In Vivo Ergogenic Properties of the Bifidobacterium longum OLP-01 Isolated from a Weightlifting Gold Medalist
Abstract
1. Introduction
2. Materials and Methods
2.1. OLP-01 Preparation
2.2. Animals and Experimental Design
2.3. Forelimb Grip Strength
2.4. Exercise Endurance Test
2.5. Determination of Fatigue-Associated Biochemical Variables
2.6. Resting Biochemical Profiles at the End of the Study
2.7. Body Composition, Glycogen Content, and Histopathology
2.8. Bacterial DNA Extraction and 16S rRNA Sequencing
2.9. Statistical Analysis
3. Results
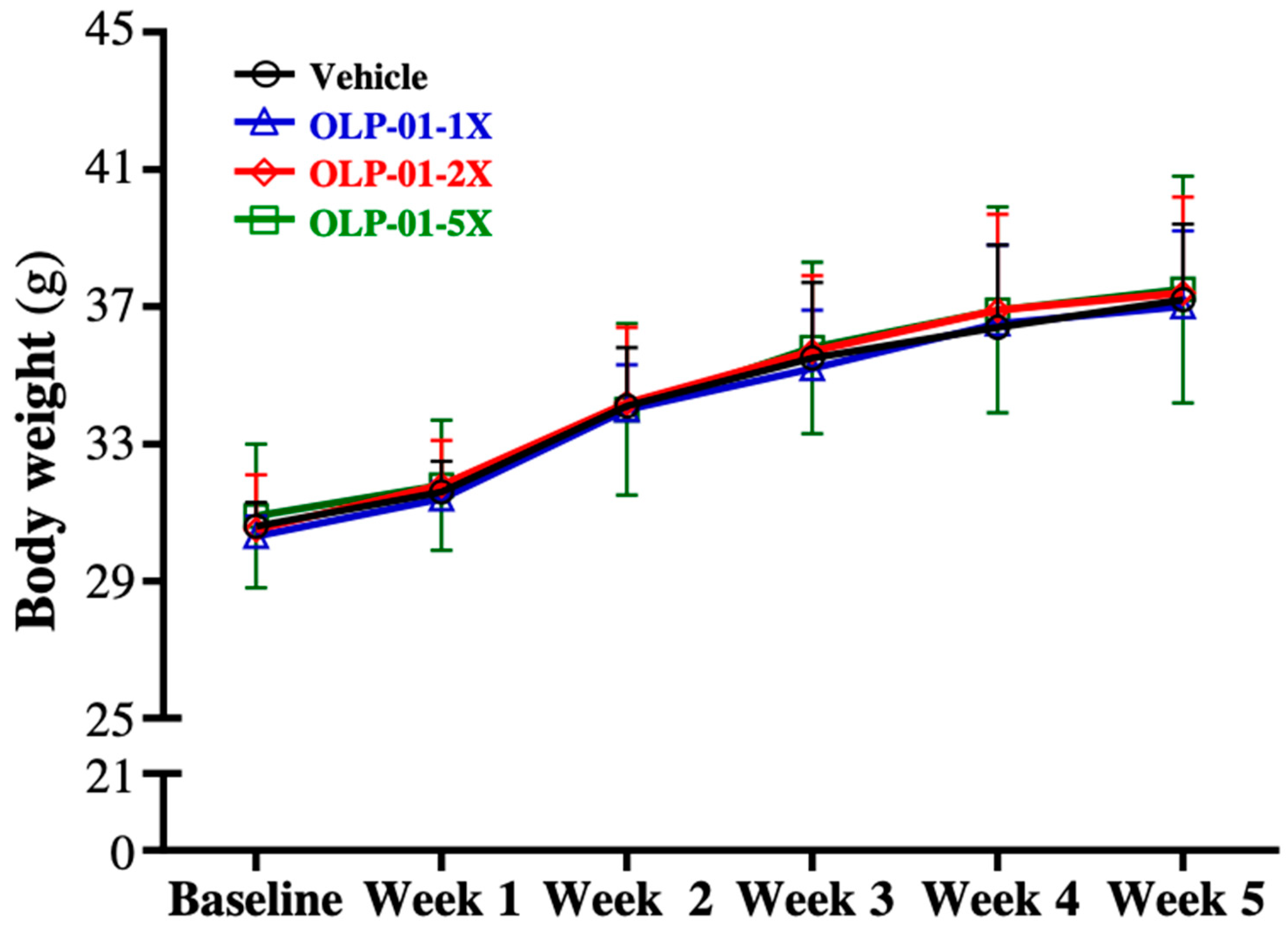
3.1. Effect of OLP-01 Supplementation on Body Weight, Food and Water Intake, and Organ Weights
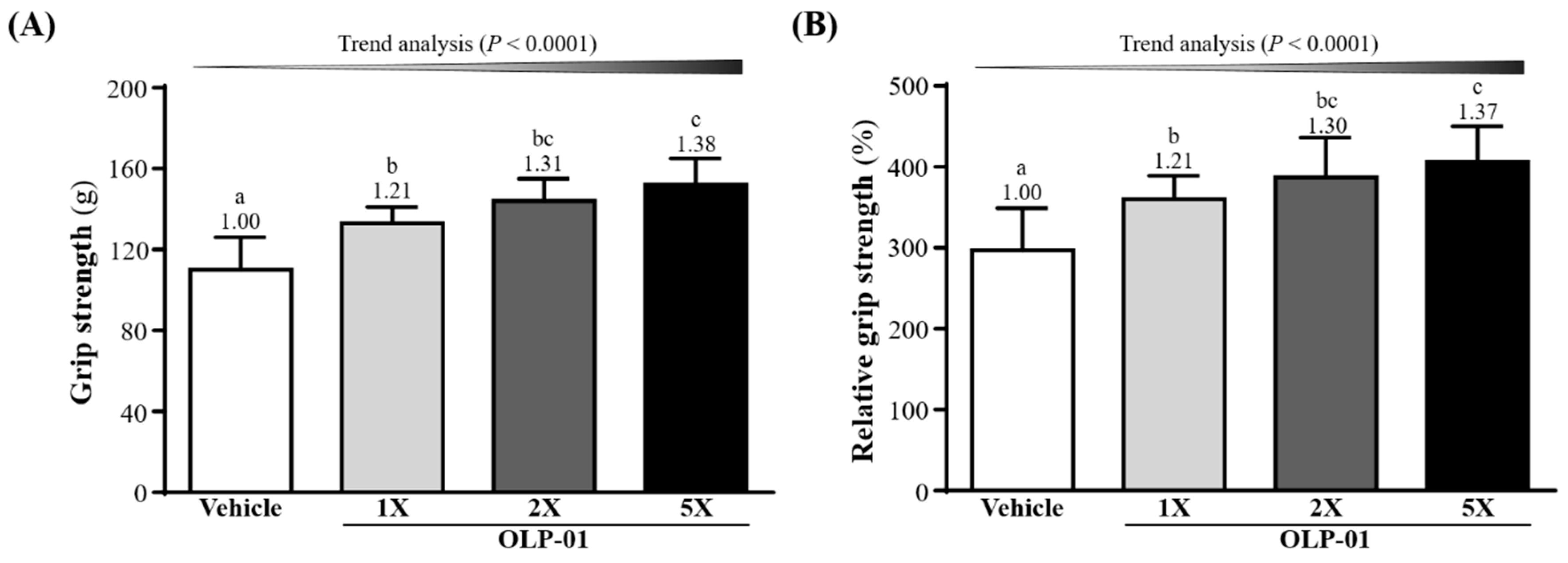
3.2. Effect of OLP-01 Supplementation on Grip Strength
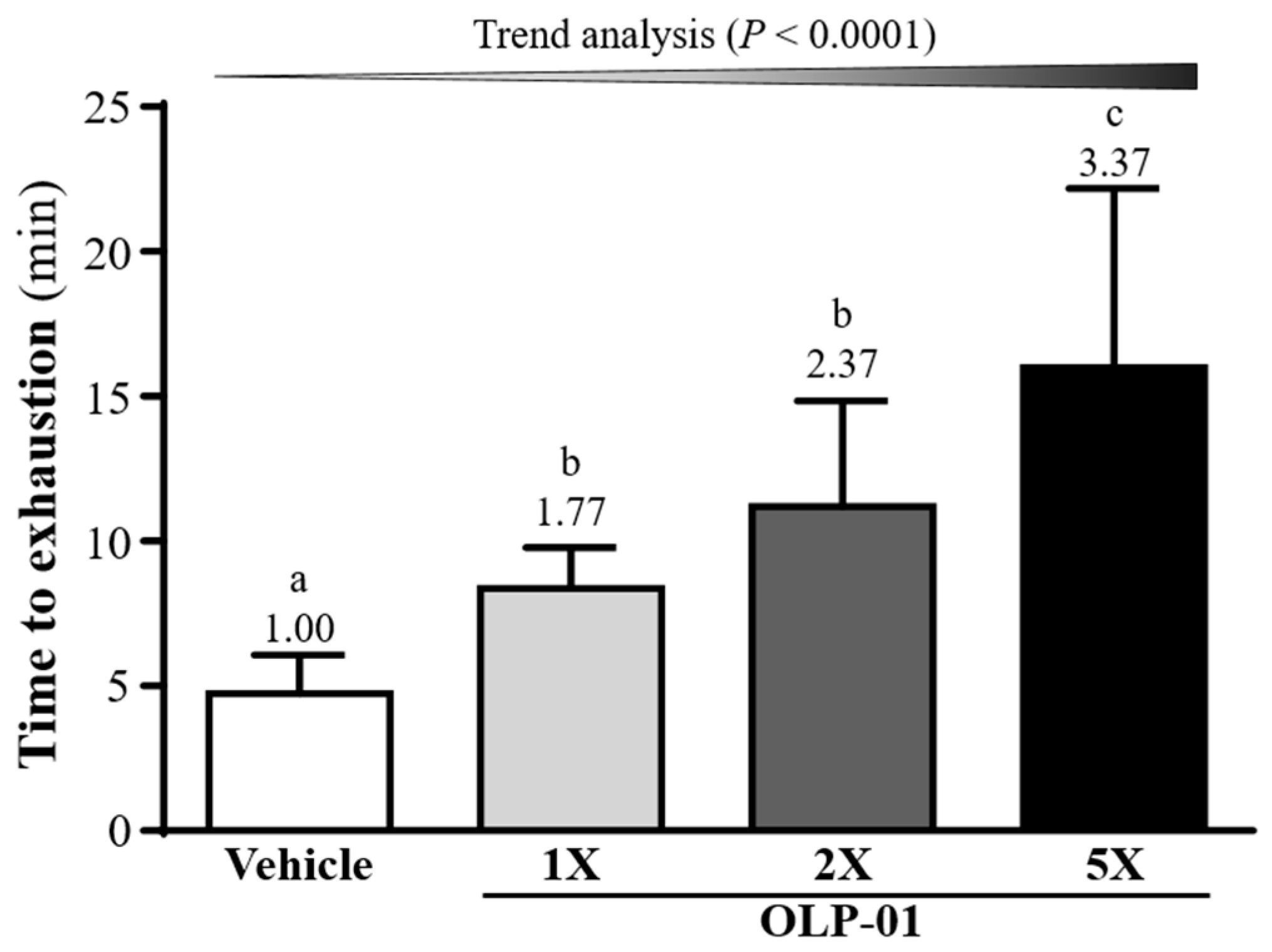
3.3. Effect of OLP-01 Supplementation on Endurance Capacity in the Swim-to-ExhaustionTest
3.4. Effect of OLP-01 Supplementation on Lactate after the 10 Min Swim Test
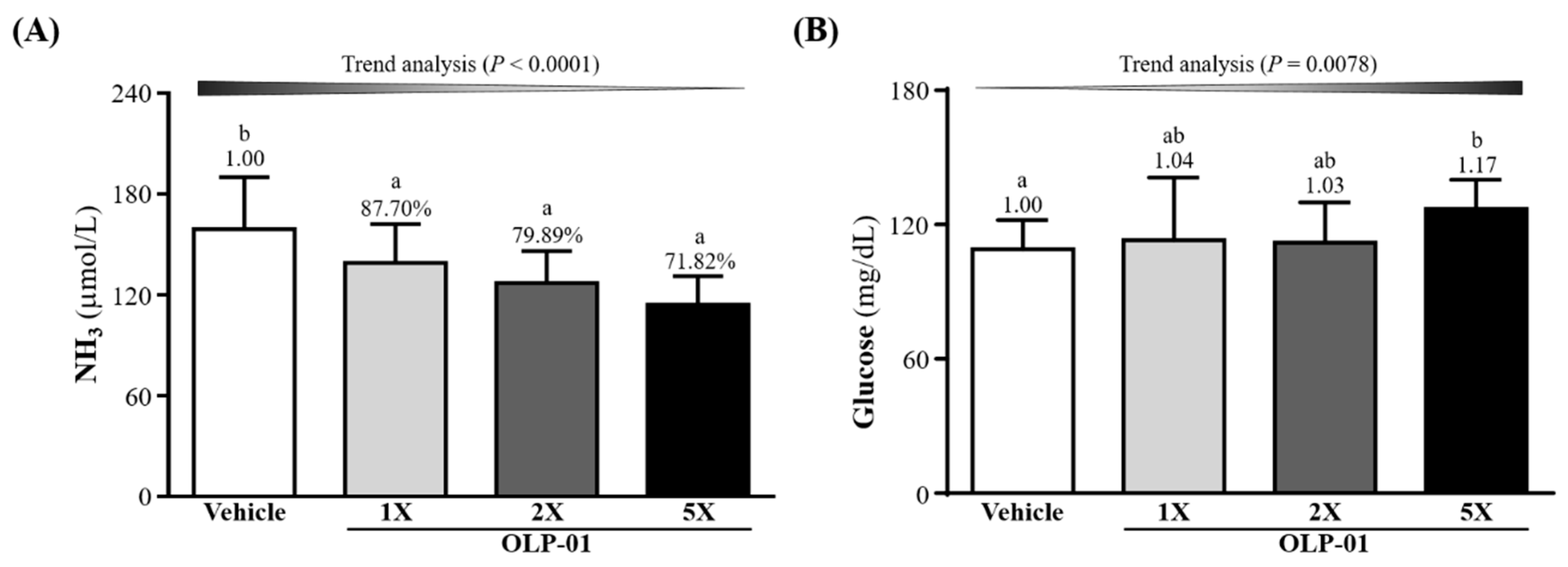
3.5. Effect of OLP-01 Supplementation on Ammonia and Glucose after the 10 Min Swim Test
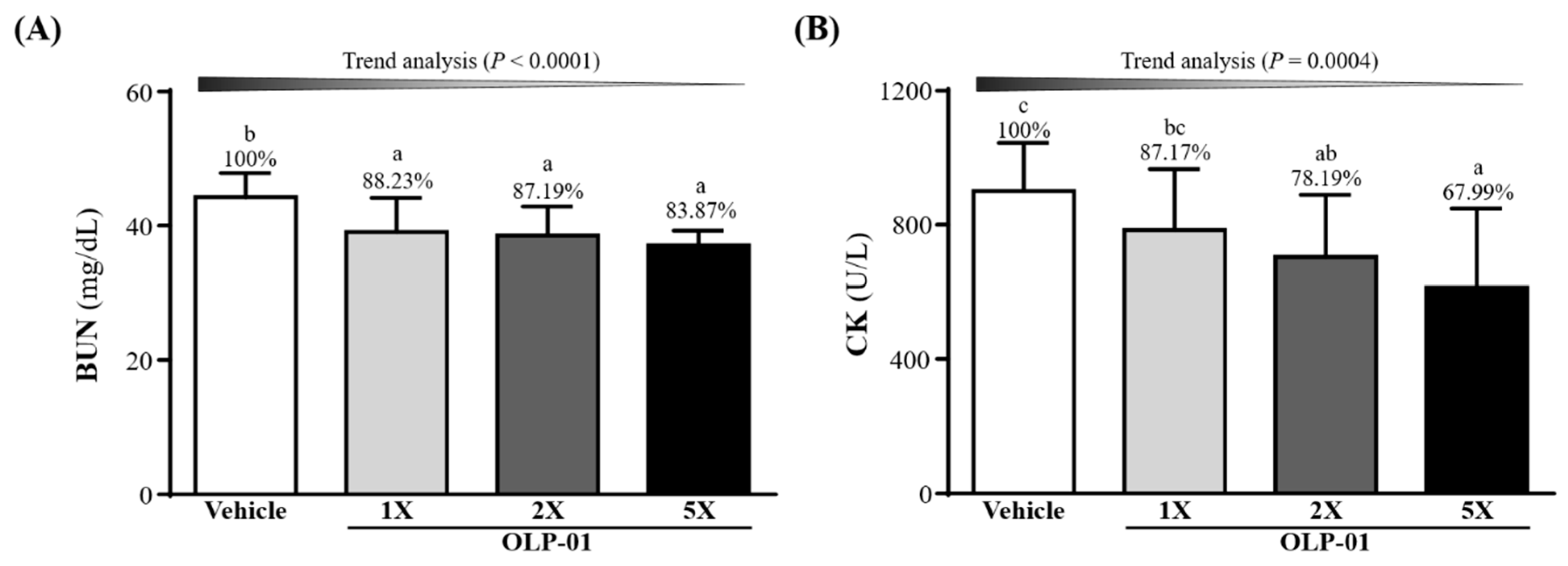
3.6. Effect of OLP-01 Supplementation on BUN and CK after a 90 Min Swim Test and 60 Min Rest Period
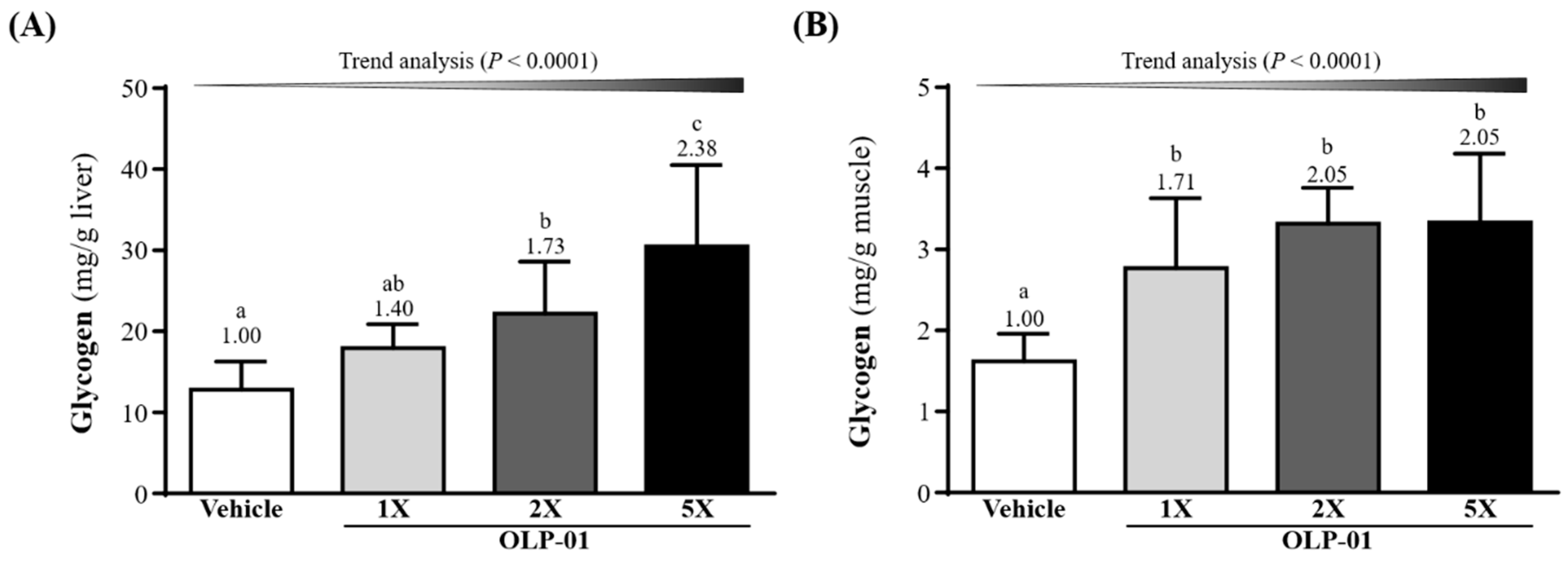
3.7. Effect of OLP-01 Supplementation on Liver and Muscle Glycogen
3.8. Effect of OLP-01 Supplementation on Biochemical Profiles at the End of the Study
3.9. Effect of OLP-01 Supplementation on the Gut Microbiota
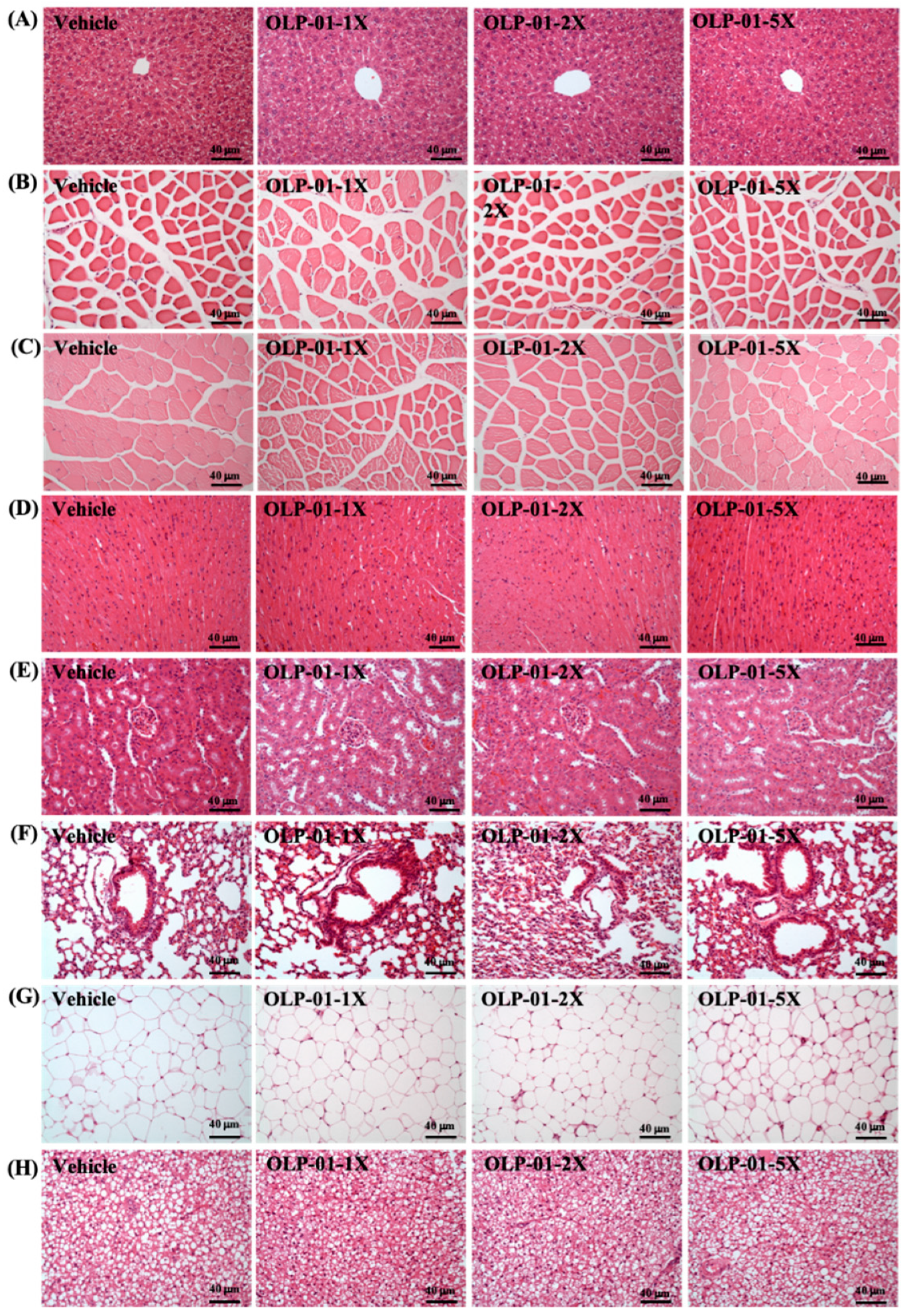
3.10. Effect of OLP-01 Supplementation on Tissue Histology at the End of the Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmidt, T.S.B.; Raes, J.; Bork, P. The human gut microbiome: From association to modulation. Cell 2018, 172, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Havenaar, R.; Huis, J.H. Probiotics: A general view. In The Lactic Acid Bacteria Volume 1; Springer: Boston, MA, USA, 1992; pp. 151–170. [Google Scholar]
