The Nutraceutical Value of Olive Oil and Its Bioactive Constituents on the Cardiovascular System. Focusing on Main Strategies to Slow Down Its Quality Decay during Production and Storage
Abstract
1. Introduction
2. Methodology
3. Unsaponifiable Fraction
3.1. Polyphenolic Components
3.1.1. Beneficial Effects of Polyphenols: Clinical Evidence
3.1.2. Beneficial Effects of Polyphenols: Preclinical Evidence
3.2. Vitamin E
3.2.1. Beneficial Effects of Vitamin E: Clinical Evidence
3.2.2. Beneficial Effects of Vitamin E: Preclinical Evidence
4. Saponifiable Fraction
4.1. MUFA
4.1.1. Beneficial Effects of Oleic Acid: Clinical Evidence
4.1.2. Preclinical Evidence of Beneficial Effects of Oleic Acid
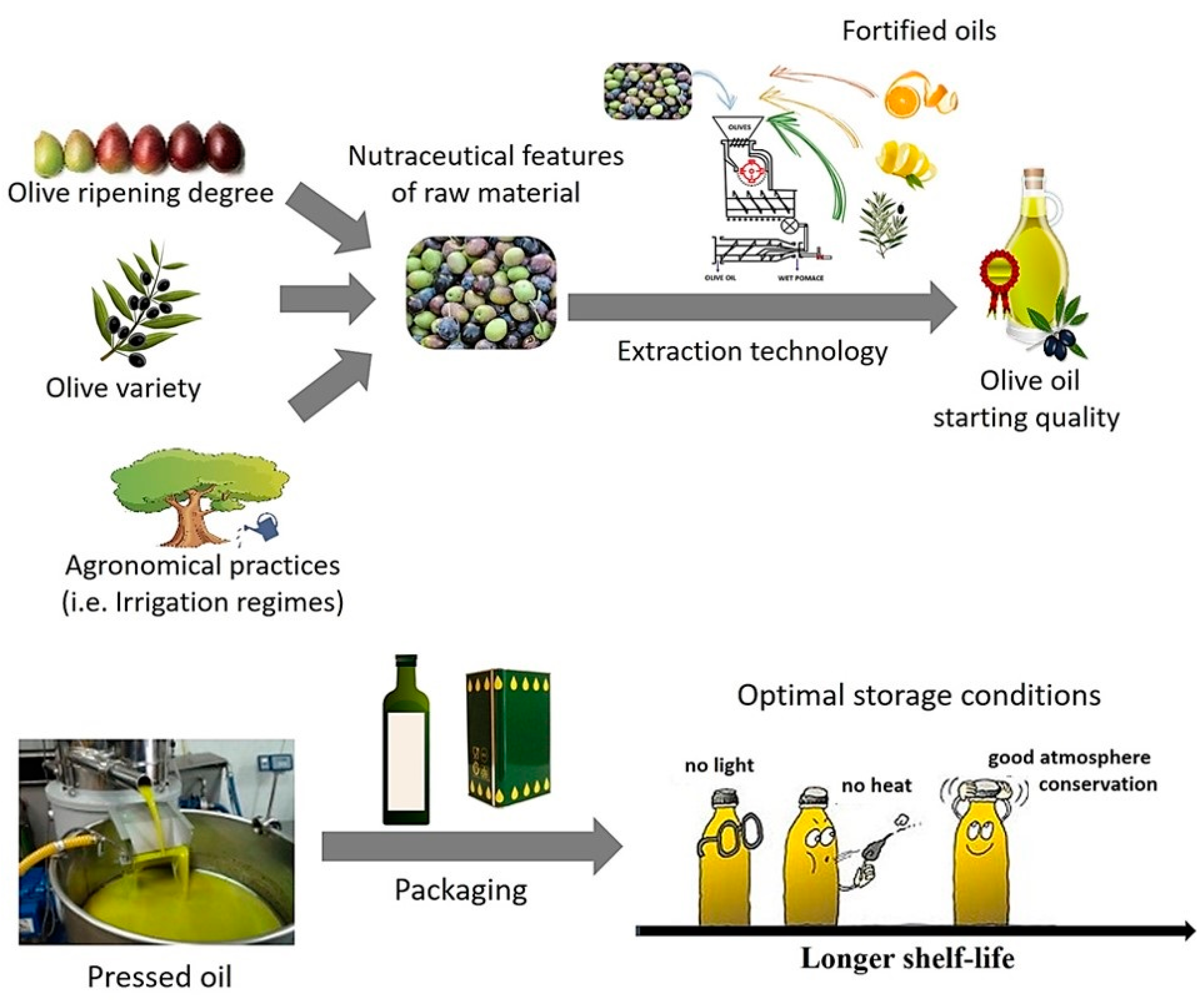
5. Focus on The Main Operating Conditions Adopted for EVOO Production and/or Storage: Influence on The Initial Concentration of Health Compounds and on The Kinetics of Their Degradation during Storage
5.1. Chemical Composition of Olive oil at Starting of Storage Time
5.1.1. Characteristic of Raw Materials: Olive Cultivar, Ripening Degree, and Agronomic Practices
5.1.2. Extraction Technology
5.2. Main Parameters Affecting the Degradation Rate of Health Compounds During EVOO Storage
5.2.1. Influence of Storage Atmosphere
5.2.2. Characteristics of Packaging and Storage Temperature
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corra, U.; Cosyns, B.; Deaton, C. European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D. Research related to underlying mechanisms in atherosclerosis. Circulation 1979, 60, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, T.; Mahoney, E.M.; Steinberg, D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: Recognition by receptors for acetylated low density lipoproteins. Proc. Natl. Acad. Sci. USA 1981, 78, 6499–6503. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, S.; Quinn, M.T.; Steinberg, D. Is oxidised low density lipoprotein involved in the recruitment and retention of monocyte/macrophages in the artery wall during the initiation of atherosclerosis. In Oxygen Radicals in Biology and Medicine; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Landmesser, U.; Harrison, D.G. Oxidant stress as a marker for cardiovascular events: Ox marks the spot. Circulation 2001, 104, 2638–2640. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sethi, R.; Takeda, N.; Nagano, M.; Dhalla, N.S. Beneficial effects of vitamin E treatment in acute myocardial infarction. J. Cardiovasc. Pharmacol. Ther. 2000, 5, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M.J.; Hennekens, C.H.; Manson, J.E.; Colditz, G.A.; Rosner, B.; Willett, W.C. Vitamin E consumption and the risk of coronary disease in women. N. Engl. J. Med. 1993, 328, 1444–1449. [Google Scholar] [CrossRef]
- Degirolamo, C.; Rudel, L.L. Dietary monounsaturated fatty acids appear not to provide cardioprotection. Curr. Atheroscler. Rep. 2010, 12, 391–396. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Covas, M.I.; Fitó, M.; Kušar, A.; Pravst, I. Health effects of olive oil polyphenols: Recent advances and possibilities for the use of health claims. Mol. Nutr. Food Res. 2013, 57, 760–771. [Google Scholar] [CrossRef]
