Fish Consumption at One Year of Age Reduces the Risk of Eczema, Asthma and Wheeze at Six Years of Age
Abstract
1. Introduction
2. Materials and Methods
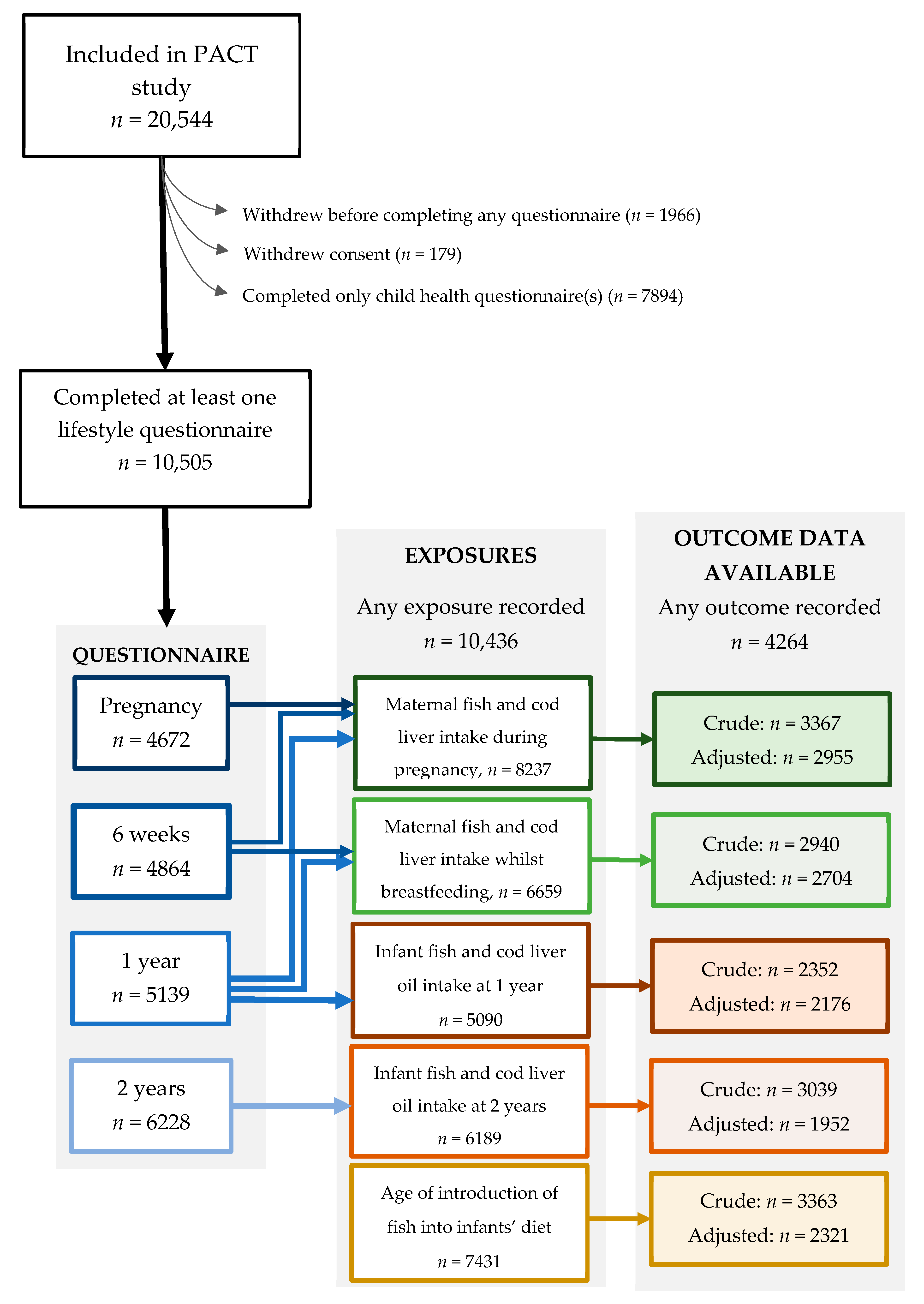
2.1. Study Population
2.2. Exposure Variables: Dietary Fish and Cod Liver Oil
2.3. Outcome Variable: Eczema, Asthma, Wheeze and Allergic Rhinoconjunctivitis
2.4. Covariates
2.5. Statistical Analysis
2.5.1. Description of Population and Dietary Correlations
2.5.2. Main Analyses
2.5.3. Sensitivity Analyses
2.6. Ethics Approval
3. Results
3.1. Participant Characteristics
3.2. Maternal and Infant Dietary Fish and Cod Liver Oil Intake: Description and Correlations
3.3. Maternal Fish and Cod Liver Oil Intake During Pregnancy and Allergy-Related Outcomes at Six Years
3.4. Maternal Fish and Cod Liver Oil Intake Whilst Breastfeeding and Allergy-Related Outcomes at Six Years
3.5. Fish and Cod Liver Consumption by Infants at One Year and Two Years and Allergy-Related Outcomes at Six Years
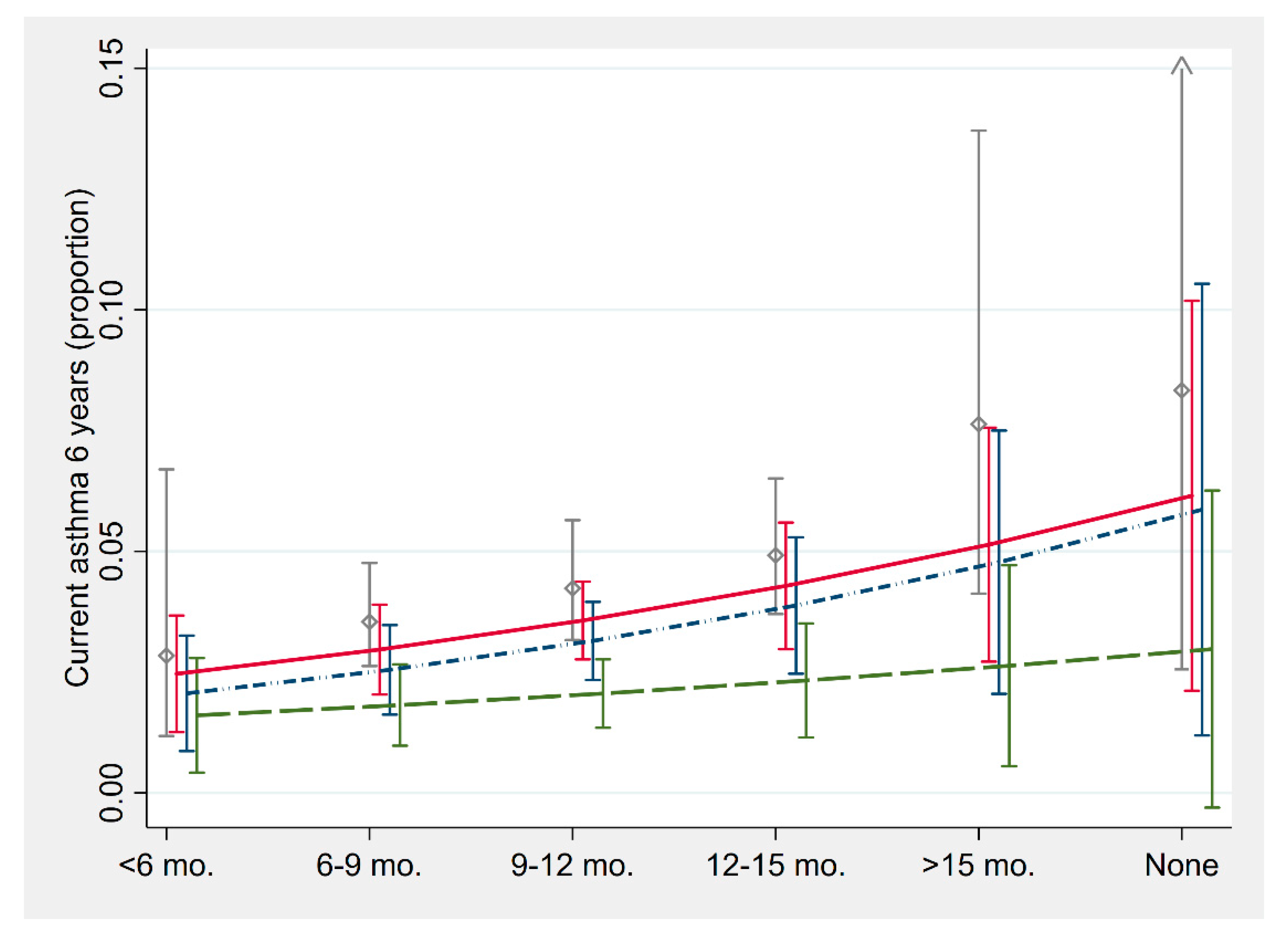
3.6. Age of Introduction of Fish into Infant Diet and Allergy-Related Outcomes at Six Years
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asher, M.I.; Montefort, S.; Bjorksten, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Mallol, J.; Crane, J.; Von Mutius, E.; Odhiambo, J.; Keil, U.; Stewart, A. The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: A global synthesis. Allergol. Immunopathol. 2013, 41, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Black, P.; Sharpe, S. Dietary fat and asthma: Is there a connection? Eur. Respir. J. 1997, 10, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Kremmyda, L.S.; Vlachava, M.; Noakes, P.S.; Diaper, N.D.; Miles, E.A.; Calder, P.C. Atopy risk in infants and children in relation to early exposure to fish, oily fish, or long-chain omega-3 fatty acids: A systematic review. Clin. Rev. Allergy Immunol. 2011, 41, 36–66. [Google Scholar] [CrossRef]
