| African American (AA) | White | |||||
|---|---|---|---|---|---|---|
| Control (n = 20) | 8 KKW (n = 20) | 20 KKW (n = 13) | Control (n = 37) | 8 KKW (n = 38) | 20 KKW (n = 36) | |
| Hunger before lunch | 3 (4) a | −2 (4) a | −1 (5) a | 7 (3) | 10 (3) | 2 (3) |
| Fullness before lunch | 0 (4) | 4 (4) | −4 (5) | −3 (3) | −6 (3) | 4 (3) |
| Prospective food consumption before lunch | 9 (3) | 3 (3) | 0 (4) b | 6 (2) | 7 (2) | 0 (2) b |
| Desire to eat before lunch | 9 (4) | 1 (4) | 2 (5) | 4 (3) | 7 (3) | 3 (3) |
| Satisfaction before lunch | −12 (4) | −3 (4) | −7 (5) | −3 (3) | −7 (3) | 2 (3) |
| Satiety quotient AUC | −1 (6) a | −9 (6) a | −5 (7) a | 10 (4) | 8 (4) | 1 (4) |
| Lunch energy intake (kJ) | 213 (18) | 161 (184) | −174 (221) | −360 (132) | 197 (132) | −133 (133) |
| Dinner energy intake (kJ) | −779 (287) | −783 (299) | −258 (351) | −160 (213) | −299 (210) | −277 (212) |
| Total energy intake (kJ) | −525 (362) | −861 (370) | −423 (444) | −521 (266) | −120 (264) | −409 (268) |
| Total carbohydrate intake (g) | −11 (11) | −11 (12) | −7 (14) | −16 (8) | 1 (8) | −8 (9) |
| Total protein intake (g) | −7 (4) | −15 (4) | −9 (5) | −7 (3) | −3 (3) | −6 (3) |
| Total fat intake (g) | −6 (4) | −11 (4) | −4 (5) | −4 (3) | −3 (3) | −5 (3) |
| African American (AA) | White | |||||
|---|---|---|---|---|---|---|
| Control (n = 20) | 8 KKW (n = 20) | 20 KKW (n = 13) | Control (n = 37) | 8 KKW (n = 38) | 20 KKW (n = 36) | |
| Weight (kg) | 0.2 (0.7) | −0.7 (0.7) | −2.1 (0.8) | −0.9 (0.5) | −0.2 (0.5) | −1.6 (0.5) |
| Waist circumference (cm) | −0.1 (0.9) | −0.8 (0.9) | −2.3 (1.1) | −1.7 (0.6) | −0.1 (0.6) | −1.4 (0.6) |
| Fat-free mass (kg) | −0.1 (0.3) | 0.0 (0.3) | −0.3 (0.3) | −0.6 (0.2) | −0.2 (0.2) | −0.3 (0.2) |
| Fat mass (kg) | 0.1 (0.6) | −0.9 (0.6) | −2.1 (0.7) a | −0.4 (0.4) | −0.1 (0.4) | −1.4 (0.4) a |
| Fat percentage (%) | −0.1 (0.4) | −0.5 (0.4) | −1.4 (0.5) b | −0.1 (0.3) | 0.0 (0.3) | −0.8 (0.3) b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorling, J.L.; Church, T.S.; Myers, C.A.; Höchsmann, C.; White, U.A.; Hsia, D.S.; Martin, C.K.; Apolzan, J.W. Racial Variations in Appetite-Related Hormones, Appetite, and Laboratory-Based Energy Intake from the E-MECHANIC Randomized Clinical Trial. Nutrients 2019, 11, 2018. https://doi.org/10.3390/nu11092018
Dorling JL, Church TS, Myers CA, Höchsmann C, White UA, Hsia DS, Martin CK, Apolzan JW. Racial Variations in Appetite-Related Hormones, Appetite, and Laboratory-Based Energy Intake from the E-MECHANIC Randomized Clinical Trial. Nutrients. 2019; 11(9):2018. https://doi.org/10.3390/nu11092018
Chicago/Turabian StyleDorling, James L., Timothy S. Church, Candice A. Myers, Christoph Höchsmann, Ursula A. White, Daniel S. Hsia, Corby K. Martin, and John W. Apolzan. 2019. "Racial Variations in Appetite-Related Hormones, Appetite, and Laboratory-Based Energy Intake from the E-MECHANIC Randomized Clinical Trial" Nutrients 11, no. 9: 2018. https://doi.org/10.3390/nu11092018
APA StyleDorling, J. L., Church, T. S., Myers, C. A., Höchsmann, C., White, U. A., Hsia, D. S., Martin, C. K., & Apolzan, J. W. (2019). Racial Variations in Appetite-Related Hormones, Appetite, and Laboratory-Based Energy Intake from the E-MECHANIC Randomized Clinical Trial. Nutrients, 11(9), 2018. https://doi.org/10.3390/nu11092018