- Vargas, A.J.; Neuhouser, M.L.; George, S.M.; Thomson, C.A.; Ho, G.Y.; Rohan, T.E.; Kato, I.; Nassir, R.; Hou, L.; Manson, J.E. Diet quality and colorectal cancer risk in the Women’s Health Initiative Observational Study. Am. J. Epidemiol. 2016, 184, 23–32. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Davalos, A.; López de las Hazas, M.C.; Crespo, M.C.; Tomé-Carneiro, J. An overview of the pharmacology of olive oil and its active ingredients. Br. J. Pharmacol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Piroddi, M.; Albini, A.; Fabiani, R.; Giovannelli, L.; Luceri, C.; Natella, F.; Rosignoli, P.; Rossi, T.; Taticchi, A.; Servili, M. Nutrigenomics of extra-virgin olive oil: A review. Biofactors 2017, 43, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—A review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Domínguez, M.L.; Raigón, M.D.; Prohens, J. Diversity for olive oil composition in a collection of varieties from the region of Valencia (Spain). Food Res. Int. 2013, 54, 1941–1949. [Google Scholar] [CrossRef]
- Kris-Etherton, P.; Derr, J.; Mitchell, D.C.; Mustad, V.A.; Russell, M.E.; McDonnell, E.T.; Salabsky, D.; Pearson, T.A. The role of fatty acid saturation on plasma lipids, lipoproteins, and apolipoproteins: I. Effects of whole food diets high in cocoa butter, olive oil, soybean oil, dairy butter, and milk chocolate on the plasma lipids of young men. Metabolism 1993, 42, 121–129. [Google Scholar] [CrossRef]
- Jansen, S.; López-Miranda, J.; Castro, P.; López-Segura, F.; Marín, C.; Ordovás, J.M.; Paz, E.; Jiménez-Perepérez, J.; Fuentes, F.; Pérez-Jiménez, F. Low-fat and high–monounsaturated fatty acid diets decrease plasma cholesterol ester transfer protein concentrations in young, healthy, normolipemic men. Am. J. Clin. Nutr. 2000, 72, 36–41. [Google Scholar] [CrossRef][Green Version]
- Fuentes, F.; López-Miranda, J.; Sánchez, E.; Sánchez, F.; Paez, J.; Paz-Rojas, E.; Marín, C.; Gómez, P.; Jimenez-Perepérez, J.; Ordovás, J.M. Mediterranean and low-fat diets improve endothelial function in hypercholesterolemic men. Ann. Intern. Med. 2001, 134, 1115–1119. [Google Scholar] [CrossRef]
- Mata, P.; Garrido, J.A.; Ordovas, J.M.; Blazquez, E.; Alvarez-Sala, L.A.; Rubio, M.J.; Alonso, R.; De Oya, M. Effect of dietary monounsaturated fatty acids on plasma lipoproteins and apolipoproteins in women. Am. J. Clin. Nutr. 1992, 56, 77–83. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M. Monounsaturated fatty acids and risk of cardiovascular disease. Circulation 1999, 100, 1253–1258. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the substantiation of health claims related to olive oil and maintenance of normal blood LDL-cholesterol concentrations (ID 1316, 1332), maintenance of normal (fasting) blood concentrations of triglycerides (ID 1316, 1332), maintenance of normal blood HDL cholesterol concentrations (ID 1316, 1332) and maintenance of normal blood glucose concentrations (ID 4244) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2044. [CrossRef]
- FDA. FDA Allows Qualified Health Claim to Decrease Risk of Coronary Heart Disease. 2004. Available online: http://www. fda.gov/bbs/topics/news/2004/NEW01129. html (accessed on 15 May 2019).