- Devereux, G.; Seaton, A. Diet as a risk factor for atopy and asthma. J. Allergy Clin. Immunol. 2005, 115, 1109–1117. [Google Scholar] [CrossRef]
- Blumer, N.; Renz, H. Consumption of omega3-fatty acids during perinatal life: Role in immuno-modulation and allergy prevention. J. Perinat. Med. 2007, 35 (Suppl. 1), S12–S18. [Google Scholar] [CrossRef]
- Best, K.P.; Sullivan, T.R.; Palmer, D.J.; Gold, M.; Martin, J.; Kennedy, D.; Makrides, M. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood—A longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ. J. 2018, 11, 10. [Google Scholar] [CrossRef]
- Thorsen, J.; Bisgaard, H.; Chawes, B.L.; Vissing, N.H.; Schoos, A.-M.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Følsgaard, N.V.; Fink, N.R.; et al. Fish Oil–Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N. Engl. J. Med. 2016, 375, 2530–2539. [Google Scholar]
- Garcia-Larsen, V.; Ierodiakonou, D.; Jarrold, K.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Ruparelia, A.; Devani, P.; Trivella, M.; et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: A systematic review and meta-analysis. PLoS Med. 2018, 15, e1002507. [Google Scholar] [CrossRef]
- Anandan, C.; Nurmatov, U.; Sheikh, A. Omega 3 and 6 oils for primary prevention of allergic disease: Systematic review and meta-analysis. Allergy 2009, 64, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Calder, P.C. Can Early Omega-3 Fatty Acid Exposure Reduce Risk of Childhood Allergic Disease? Nutrients 2017, 9, 784. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.; Sinn, J.K.H.; Osborn, D.A. Polyunsaturated fatty acid supplementation in infancy for the prevention of allergy. Cochrane Database Syst. Rev. 2016, 10, CD010112. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.T.; Li, Y.F.; Langholz, B.; Gilliland, F.D. Maternal fish consumption during pregnancy and risk of early childhood asthma. J. Asthma 2005, 42, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Torrent, M.; Garcia-Esteban, R.; Ferrer, C.; Ribas-Fitó, N.; Antó, J.M.; Sunyer, J.; Garcia-Esteban, R.; Ribas-Fitó, N. Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin. Exp. Allergy 2007, 37, 518–525. [Google Scholar] [CrossRef] [PubMed]