- Currò, D.; Ianiro, G.; Pecere, S.; Bibbò, S.; Cammarota, G. Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. Br. J. Pharmacol. 2017, 174, 1426–1449. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Huang, E.; Park, S.; Holzapfel, W.; Lim, S.D. Physiological Characteristics and Anti-obesity Effect of Lactobacillus plantarum K10. Korean J. Food Sci. Anim. Resour. 2018, 38, 554–569. [Google Scholar] [PubMed]
- Grover, S.; Rashmi, H.M.; Srivastava, A.K.; Batish, V.K. Probiotics for human health -new innovations and emerging trends. Gut Pathog. 2012, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Karimi, G.; Sabran, M.R.; Jamaluddin, R.; Parvaneh, K.; Mohtarrudin, N.; Ahmad, Z.; Khazaai, H.; Khodavandi, A. The anti-obesity effects of Lactobacillus casei strain Shirota versus Orlistat on high fat diet-induced obese rats. Food Nutr. Res. 2015, 59, 29273. [Google Scholar] [CrossRef]
- Son, S.H.; Yang, S.J.; Jeon, H.L.; Yu, H.S.; Lee, N.K.; Park, Y.S.; Paik, H.D. Antioxidant and immunostimulatory effect of potential probiotic Lactobacillus paraplantarum SC61 isolated from Korean traditional fermented food, jangajji. Microb. Pathog. 2018, 125, 486–492. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- George Kerry, R.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef]
- Vemuri, R.; Shinde, T.; Shastri, M.D.; Perera, A.P.; Tristram, S.; Martoni, C.J.; Gundamaraju, R.; Ahuja, K.D.K.; Ball, M.; Eri, R. A human origin strain Lactobacillus acidophilus DDS-1 exhibits superior in vitro probiotic efficacy in comparison to plant or dairy origin probiotics. Int. J. Med. Sci. 2018, 15, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Wang, S.; Ahmadi, S.; Hayes, J.; Gagliano, J.; Subashchandrabose, S.; Kitzman, D.W.; Becton, T.; Read, R.; Yadav, H. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci. Rep. 2018, 8, 12649. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; O’Sullivan, D.J. Genomic insights into bifidobacteria. Microbiol. Mol. Biol. Rev. 2010, 74, 378–416. [Google Scholar] [CrossRef] [PubMed]