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Stefani, M. Nutraceutical properties of olive oil polyphenols. An itinerary from cultured cells through animal models to humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R. Anti-cancer properties of olive oil secoiridoid phenols: A systematic review of in vivo studies. Food Funct. 2016, 7, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol exerts anti-inflammatory and anti-oxidant activities in a mouse model of systemic inflammation. Molecules 2018, 23, 3212. [Google Scholar] [CrossRef] [PubMed]
- Margheri, F.; Menicacci, B.; Laurenzana, A.; Del Rosso, M.; Fibbi, G.; Cipolleschi, M.G.; Ruzzolini, J.; Nediani, C.; Mocali, A.; Giovannelli, L. Oleuropein aglycone attenuates the pro-angiogenic phenotype of senescent fibroblasts: A functional study in endothelial cells. J. Funct. Foods 2019, 53, 219–226. [Google Scholar] [CrossRef]
- Mantilla-Escalante, D.C.; López de las Hazas, M.-C.; Gil-Zamorano, J.; del Pozo-Acebo, L.; Crespo, M.C.; Martín-Hernández, R.; del Saz, A.; Tomé-Carneiro, J.; Cardona, F.; Cornejo-Pareja, I. Postprandial Circulating miRNAs in Response to a Dietary Fat Challenge. Nutrients 2019, 11, 1326. [Google Scholar] [CrossRef]
- Terzuoli, E.; Nannelli, G.; Giachetti, A.; Morbidelli, L.; Ziche, M.; Donnini, S. Targeting endothelial-to-mesenchymal transition: The protective role of hydroxytyrosol sulfate metabolite. Eur. J. Nutr. 2019, 1–11. [Google Scholar] [CrossRef]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation–A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- de las Hazas, M.-C.L.; Piñol, C.; Macià, A.; Romero, M.-P.; Pedret, A.; Solà, R.; Rubió, L.; Motilva, M.-J. Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef]
- Imran, M.; Nadeem, M.; Gilani, S.A.; Khan, S.; Sajid, M.W.; Amir, R.M. Antitumor perspectives of oleuropein and its metabolite hydroxytyrosol: Recent updates. J. Food Sci. 2018, 83, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- de las Hazas, M.-C.L.; Godinho-Pereira, J.; Macià, A.; Almeida, A.F.; Ventura, M.R.; Motilva, M.-J.; Santos, C.N. Brain uptake of hydroxytyrosol and its main circulating metabolites: Protective potential in neuronal cells. J. Funct. Foods 2018, 46, 110–117. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Biological relevance of extra virgin olive oil polyphenols metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ávila, C.; Montes, R.; Castellote, A.; Chisaguano, A.; Fitó, M.; Covas, M.; Muñoz-Aguallo, D.; Nyyssönen, K.; Zunft, H.; López-Sabater, M. Fast determination of virgin olive oil phenolic metabolites in human high-density lipoproteins. Biomed. Chromatogr. 2015, 29, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Perez-Mana, C.; Khymenets, O.; Fitó, M.; Farré, M. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Robledo, P.; Tanner, J.-A.; Boronat, A.; Pérez-Mañá, C.; Chen, C.-Y.O.; Tyndale, R.F.; de la Torre, R. CYP2D6 and CYP2A6 biotransform dietary tyrosol into hydroxytyrosol. Food Chem. 2017, 217, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Martín-Peláez, S.; Macià, A.; Farràs, M.; Valls, R.M.; Catalán, Ú.; Motilva, M.J. Faecal microbial metabolism of olive oil phenolic compounds: In vitro and in vivo approaches. Mol. Nutr. Food Res. 2014, 58, 1809–1819. [Google Scholar] [CrossRef]
- Cicero, A.F.; Nascetti, S.; López-Sabater, M.C.; Elosua, R.; Salonen, J.T.; Nyyssönen, K.; Poulsen, H.E.; Zunft, H.-J.F.; Kiesewetter, H.; de la Torre, K. Changes in LDL fatty acid composition as a response to olive oil treatment are inversely related to lipid oxidative damage: The EUROLIVE study. J. Am. Coll. Nutr. 2008, 27, 314–320. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781),“anti-inflammatory properties”(ID 1882),“contributes to the upper respiratory tract health”(ID 3468),“can help to maintain a normal function of gastrointestinal tract”(3779), and “contributes to body defences against external agents”(ID 3467) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [CrossRef]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar] [PubMed]
- Crespo, M.C.; Tomé-Carneiro, J.; Burgos-Ramos, E.; Kohen, V.L.; Espinosa, M.I.; Herranz, J.; Visioli, F. One-week administration of hydroxytyrosol to humans does not activate Phase II enzymes. Pharmacol. Res. 2015, 95, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Valls, R.-M.; Farràs, M.; Suárez, M.; Fernández-Castillejo, S.; Fitó, M.; Konstantinidou, V.; Fuentes, F.; López-Miranda, J.; Giralt, M.; Covas, M.-I. Effects of functional olive oil enriched with its own phenolic compounds on endothelial function in hypertensive patients. A randomised controlled trial. Food Chem. 2015, 167, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, C.-D.; Cramer, H.; Michalsen, A.; Kessler, C.; Steckhan, N.; Choi, K.; Dobos, G. Effects of high phenolic olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Phytomedicine 2015, 22, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef] [PubMed]