- LISA Study Group; Sausenthaler, S.; Koletzko, S.; Schaaf, B.; Lehmann, I.; Borte, M.; Herbarth, O.; Von Berg, A.; Wichmann, H.-E.; Heinrich, J. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am. J. Clin. Nutr. 2007, 85, 530–537. [Google Scholar] [CrossRef]
- Willers, S.M.; Devereux, G.; Craig, L.C.A.; McNeill, G.; Wijga, A.H.; El-Magd, W.A.; Turner, S.W.; Helms, P.J.; Seaton, A. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax 2007, 62, 773–779. [Google Scholar] [CrossRef]
- Virtanen, S.M.; Kaila, M.; Pekkanen, J.; Kenward, M.G.; Uusitalo, U.; Pietinen, P.; Kronberg-Kippilä, C.; Hakulinen, T.; Simell, O.; Ilonen, J.; et al. Early introduction of oats associated with decreased risk of persistent asthma and early introduction of fish with decreased risk of allergic rhinitis. Br. J. Nutr. 2010, 103, 266–273. [Google Scholar] [CrossRef]
- Nafstad, P.; Nystad, W.; Magnus, P.; Jaakkola, J.J.K. Asthma and Allergic Rhinitis at 4 Years of Age in Relation to Fish Consumption in Infancy. J. Asthma 2003, 40, 343–348. [Google Scholar] [CrossRef]
- Kull, I.; Bergström, A.; Lilja, G.; Pershagen, G.; Wickman, M. Fish consumption during the first year of life and development of allergic diseases during childhood. Allergy 2006, 61, 1009–1015. [Google Scholar] [CrossRef]
- Noakes, P.S.; Vlachava, M.; Kremmyda, L.-S.; Diaper, N.D.; Miles, E.; Erlewyn-Lajeunesse, M.; Williams, A.P.; Godfrey, K.M.; Calder, P.C. Increased intake of oily fish in pregnancy: Effects on neonatal immune responses and on clinical outcomes in infants at 6 mo. Am. J. Clin. Nutr. 2012, 95, 395–404. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Liu, B.; Li, J.; Luo, C.Q.; Zhang, Q.; Chen, J.L.; Sinha, A.; Li, Z.Y. Fish intake during pregnancy or infancy and allergic outcomes in children: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2017, 28, 152–161. [Google Scholar] [CrossRef]