- Garrido, D.; Ruiz-Moyano, S.; Jimenez-Espinoza, R.; Eom, H.J.; Block, D.E.; Mills, D.A. Utilization of galactooligosaccharides by Bifidobacterium longum subsp. infantis Isolates. Food Microbiol. 2013, 33, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhu, L.; Liu, X.; Li, T.; Zhang, Y.; Ying, T.; Wang, B.; Wang, J.; Dong, H.; Feng, E.; et al. A proteome reference map and proteomic analysis of Bifidobacterium longum NCC2705. Mol. Cell Proteom. 2006, 5, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kobayashi, Y.; Mori, N.; Sakagawa, M.; Xiao, J.Z.; Moritani, T.; Sakane, N.; Nagai, N. Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef. Microbes 2018, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.S.; Muhamad, A.S.; Ooi, F.K.; Meor-Osman, J.; Chen, C.K. The effects of combined probiotic ingestion and circuit training on muscular strength and power and cytokine responses in young males. Appl. Physiol. Nutr. Metab. 2018, 43, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Li, Z.; Ji, G.E. Anti-Obesity Effects of a Mixture of Fermented Ginseng, Bifidobacterium longum BORI, and Lactobacillus paracasei CH88 in High-Fat Diet-Fed Mice. J. Microbiol. Biotechnol. 2018, 28, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.E.; Jeong, J.J.; Kim, J.K.; Han, M.J.; Kim, D.H. Simultaneous Amelioratation of Colitis and Liver Injury in Mice by Bifidobacterium longum LC67 and Lactobacillus plantarum LC27. Sci. Rep. 2018, 8, 7500. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Huang, W.C.; Lin, J.S.; Chen, Y.M.; Ho, S.T.; Huang, C.C.; Tung, Y.T. Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice. Nutrients 2018, 10, 862. [Google Scholar] [CrossRef]