- González-Correa, J.A.; Navas, M.D.; Muñoz-Marϭn, J.; Trujillo, M.; Fernández-Bolaños, J.; de la Cruz, J.P. Effects of hydroxytyrosol and hydroxytyrosol acetate administration to rats on platelet function compared to acetylsalicylic acid. J. Agric. Food Chem. 2008, 56, 7872–7876. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Maschi, O.; Galli, G.V.; Fagnani, R.; Dal Cero, E.; Caruso, D.; Bosisio, E. Inhibition of platelet aggregation by olive oil phenols via cAMP-phosphodiesterase. Br. J. Nutr. 2008, 99, 945–951. [Google Scholar] [CrossRef]
- Buckland, G.; Travier, N.; Agudo, A.; Fonseca-Nunes, A.; Navarro, C.; Lagiou, P.; Demetriou, C.; Amiano, P.; Dorronsoro, M.; Chirlaque, M.D. Olive oil intake and breast cancer risk in the Mediterranean countries of the European Prospective Investigation into Cancer and Nutrition study. Int. J. Cancer 2012, 131, 2465–2469. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: A randomised clinical trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; De la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J. Mediterranean diet and age-related cognitive decline: A randomised clinical trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; San Julian, B.; Sanchez-Tainta, A.; Corella, D.; Lamuela-Raventos, R.; Martinez, J.; Martinez-Gonzalez, M. Virgin olive oil supplementation and long-term cognition: The PREDIMED-NAVARRA randomised, trial. J. Nutr. Health Aging 2013, 17, 544–552. [Google Scholar] [CrossRef] [PubMed]
- de las Hazas, M.C.; Rubio, L.; Macia, A.; Motilva, M.J. Hydroxytyrosol: Emerging trends in potential therapeutic applications. Curr. Pharm. Des. 2018, 24, 2157–2179. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Huertas, E.; Fonolla, J. Hydroxytyrosol supplementation increases vitamin C levels in vivo. A human volunteer trial. Redox Biol. 2017, 11, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-D.; Chen, Z.-Z.; Li, N.; Lu, W.-F.; Xu, Y.-H.; Lin, Y.-Y.; Shao, L.-H.; Wang, Q.-T.; Guo, L.-Y.; Gao, Y.-Q. Hydroxytyrosol nicotinate, a new multifunctional hypolipidemic and hypoglycemic agent. Biomed. Pharmacother. 2018, 99, 715–724. [Google Scholar] [CrossRef] [PubMed]
- D’Addato, S.; Scandiani, L.; Mombelli, G.; Focanti, F.; Pelacchi, F.; Salvatori, E.; Di Loreto, G.; Comandini, A.; Maffioli, P.; Derosa, G. Effect of a food supplement containing berberine, monacolin K, hydroxytyrosol and coenzyme Q10 on lipid levels: A randomised, double-blind, placebo controlled study. Drug Des. Dev. Ther. 2017, 11, 1585. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Mosele, J.I.; Pizarro, N.; Farràs, M.; de la Torre, R.; Subirana, I.; Pérez-Cano, F.J.; Castañer, O.; Solà, R.; Fernandez-Castillejo, S. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. Eur. J. Nutr. 2017, 56, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Ruano, J.; López-Miranda, J.; de la Torre, R.; Delgado-Lista, J.; Fernández, J.; Caballero, J.; Covas, M.I.; Jiménez, Y.; Pérez-Martínez, P.; Marín, C. Intake of phenol-rich virgin olive oil improves the postprandial prothrombotic profile in hypercholesterolemic patients. Am. J. Clin. Nutr. 2007, 86, 341–346. [Google Scholar] [CrossRef]
- Muhindo, C.T.; Ahn, S.A.; Rousseau, M.F.; Dierckxsens, Y.; Hermans, M.P. Efficacy and safety of a combination of red yeast rice and olive extract in hypercholesterolemic patients with and without statin-associated myalgia. Complement. Ther. Med. 2017, 35, 140–144. [Google Scholar] [CrossRef] [PubMed]
- de Bock, M.; Derraik, J.G.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomised, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef] [PubMed]