- Itsiopoulos, C.; Erbas, B.; Papamichael, M.M.; Shrestha, S.K. The role of fish intake on asthma in children: A meta-analysis of observational studies. Pediatr. Allergy Immunol. 2018, 29, 350–360. [Google Scholar]
- Dotterud, C.K.; Storrø, O.; Simpson, M.R.; Johnsen, R.; Øien, T. The impact of pre- and postnatal exposures on allergy related diseases in childhood: A controlled multicentre intervention study in primary health care. BMC Public Health 2013, 13, 123. [Google Scholar] [CrossRef]
- Oien, T.; Storro, O.; Johnsen, R. Do early intake of fish and fish oil protect against eczema and doctor-diagnosed asthma at 2 years of age? A cohort study. J. Epidemiol. Community Health 2010, 64, 124–129. [Google Scholar] [CrossRef]
- Storro, O.; Oien, T.; Dotterud, C.K.; Jenssen, J.A.; Johnsen, R. A primary health-care intervention on pre- and postnatal risk factor behavior to prevent childhood allergy. The Prevention of Allergy among Children in Trondheim (PACT) study. BMC Public Health 2010, 10, 443. [Google Scholar] [CrossRef]
- Johansson, S.G.O.; Hourihane, J.; Bousquet, J.; Bruijnzeel-Koomen, C.; Dreborg, S.; Haahtela, T.; Kowalski, M.L.; Mygind, N.; Ring, J.; Van Cauwenberge, P.; et al. A revised nomenclature for allergy: An EAACI position statement from the EAACI nomenclature task force. Allergy 2001, 56, 813–824. [Google Scholar] [CrossRef]
- Øien, T.; Storrø, O.; Johnsen, R. Assessing atopic disease in children two to six years old: Reliability of a revised questionnaire. Prim. Care Respir. J. 2008, 17, 164–168. [Google Scholar] [CrossRef][Green Version]
- Hasselberg, P.K.J.; Agerlie, K.; Berre, T.; Johansen, T. Hvor tjener folk i Trondheim Mest? [Norwegian: Where do people earn the most in Trondheim?]. 2010. Available online: https://www.nrk.no/trondelag/hvor-tjener-folk-i-trondheim-mest_-1.7344254 (accessed on 25 May 2018).