- Kan, N.W.; Lee, M.C.; Tung, Y.T.; Chiu, C.C.; Huang, C.C.; Huang, W.C. The Synergistic Effects of Resveratrol combined with Resistant Training on Exercise Performance and Physiological Adaption. Nutrients 2018, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wen, Y.T.; Lee, M.C.; Ho, H.M.; Huang, C.C.; Hsu, Y.J. Effects of isolated soy protein and strength exercise training on exercise performance and biochemical profile in postpartum mice. Metabolism 2019, 94, 18–27. [Google Scholar] [CrossRef]
- Huang, W.C.; Huangm, H.Y.; Hsu, Y.J.; Su, W.H.; Shen, S.Y.; Lee, M.C.; Lin, C.L.; Huang, C.C. The Effects of Thiamine Tetrahydrofurfuryl Disulfide on Physiological Adaption and Exercise Performance Improvement. Nutrients 2018, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.W. Probiotics and athletic performance: A systematic review. Curr. Sports Med. Rep. 2007, 6, 269–273. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef]
- de Vries, W.; Stouthamer, A.H. Pathway of glucose fermentation in relation to the taxonomy of bifidobacteria. J. Bacteriol. 1967, 93, 574–576. [Google Scholar]
- Sneath, P.H.; Mair, N.S.; Sharpe, M.E.; Holt, J.G. Bergey’s Manual of Systematic Bacteriology; Williams & Wilkins: Philadelphia, PA, USA, 1986; Volume 2. [Google Scholar]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Al-Obaidi, S.; Al-Sayegh, N.; Nadar, M. Smoking impact on grip strength and fatigue resistance: Implications for exercise and hand therapy practice. J. Phys. Act. Health 2014, 11, 1025–1031. [Google Scholar]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Chen, Y.M.; Lee, H.C.; Chen, M.T.; Huang, C.C.; Chen, W.C. Dehydroepiandrosterone supplementation combined with Weight-Loading Whole-Body Vibration Training (WWBV) affects exercise performance and muscle glycogen storage in middle-aged C57BL/6 mice. Int. J. Med. Sci. 2018, 15, 564–573. [Google Scholar] [CrossRef]