- Hermans, N.; Van der Auwera, A.; Breynaert, A.; Verlaet, A.; De Bruyne, T.; Van Gaal, L.; Pieters, L.; Verhoeven, V. A red yeast rice-olive extract supplement reduces biomarkers of oxidative stress, OxLDL and Lp-PLA 2, in subjects with metabolic syndrome: A randomised, double-blind, placebo-controlled trial. Trials 2017, 18, 302. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, V.; Van der Auwera, A.; Van Gaal, L.; Remmen, R.; Apers, S.; Stalpaert, M.; Wens, J.; Hermans, N. Can red yeast rice and olive extract improve lipid profile and cardiovascular risk in metabolic syndrome?: A double blind, placebo controlled randomised trial. BMC Complement. Altern. Med. 2015, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Pais, P.; Villar, A.; Rull, S. Impact of a proprietary standardised olive fruit extract (SOFE) on cardio-ankle vascular index, visual analog scale and c-reactive protein assessments in subjects with arterial stiffness risk. Drugs RD 2016, 16, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Di Renzo, L.; Trombetta, D.; Smeriglio, A.; Bernardini, S.; Cioccoloni, G.; Costa de Miranda, R.; Gualtieri, P.; Sinibaldi Salimei, P.; De Lorenzo, A. Antioxidant effects of a hydroxytyrosol-based pharmaceutical formulation on body composition, metabolic state, and gene expression: A randomised double-blinded, placebo-controlled crossover trial. Oxid. Med. Cell. Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Crespo, M.C.; Iglesias-Gutierrez, E.; Martín, R.; Gil-Zamorano, J.; Tomas-Zapico, C.; Burgos-Ramos, E.; Correa, C.; Gómez-Coronado, D.; Lasunción, M.A. Hydroxytyrosol supplementation modulates the expression of miRNAs in rodents and in humans. J. Nutr. Biochem. 2016, 34, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Corona, G.; Yaqoob, P.; Spencer, J.P.; Rowland, I. Secoiridoids delivered as olive leaf extract induce acute improvements in human vascular function and reduction of an inflammatory cytokine: A randomised, double-blind, placebo-controlled, cross-over trial. Br. J. Nutr. 2015, 114, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Oliveras-López, M.-J.; Molina, J.J.M.; Mir, M.V.; Rey, E.F.; Martín, F.; de la Serrana, H.L.-G. Extra virgin olive oil (EVOO) consumption and antioxidant status in healthy institutionalised elderly humans. Arch. Gerontol. Geriatr. 2013, 57, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Castaner, O.; Covas, M.-I.; Khymenets, O.; Nyyssonen, K.; Konstantinidou, V.; Zunft, H.-F.; de la Torre, R.; Munoz-Aguayo, D.; Vila, J.; Fito, M. Protection of LDL from oxidation by olive oil polyphenols is associated with a downregulation of CD40-ligand expression and its downstream products in vivo in humans. Am. J. Clin. Nutr. 2012, 95, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.-I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.-J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H. The effect of polyphenols in olive oil on heart disease risk factors: A randomised trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.-I.; de la Torre, K.; Farré-Albaladejo, M.; Kaikkonen, J.; Fitó, M.; López-Sabater, C.; Pujadas-Bastardes, M.A.; Joglar, J.; Weinbrenner, T.; Lamuela-Raventós, R.M. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Radic. Biol. Med. 2006, 40, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Marrugat, J.; Covas, M.-I.; Fitó, M.; Schröder, H.; Miró-Casas, E.; Gimeno, E.; López-Sabater, M.C.; de la Torre, R.; Farré, M. Effects of differing phenolic content in dietary olive oils on lipids and LDL oxidation. Eur. J. Nutr. 2004, 43, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Jemai, H.; Bouaziz, M.; Fki, I.; El Feki, A.; Sayadi, S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem. Biol. Interact. 2008, 176, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Catalán, Ú.; de las Hazas, M.-C.L.; Piñol, C.; Rubió, L.; Motilva, M.-J.; Fernandez-Castillejo, S.; Solà, R. Hydroxytyrosol and its main plasma circulating metabolites attenuate the initial steps of atherosclerosis through inhibition of the MAPK pathway. J. Funct. Foods 2018, 40, 280–291. [Google Scholar] [CrossRef]
- Kim, S.W.; Hur, W.; Li, T.Z.; Lee, Y.K.; Choi, J.E.; Hong, S.W.; Lyoo, K.-S.; You, C.R.; Jung, E.S.; Jung, C.K. Oleuropein prevents the progression of steatohepatitis to hepatic fibrosis induced by a high-fat diet in mice. Exp. Mol. Med. 2014, 46, e92. [Google Scholar] [CrossRef] [PubMed]
- Porcu, C.; Sideri, S.; Martini, M.; Cocomazzi, A.; Galli, A.; Tarantino, G.; Balsano, C. Oleuropein induces AMPK-Dependent autophagy in NAFLD mice, regardless of the gender. Int. J. Mol. Sci. 2018, 19, 3948. [Google Scholar] [CrossRef] [PubMed]
- Soto-Alarcon, S.A.; Valenzuela, R.; Valenzuela, A.; Videla, L.A. Liver protective effects of extra virgin olive oil: Interaction between its chemical composition and the cell-signaling pathways involved in protection. Endocr. Metab. Immune Disord. Drug Targets Former. Curr. Drug Targets-Immune Endocr. Metab. Disord. 2017, 18, 75–84. [Google Scholar] [CrossRef]
- Pirozzi, C.; Lama, A.; Simeoli, R.; Paciello, O.; Pagano, T.B.; Mollica, M.P.; Di Guida, F.; Russo, R.; Magliocca, S.; Canani, R.B. Hydroxytyrosol prevents metabolic impairment reducing hepatic inflammation and restoring duodenal integrity in a rat model of NAFLD. J. Nutr. Biochem. 2016, 30, 108–115. [Google Scholar] [CrossRef]