- Stratakis, N.; Roumeliotaki, T.; Oken, E.; Ballester, F.; Barros, H.; Basterrechea, M.; Cordier, S.; De Groot, R.; Dekker, H.T.D.; Duijts, L.; et al. Fish and seafood consumption during pregnancy and the risk of asthma and allergic rhinitis in childhood: A pooled analysis of 18 European and US birth cohorts. Int. J. Epidemiol. 2017, 46, 1465–1477. [Google Scholar] [CrossRef]
- Goksör, E.; Alm, B.; Thengilsdottir, H.; Pettersson, R.; Åberg, N.; Wennergren, G. Preschool wheeze—Impact of early fish introduction and neonatal antibiotics. Acta Paediatr. 2011, 100, 1561–1566. [Google Scholar] [CrossRef]
- Mermiri, D.-Z.T.; Lappa, T.; Papadopoulou, A.L. Review suggests that the immunoregulatory and anti-inflammatory properties of allergenic foods can provoke oral tolerance if introduced early to infants’ diets. Acta Paediatr. 2017, 106, 721–726. [Google Scholar] [CrossRef]
- Caffarelli, C.; Di Mauro, D.; Mastrorilli, C.; Bottau, P.; Cipriani, F.; Ricci, G. Solid Food Introduction and the Development of Food Allergies. Nutrients 2018, 10, 1790. [Google Scholar] [CrossRef]
- Logan, K.; Tseng, A.; Raji, B.; Marrs, T.; Lack, G.; Perkin, M.R.; Ayis, S.; Peacock, J.; Brough, H.; Radulovic, S.; et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N. Engl. J. Med. 2016, 374, 1733–1743. [Google Scholar]
- Lee-Sarwar, K.; Kelly, R.S.; Lasky-Su, J.; Kachroo, P.; Zeiger, R.S.; O’Connor, G.T.; Sandel, M.T.; Bacharier, L.B.; Beigelman, A.; Laranjo, N.; et al. Dietary and Plasma Polyunsaturated Fatty Acids Are Inversely Associated with Asthma and Atopy in Early Childhood. J. Allergy Clin. Immunol. Pract. 2019, 7, 529–538. [Google Scholar] [CrossRef]
- Kachroo, P.; Kelly, R.S.; Mirzakhani, H.; Lee-Sarwar, K.; Chawes, B.L.; Blighe, K.; Davaasambuu, G.; Bisgaard, H.; Litonjua, A.A.; Weiss, S.T.; et al. Fish oil supplementation during pregnancy is protective against asthma/wheeze in offspring. J. Allergy Clin. Immunol. Pract. 2019. [Google Scholar] [CrossRef]
| Included (N = 4264) | Drop-outs (N = 6241) | ||||||
|---|---|---|---|---|---|---|---|
| Binary Covariates | N | n | % | N | n | % | p-Value |
| Sex, male | 4261 | 2131 | 50.0 | 5339 | 2677 | 50.1 | 0.900 |
| Family history | 3224 | 2264 | 70.2 | 4535 | 3332 | 73.5 | 0.002 |
| Older siblings | 3229 | 1823 | 56.5 | 4525 | 2323 | 51.3 | <0.001 |
| Breastfeeding at 6 mo | 3702 | 3134 | 84.7 | 4442 | 3571 | 80.4 | <0.001 |
| Mother smoking | |||||||
| Pregnancy | 1896 | 130 | 6.9 | 2488 | 213 | 8.6 | 0.037 |