- Cairns, S.P. Lactic acid and exercise performance. Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Huang, W.C.; Chiu, C.C.; Liu, Y.L.; Chiu, W.C.; Chiu, C.H.; Chiu, Y.S.; Huang, C.C. Capsaicin Supplementation Reduces Physical Fatigue and Improves Exercise Performance in Mice. Nutrients 2016, 8, 648. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Deguchi, Y.; Makino, K.; Iwabuchi, A.; Watanuki, M.; Yamashita, T. Selection of ammonia-assimilating bifidobacteria and their effect on ammonia levels in rat caecal contents and blood. Microb. Ecol. Health Dis. 1993, 6, 85–94. [Google Scholar] [CrossRef]
- Korzeniewski, B. AMP deamination delays muscle acidification during heavy exercise and hypoxia. J. Biol. Chem. 2006, 281, 3057–3066. [Google Scholar] [CrossRef]
- Ørtenblad, N.; Westerblad, H.; Nielsen, J. Muscle glycogen stores and fatigue. J. Physiol. 2013, 591, 4405–4413. [Google Scholar] [CrossRef]
- Hearris, M.A.; Hammond, K.M.; Fell, J.M.; Morton, J.P. Regulation of Muscle Glycogen Metabolism during Exercise: Implications for Endurance Performance and Training Adaptations. Nutrients 2018, 10, 298. [Google Scholar] [CrossRef]
- Parche, S.; Amon, J.; Jankovic, I.; Rezzonico, E.; Beleut, M.; Barutçu, H.; Schendel, I.; Eddy, M.P.; Burkovski, A.; Arigoni, F.; et al. Sugar transport systems of Bifidobacterium longum NCC2705. J. Mol. Microbiol. Biotechnol. 2007, 12, 9–19. [Google Scholar] [CrossRef]
| Characteristic | Vehicle | OLP-01-1X | OLP-01-2X | OLP-01-5X | Trend Analysis |
|---|---|---|---|---|---|
| Initial BW (g) | 30.6 ± 0.7 | 30.3 ± 0.6 | 30.5 ± 1.6 | 30.9 ± 2.1 | 0.4253 |
| Final BW (g) | 38.5 ± 2.3 | 38.1 ± 2.7 | 38.8 ± 2.9 | 38.8 ± 3.2 | 0.5491 |
| Water intake (mL/mouse/day) | 7.4 ± 0.7 | 7.5 ± 0.8 | 7.4 ± 1.0 | 7.4 ± 1.4 | 0.3575 |
| Diet intake (g/mouse/day) | 7.9 ± 1.1 | 7.9 ± 1.0 | 7.8 ± 1.8 | 7.9 ± 1.5 | 0.7006 |
| Liver (g) | 2.16 ± 0.15 | 2.19 ± 0.27 | 2.22 ± 0.16 | 2.22 ± 0.25 | 0.5576 |
| Muscle (g) | 0.39 ± 0.03 | 0.39 ± 0.03 | 0.39 ± 0.03 | 0.39 ± 0.05 | 0.8603 |
| Quadriceps (g) | 0.51 ± 0.05 | 0.51 ± 0.04 | 0.52 ± 0.04 | 0.51 ± 0.05 | 0.8678 |