- Kuem, N.; Song, S.J.; Yu, R.; Yun, J.W.; Park, T. Oleuropein attenuates visceral adiposity in high-fat diet-induced obese mice through the modulation of WNT10b-and galanin-mediated signalings. Mol. Nutr. Food Res. 2014, 58, 2166–2176. [Google Scholar] [CrossRef]
- Valenzuela, R.; Echeverria, F.; Ortiz, M.; Rincón-Cervera, M.Á.; Espinosa, A.; Hernandez-Rodas, M.C.; Illesca, P.; Valenzuela, A.; Videla, L.A. Hydroxytyrosol prevents reduction in liver activity of Δ-5 and Δ-6 desaturases, oxidative stress, and depletion in long chain polyunsaturated fatty acid content in different tissues of high-fat diet fed mice. Lipids Health Dis. 2017, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Illesca, P.; Echeverría, F.; Espinosa, A.; Rincón-Cervera, M.Á.; Ortiz, M.; Hernandez-Rodas, M.C.; Valenzuela, A.; Videla, L.A. Molecular adaptations underlying the beneficial effects of hydroxytyrosol in the pathogenic alterations induced by a high-fat diet in mouse liver: PPAR-α and Nrf2 activation, and NF-κB down-regulation. Food Funct. 2017, 8, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Valenzuela, R.; Bustamante, A.; Álvarez, D.; Ortiz, M.; Soto-Alarcon, S.A.; Muñoz, P.; Corbari, A.; Videla, L.A. Attenuation of high-fat diet-induced rat liver oxidative stress and steatosis by combined hydroxytyrosol-(HT-) eicosapentaenoic acid supplementation mainly relies on HT. Oxid. Med. Cell. Longev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lemonakis, N.; Poudyal, H.; Halabalaki, M.; Brown, L.; Tsarbopoulos, A.; Skaltsounis, A.-L.; Gikas, E. The LC–MS-based metabolomics of hydroxytyrosol administration in rats reveals amelioration of the metabolic syndrome. J. Chromatogr. B 2017, 1041, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Campbell, F.; Brown, L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate–, high fat–fed rats. J. Nutr. 2010, 140, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Fagnani, R.; Mitro, N.; Scurati, S.; Masciadri, M.; Mussoni, L.; Galli, G.V.; Bosisio, E.; Crestani, M.; De Fabiani, E. Minor components of olive oil modulate proatherogenic adhesion molecules involved in endothelial activation. J. Agric. Food Chem. 2006, 54, 3259–3264. [Google Scholar] [CrossRef] [PubMed]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Terzuoli, E.; Donnini, S.; Nicolella, C.; Zinnai, A. Development of phenol-enriched olive oil with phenolic compounds extracted from wastewater produced by physical refining. Nutrients 2017, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Gnoni, A.; Stanca, E.; Cavallo, A.; Damiano, F.; Siculella, L.; Carluccio, M.A. Hydroxytyrosol ameliorates endothelial function under inflammatory conditions by preventing mitochondrial dysfunction. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Giordano, E.; Dangles, O.; Rakotomanomana, N.; Baracchini, S.; Visioli, F. 3-O-Hydroxytyrosol glucuronide and 4-O-hydroxytyrosol glucuronide reduce endoplasmic reticulum stress in vitro. Food Funct. 2015, 6, 3275–3281. [Google Scholar] [CrossRef]
- Deiana, M.; Incani, A.; Rosa, A.; Atzeri, A.; Loru, D.; Cabboi, B.; Melis, M.P.; Lucas, R.; Morales, J.C.; Dessi, M.A. Hydroxytyrosol glucuronides protect renal tubular epithelial cells against H2O2 induced oxidative damage. Chem. Biol. Interact. 2011, 193, 232–239. [Google Scholar] [CrossRef]
- Atzeri, A.; Lucas, R.; Incani, A.; Peñalver, P.; Zafra-Gómez, A.; Melis, M.P.; Pizzala, R.; Morales, J.C.; Deiana, M. Hydroxytyrosol and tyrosol sulfate metabolites protect against the oxidised cholesterol pro-oxidant effect in Caco-2 human enterocyte-like cells. Food Funct. 2016, 7, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Catalán, Ú.; Rubió, L.; López de las Hazas, M.C.; Herrero, P.; Nadal, P.; Canela, N.; Pedret, A.; Motilva, M.J.; Solà, R. Hydroxytyrosol and its complex forms (secoiridoids) modulate aorta and heart proteome in healthy rats: Potential cardio-protective effects. Mol. Nutr. Food Res. 2016, 60, 2114–2129. [Google Scholar] [CrossRef] [PubMed]