| 6 weeks | 2178 | 165 | 7.6 | 2405 | 240 | 10.0 | 0.004 |
| 1 year | 2319 | 348 | 15.0 | 2678 | 480 | 17.9 | 0.006 |
| Ever in first year | 3334 | 466 | 14.0 | 4793 | 752 | 15.7 | 0.033 |
| Father smoking | |||||||
| Pregnancy | 1852 | 296 | 16.0 | 2395 | 481 | 20.1 | 0.001 |
| 6 weeks | 2072 | 340 | 16.4 | 2299 | 451 | 19.6 | 0.006 |
| 1 year | 2164 | 371 | 17.1 | 2490 | 506 | 20.3 | 0.006 |
| Ever in first year | 3230 | 665 | 20.6 | 4573 | 1059 | 23.2 | 0.007 |
| Parental smoking | |||||||
| Pregnancy | 1830 | 316 | 17.3 | 2371 | 511 | 21.6 | 0.001 |
| 6 weeks | 2040 | 367 | 18.0 | 2225 | 458 | 20.6 | 0.032 |
| 1 year | 2144 | 460 | 21.5 | 2459 | 612 | 24.9 | 0.006 |
| Ever in first year | 3209 | 779 | 24.3 | 4516 | 1204 | 26.7 | 0.018 |
| Cohort, intervention | 4264 | 1204 | 28.2 | 6241 | 1714 | 27.5 | 0.385 |
| Continuous covariates | N | mean | SD | N | mean | SD | |
| Maternal age, yrs | 4244 | 30.3 | 4.4 | 5258 | 29.3 | 4.7 | <0.001 |
| Birthweight, gm | 3944 | 3583 | 565 | 5100 | 3579 | 597 | 0.716 |
| Breastfeeding duration, mo. | 3392 | 10.7 | 5.6 | 3966 | 9.8 | 5.7 | <0.001 |
| Income, NOK | 4158 | 257,043 | 28,563 | 5664 | 250,535 | 31,617 | <0.001 |
| Maternal education, yrs | 2573 | 15.7 | 2.6 | 1331 | 15.5 | 2.6 | 0.051 |
| Paternal education, yrs | 2543 | 15.1 | 3.0 | 1314 | 15.1 | 3.0 | 0.896 |
| ≥1 Time/Week | <1 Time Per Week | Crude | Adjusted (Model 1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N1 | n1 | % | N0 | n0 | % | OR | aOR | 95% CI | p-Value | |
| Any fish | |||||||||||
| Current eczema | 2125 | 1108 | 125 | 11.3 | 1017 | 153 | 15.0 | 0.73 | 0.72 | 0.56–0.93 | 0.011 |
| Current asthma | 2162 | 1129 | 37 | 3.3 | 1033 | 57 | 5.5 | 0.58 | 0.60 | 0.39–0.91 | 0.018 |
| Current wheeze | 2139 | 1115 | 93 | 8.3 | 1024 | 131 | 12.8 | 0.61 | 0.66 | 0.50–0.88 | 0.005 |
| Ever ARC | 2023 | 1067 | 121 | 11.3 | 956 | 118 | 12.3 | 0.93 | 0.92 | 0.70–1.21 | 0.534 |
| Oily fish | |||||||||||
| Current eczema | 2126 | 579 | 74 | 12.8 | 1547 | 204 | 13.2 | 1.03 | 0.95 | 0.71–1.26 | 0.713 |
| Current asthma | 2163 | 590 | 20 | 3.4 | 1573 | 74 | 4.7 | 0.71 | 0.72 | 0.43–1.20 | 0.211 |
| Current wheeze | 2140 | 583 | 51 | 8.7 | 1557 | 173 | 11.1 | 0.74 | 0.83 | 0.60–1.16 | 0.276 |
| Ever ARC | 2024 | 556 | 69 | 12.4 | 1468 | 170 | 11.6 | 1.11 | 1.07 | 0.79–1.45 | 0.657 |
| Lean fish | |||||||||||
| Current eczema | 2129 | 966 | 110 | 11.4 | 1163 | 169 | 14.5 | 0.74 | 0.76 | 0.59–0.99 | 0.043 |