| Kidney (g) | 0.70 ± 0.09 | 0.70 ± 0.08 | 0.71 ± 0.12 | 0.71 ± 0.09 | 0.3795 |
| Heart (g) | 0.20 ± 0.03 | 0.20± 0.03 | 0.20 ± 0.02 | 0.20 ± 0.02 | 0.7699 |
| Lung (g) | 0.23 ± 0.03 | 0.24 ± 0.03 | 0.23 ± 0.03 | 0.24 ± 0.03 | 0.8482 |
| EFP (g) | 0.38 ± 0.16 | 0.29 ± 0.10 | 0.29 ± 0.09 | 0.29 ± 0.07 | 0.3066 |
| BAT (g) | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.03 | 0.09 ± 0.01 | 0.2921 |
| Cecum (g) | 0.79 ± 0.20 | 0.77 ± 0.05 | 0.78 ± 0.10 | 0.79 ± 0.16 | 0.5076 |
| Relative liver weight (%) | 5.62 ± 0.15 | 5.73 ± 0.32 | 5.72 ± 0.12 | 5.71 ± 0.23 | 0.2903 |
| Relative muscle weight (%) | 1.01 ± 0.04 | 1.01 ± 0.03 | 1.00 ± 0.02 | 1.01 ± 0.05 | 0.6849 |
| Relative quadriceps weight (%) | 1.33 ± 0.07 | 1.34 ± 0.03 | 1.33 ± 0.06 | 1.33 ± 0.03 | 0.0896 |
| Relative kidney weight (%) | 1.80 ± 0.14 | 1.83 ± 0.12 | 1.83 ± 0.22 | 1.83 ± 0.10 | 0.7062 |
| Relative heart weight (%) | 0.52 ± 0.04 | 0.52 ± 0.04 | 0.50 ± 0.03 | 0.51 ± 0.03 | 0.4263 |
| Relative lung weight (%) | 0.60 ± 0.05 | 0.62 ± 0.04 | 0.60 ± 0.03 | 0.61 ± 0.03 | 0.8376 |
| Relative EFP weight (%) | 0.97 ± 0.36 b | 0.74 ± 0.21 a | 0.73 ± 0.18 a | 0.74 ± 0.12 a | 0.1584 |
| Relative BAT weight (%) | 0.22 ± 0.03 | 0.20 ± 0.01 | 0.22 ± 0.05 | 0.23 ± 0.02 | 0.5144 |
| Relative cecum weight (%) | 2.02 ± 0.42 | 2.01 ± 0.30 | 2.00 ± 0.12 | 2.03 ± 0.28 | 0.9822 |
| Time Point | Vehicle | OLP1-01-1X | OLP-01-2X | OLP-01-5X | Trend Analysis |
|---|---|---|---|---|---|
| Lactate (mmol/L) | |||||
| Before swimming (A) | 3.42 ± 0.37 | 3.43 ± 0.36 | 3.41 ± 0.30 | 3.43 ± 0.24 | 0.7111 |
| After swimming (B) | 6.74 ± 0.28 b | 5.64 ± 0.57 a | 5.40 ± 0.83 a | 5.12 ± 0.59 a | <0.0001 |
| After a 20 min rest(C) | 6.05 ± 0.25 b | 5.04 ± 0.65 a | 4.76 ± 0.67 a | 4.53 ± 0.57 a | <0.0001 |
| Rate of lactate production and clearance | |||||
| Production rate = B/A | 1.99 ± 0.23 c | 1.64 ± 0.05 b | 1.58 ± 0.12 ab | 1.49 ± 0.10 a | <0.0001 |