- Berrougui, H.; Ikhlef, S.; Khalil, A. Extra virgin olive oil polyphenols promote cholesterol efflux and improve HDL functionality. Evid. Based Complement. Altern. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Perona, J.S.; Cabello-Moruno, R.; Ruiz-Gutierrez, V. The role of virgin olive oil components in the modulation of endothelial function. J. Nutr. Biochem. 2006, 17, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.M.; Thirunavukkarasu, M.; Penumathsa, S.V.; Paul, D.; Maulik, N. Akt/FOXO3a/SIRT1-mediated cardioprotection by n-tyrosol against ischemic stress in rat in vivo model of myocardial infarction: Switching gears toward survival and longevity. J. Agric. Food Chem. 2008, 56, 9692–9698. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, M.B.; Aliev, O.I.; Sidekhmenova, A.V.; Shamanaev, A.Y.; Anishchenko, A.M.; Fomina, T.I.; Plotnikova, T.M.; Arkhipov, A.M. Effect of p-tyrosol on hemorheological parameters and cerebral capillary network in young spontaneously hypertensive rats. Microvasc. Res. 2018, 119, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Warnke, I.; Goralczyk, R.; Fuhrer, E.; Schwager, J. Dietary constituents reduce lipid accumulation in murine C3H10 T1/2 adipocytes: A novel fluorescent method to quantify fat droplets. Nutr. Metab. 2011, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Dagla, I.; Benaki, D.; Baira, E.; Lemonakis, N.; Poudyal, H.; Brown, L.; Tsarbopoulos, A.; Skaltsounis, A.-L.; Mikros, E.; Gikas, E. Alteration in the liver metabolome of rats with metabolic syndrome after treatment with Hydroxytyrosol. A Mass Spectrometry And Nuclear Magnetic Resonance-based metabolomics study. Talanta 2018, 178, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Stefanon, B.; Colitti, M. Hydroxytyrosol, an ingredient of olive oil, reduces triglyceride accumulation and promotes lipolysis in human primary visceral adipocytes during differentiation. Exp. Biol. Med. 2016, 241, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Priore, P.; Siculella, L.; Gnoni, G.V. Extra virgin olive oil phenols down-regulate lipid synthesis in primary-cultured rat-hepatocytes. J. Nutr. Biochem. 2014, 25, 683–691. [Google Scholar] [CrossRef]
- Sarna, L.K.; Sid, V.; Wang, P.; Siow, Y.L.; House, J.D.; Karmin, O. Tyrosol attenuates high fat diet-induced hepatic oxidative stress: Potential involvement of cystathionine β-synthase and cystathionine γ-lyase. Lipids 2016, 51, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Quinn, P.J. Vitamin E and its function in membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Todd, S.; Woodward, M.; Tunstall-Pedoe, H.; Bolton-Smith, C. Dietary antioxidant vitamins and fiber in the etiology of cardiovascular disease and all-causes mortality: Results from the Scottish Heart Health Study. Am. J. Epidemiol. 1999, 150, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Stampfer, M.J.; Ascherio, A.; Giovannucci, E.; Colditz, G.A.; Willett, W.C. Vitamin E consumption and the risk of coronary heart disease in men. N. Engl. J. Med. 1993, 328, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Muntwyler, J.; Hennekens, C.H.; Manson, J.E.; Buring, J.E.; Gaziano, J.M. Vitamin supplement use in a low-risk population of US male physicians and subsequent cardiovascular mortality. Arch. Intern. Med. 2002, 162, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Kushi, L.H.; Folsom, A.R.; Prineas, R.J.; Mink, P.J.; Wu, Y.; Bostick, R.M. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N. Engl. J. Med. 1996, 334, 1156–1162. [Google Scholar] [CrossRef]
- Knekt, P.; Reunanen, A.; Jävinen, R.; Seppänen, R.; Heliövaara, M.; Aromaa, A. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am. J. Epidemiol. 1994, 139, 1180–1189. [Google Scholar] [CrossRef]
- Stephens, N.G.; Parsons, A.; Brown, M.; Schofield, P.; Kelly, F.; Cheeseman, K.; Mitchinson, M. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 1996, 347, 781–786. [Google Scholar] [CrossRef]
- Eshak, E.S.; Iso, H.; Yamagishi, K.; Cui, R.; Tamakoshi, A. Dietary intakes of fat soluble vitamins as predictors of mortality from heart failure in a large prospective cohort study. Nutrition 2018, 47, 50–55. [Google Scholar] [CrossRef]
- Boaz, M.; Smetana, S.; Weinstein, T.; Matas, Z.; Gafter, U.; Iaina, A.; Knecht, A.; Weissgarten, Y.; Brunner, D.; Fainaru, M. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): Randomised placebo-controlled trial. Lancet 2000, 356, 1213–1218. [Google Scholar] [CrossRef]
- Devaraj, S.; Traber, M.G. γ-Tocopherol, the new vitamin E? Am. J. Clin. Nutr. 2003, 77, 530–531. [Google Scholar] [CrossRef] [PubMed]
- Öhrvall, M.; Vessby, B.; Sundlöf, G. Gamma, but not alpha, tocopherol levels in serum are reduced in coronary heart disease patients. J. Intern. Med. 1996, 239, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Leonard, S.; Traber, M.G.; Jialal, I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic. Biol. Med. 2008, 44, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W.; Brinkworth, G.D.; Thompson, C.H.; Abeywardena, M.Y. Short term effects of palm-tocotrienol and palm-carotenes on vascular function and cardiovascular disease risk: A randomised controlled trial. Atherosclerosis 2016, 254, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Hercberg, S.; Galan, P.; Preziosi, P.; Bertrais, S.; Mennen, L.; Malvy, D.; Roussel, A.-M.; Favier, A.; Briançon, S. The SU. VI. MAX Study: A randomised, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch. Intern. Med. 2004, 164, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.-K.; Ju, W.; Cho, B.; Oh, S.-W.; Park, S.M.; Koo, B.-K.; Park, B.-J. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: Systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 346, f10. [Google Scholar] [CrossRef]