| Current asthma | 2166 | 985 | 30 | 3.0 | 1181 | 64 | 5.4 | 0.54 | 0.56 | 0.36–0.88 | 0.012 |
| Current wheeze | 2143 | 973 | 82 | 8.4 | 1170 | 143 | 12.2 | 0.65 | 0.69 | 0.52–0.92 | 0.012 |
| Ever ARC | 2027 | 932 | 101 | 10.8 | 1095 | 139 | 12.7 | 0.86 | 0.85 | 0.65–1.12 | 0.250 |
| Oily Fish Only Vs. None | Lean Fish Only Vs. None | Both Fish Vs. None | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | |
| Current eczema | 2125 | 0.68 | 0.39−1.17 | 0.164 | 0.61 | 0.44−0.86 | 0.005 | 0.86 | 0.62−1.20 | 0.388 |
| Current asthma | 2162 | 0.87 | 0.38−1.96 | 0.731 | 0.58 | 0.33−1.00 | 0.051 | 0.53 | 0.29−0.99 | 0.047 |
| Current wheeze | 2139 | 0.69 | 0.42−1.29 | 0.243 | 0.61 | 0.42−0.88 | 0.008 | 0.73 | 0.50−1.06 | 0.100 |
| Ever ARC | 2023 | 1.25 | 0.75−2.08 | 0.396 | 0.83 | 0.58−1.17 | 0.279 | 0.93 | 0.65−1.33 | 0.678 |
| CrudeOR ‡ | Adjusted (Model 1) | Infants with Symptoms before 6 Months Excluded | INFANTS with Symptoms before 12 Months Excluded | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | aOR ‡ | 95% CI | N | aOR ‡ | 95% CI | N | aOR ‡ | 95% CI | ||
| Current eczema | 0.88 | 2369 | 0.89 | 0.78−1.02 | 1987 | 0.90 | 0.76−1.06 | 1758 | 0.95 | 0.77−1.17 |
| Current asthma | 0.81 | 2406 | 0.83 | 0.66−1.03 | 2012 | 0.80 | 0.62−1.05 | 1777 | 0.88 | 0.62−1.25 |
| Current wheeze | 0.89 | 2382 | 0.93 | 0.80−1.07 | 1992 | 0.98 | 0.82−1.15 | 1760 | 1.03 | 0.85−1.25 |
| ARC | 0.85 | 2245 | 0.86 | 0.75−0.98 | 1903 | 0.96 | 0.81−1.14 | 1695 | 0.93 | 0.77−1.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Øien, T.; Schjelvaag, A.; Storrø, O.; Johnsen, R.; Simpson, M.R. Fish Consumption at One Year of Age Reduces the Risk of Eczema, Asthma and Wheeze at Six Years of Age. Nutrients 2019, 11, 1969. https://doi.org/10.3390/nu11091969
Øien T, Schjelvaag A, Storrø O, Johnsen R, Simpson MR. Fish Consumption at One Year of Age Reduces the Risk of Eczema, Asthma and Wheeze at Six Years of Age. Nutrients. 2019; 11(9):1969. https://doi.org/10.3390/nu11091969
Chicago/Turabian StyleØien, Torbjørn, Astrid Schjelvaag, Ola Storrø, Roar Johnsen, and Melanie Rae Simpson. 2019. "Fish Consumption at One Year of Age Reduces the Risk of Eczema, Asthma and Wheeze at Six Years of Age" Nutrients 11, no. 9: 1969. https://doi.org/10.3390/nu11091969
APA StyleØien, T., Schjelvaag, A., Storrø, O., Johnsen, R., & Simpson, M. R. (2019). Fish Consumption at One Year of Age Reduces the Risk of Eczema, Asthma and Wheeze at Six Years of Age. Nutrients, 11(9), 1969. https://doi.org/10.3390/nu11091969