| Clearance rate = (B − C)/B | 0.10 ± 0.02 | 0.11 ± 0.06 | 0.12 ± 0.03 | 0.12 ± 0.02 | 0.0222 |
| Parameter | Vehicle | OLP-01-1X | OLP-01-2X | OLP-01-5X | Trend Analysis |
|---|---|---|---|---|---|
| AST (U/L) | 68 ± 11 | 68 ± 10 | 69 ± 27 | 68 ± 13 | 0.9451 |
| ALT (U/L) | 31 ± 8 | 32 ± 10 | 31 ± 15 | 32 ± 8 | 0.4689 |
| CK (U/L) | 162 ± 52 | 129 ± 65 | 120 ± 69 | 128 ± 94 | 0.0751 |
| GLU (mg/dL) | 229 ± 21 | 231 ± 48 | 227 ± 24 | 228 ± 26 | 0.8925 |
| CREA (mg/dL) | 0.38 ±0.02 | 0.37 ± 0.02 | 0.37 ± 0.03 | 0.38 ± 0.02 | 0.9674 |
| BUN (mg/dL) | 23.4 ± 1.6 | 23.4 ± 1.7 | 23.7 ± 2.8 | 22.3 ± 1.4 | 0.8665 |
| UA (mg/dL) | 2.6 ± 0.6 | 2.5 ± 0.8 | 2.5 ± 0.5 | 2.5 ± 0.3 | 0.8331 |
| TC (mg/dL) | 138 ± 32 | 133 ± 24 | 135 ± 15 | 135± 13 | 0.9813 |
| TG (mg/dL) | 141 ± 27 | 141 ± 13 | 141 ± 20 | 142 ± 13 | 0.5683 |
| ALB (g/dL) | 3.0 ± 0.1 | 3.0 ± 0.2 | 3.0 ± 0.2 | 3.1 ± 0.1 | 0.0915 |
| TP (g/dL) | 5.3 ± 0.2 | 5.4 ± 0.2 | 5.4 ± 0.3 | 5.4 ± 0.2 | 0.0630 |
| Counts | Vehicle | OLP-01-5X | p Value | Compared with Vehicle (%) |
|---|---|---|---|---|
| Genus | ||||
| Lactobacillus | 842.36 ± 116.85 | 1732.35 ± 1290.13 | 0.2185 | 205.66 |
| Clostridium | 1028.05 ± 349.22 | 1074.37 ± 190.07 | 0.8235 | 104.51 |
| Bacillus | 581.67 ± 174.5 | 537.12 ± 94.68 | 0.6693 | 92.34 |
| Enterococcus | 179.41 ± 105.22 | 132.67 ± 59.86 | 0.4693 | 73.95 |
| Bifidobacterium | 55.05 ± 7.85 | 90.42 ± 30.04 | 0.0630 | 164.25 |
| Streptococcus | 55.5 ± 10.55 | 55.25 ± 15.36 | 0.9796 | 99.55 |
| Akkermansia | 68.07 ± 47.44 | 27.07 ± 9.71 | 0.1413 | 39.77 |
| Lactococcus | 0.23 ± 0.45 | 1.3 ± 1.69 | 0.2662 | 571.76 |
| Species | ||||
| Bifidobacterium bombi | 13.85 ± 4.68 | 21.22 ± 10.27 | 0.2392 | 153.23 |
| Bifidobacterium longum | 1.25 ± 1.72 | 15.11 ± 12.43 | 0.0693 | 1210.56 |
| Bifidobacterium asteroides | 3.15 ± 1.70 | 3.84 ± 1.50 | 0.5626 | 122.05 |
| Bifidobacterium indicum | 2.51 ± 1.36 | 2.71 ± 1.18 | 0.8295 | 108.06 |
| Bifidobacterium catenulatum | 0 ± 0 | 6.22 ± 2.75 | 0.0040 * | - |
| Bifidobacterium scardovii | 0.63 ± 0.56 | 1.89 ± 2.22 | 0.3128 | 298.82 |
| Bifidobacterium stercoris | 0.84 ± 1.69 | 1.66 ± 1.87 | 0.5412 | 196.58 |
| Bifidobacterium choerinum | 0.77 ± 0.87 | 1.08 ± 0.55 | 0.5653 | 140.76 |
| Bifidobacterium subtile | 0.16 ± 0.33 | 1.8 ± 2.19 | 0.1902 | 1092.70 |
| Bifidobacterium adolescentis | 0.17 ± 0.34 | 0.95 ± 1.36 | 0.3060 | 563.98 |
| Bifidobacterium kashiwanohense | 0.13 ± 0.26 | 0.38 ± 0.46 | 0.3859 | 288.94 |
| Bifidobacterium bifidum | 0 ± 0 | 1.26 ± 2.53 | 0.3559 | - |
| Bifidobacterium cuniculi | 0.84 ± 1.69 | 0.21 ± 0.42 | 0.4938 | 24.93 |
| Bifidobacterium merycicum | 0 ± 0 | 0.12 ± 0.23 | 0.3559 | - |
| Bifidobacterium magnum | 0.13 ± 0.27 | 0 ± 0 | 0.3559 | 0.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-C.; Hsu, Y.-J.; Chuang, H.-L.; Hsieh, P.-S.; Ho, H.-H.; Chen, W.-L.; Chiu, Y.-S.; Huang, C.-C. In Vivo Ergogenic Properties of the Bifidobacterium longum OLP-01 Isolated from a Weightlifting Gold Medalist. Nutrients 2019, 11, 2003. https://doi.org/10.3390/nu11092003
Lee M-C, Hsu Y-J, Chuang H-L, Hsieh P-S, Ho H-H, Chen W-L, Chiu Y-S, Huang C-C. In Vivo Ergogenic Properties of the Bifidobacterium longum OLP-01 Isolated from a Weightlifting Gold Medalist. Nutrients. 2019; 11(9):2003. https://doi.org/10.3390/nu11092003
Chicago/Turabian StyleLee, Mon-Chien, Yi-Ju Hsu, Hsiao-Li Chuang, Pei-Shan Hsieh, Hsieh-Hsun Ho, Wei-Ling Chen, Yen-Shuo Chiu, and Chi-Chang Huang. 2019. "In Vivo Ergogenic Properties of the Bifidobacterium longum OLP-01 Isolated from a Weightlifting Gold Medalist" Nutrients 11, no. 9: 2003. https://doi.org/10.3390/nu11092003
APA StyleLee, M.-C., Hsu, Y.-J., Chuang, H.-L., Hsieh, P.-S., Ho, H.-H., Chen, W.-L., Chiu, Y.-S., & Huang, C.-C. (2019). In Vivo Ergogenic Properties of the Bifidobacterium longum OLP-01 Isolated from a Weightlifting Gold Medalist. Nutrients, 11(9), 2003. https://doi.org/10.3390/nu11092003