- Kubota, Y.; Iso, H.; Date, C.; Kikuchi, S.; Watanabe, Y.; Wada, Y.; Inaba, Y.; Tamakoshi, A.; Group, J.S. Dietary intakes of antioxidant vitamins and mortality from cardiovascular disease: The Japan Collaborative Cohort Study (JACC) study. Stroke 2011, 42, 1665–1672. [Google Scholar] [CrossRef]
- Investigators, G.-P. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Yusuf, S.; Sleight, P.; Pogue, J.f.; Bosch, J.; Davies, R.; Dagenais, G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N. Engl. J. Med. 2000, 342, 145–153. [Google Scholar] [CrossRef]
- Chae, C.; Albert, C.; Moorthy, M.; Lee, I.; Buring, J. Vitamin E supplementation and the risk of heart failure in women. Circ. Heart Fail. 2012, 5, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Sánchez, P.; Sánchez-Villegas, A.; Ruano-Rodríguez, C.; Gea, A.; Lamuela-Raventós, R.M.; Estruch, R.; Salas-Salvadó, J.; Covas, M.; Corella, D.; Schröder, H. Dietary total antioxidant capacity and mortality in the PREDIMED study. Eur. J. Nutr. 2016, 55, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Perri, L.; Di Castelnuovo, A.; Iacoviello, L.; De Gaetano, G.; Violi, F. Supplementation with vitamin E alone is associated with reduced myocardial infarction: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Kaul, N.; Devaraj, S.; Jialal, I. α-Tocopherol and atherosclerosis. Exp. Biol. Med. 2001, 226, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Jialal, I. The effects of alpha-tocopherol on critical cells in atherogenesis. Curr. Opin. Lipidol. 1998, 9, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ricciarelli, R.; Tasinato, A.; Clemént, S.; Boscoboinik, D.; Azzi, A. α-Tocopherol specifically inactivates cellular protein kinase C α by changing its phosphorylation state. Biochem. J. 1998, 334, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Keaney, J.F., Jr.; Simon, D.I.; Freedman, J.E. Vitamin E and vascular homeostasis: Implications for atherosclerosis. FASEB J. 1999, 13, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Boscoboinik, D.; Szewczyk, A.; Azzi, A. α-Tocopherol (vitamin E) regulates vascular smooth muscle cell proliferation and protein kinase C activity. Arch. Biochem. Biophys. 1991, 286, 264–269. [Google Scholar] [CrossRef]
- Özer, N.; Palozza, P.; Boscoboinik, D.; Azzi, A. d-α-Tocopherol inhibits low density lipoprotein induced proliferation and protein kinase C activity in vascular smooth muscle cells. FEBS Lett. 1993, 322, 307–310. [Google Scholar] [CrossRef]
- Özer, N.K.; Azzi, A. Effect of vitamin E on the development of atherosclerosis. Toxicology 2000, 148, 179–185. [Google Scholar] [CrossRef]
- Cachia, O.; El Benna, J.; Pedruzzi, E.; Descomps, B.; Gougerot-Pocidalo, M.-A.; Leger, C.-L. α-tocopherol inhibits the respiratory burst in human monocytes attenuation of p47 phox membrane translocation and phosphorylation. J. Biol. Chem. 1998, 273, 32801–32805. [Google Scholar] [CrossRef] [PubMed]
- Ricciarelli, R.; Zingg, J.-M.; Azzi, A. Vitamin E reduces the uptake of oxidised LDL by inhibiting CD36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation 2000, 102, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, A.; Taddei, M.; Tamburini, I.; Bergamini, E.; Azzi, A.; Zingg, J.-M. Antagonistic Effects of Oxidised Low Density Lipoprotein and α-Tocopherol on CD36 Scavenger Receptor Expression in Monocytes INVOLVEMENT OF PROTEIN KINASE B AND PEROXISOME PROLIFERATOR-ACTIVATED RECEPTOR-γ. J. Biol. Chem. 2006, 281, 6489–6497. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-G.; Liang, C.; Han, S.-F.; Wu, Z.-G. Vitamin E ameliorates ox-LDL-induced foam cells formation through modulating the activities of oxidative stress-induced NF-κB pathway. Mol. Cell. Biochem. 2012, 363, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ferré, N.; Camps, J.; Paul, A.; Cabré, M.; Calleja, L.; Osada, J.; Joven, J. Effects of high-fat, low-cholesterol diets on hepatic lipid peroxidation and antioxidants in apolipoprotein E-deficient mice. Mol. Cell. Biochem. 2001, 218, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.R.; Leichtweis, S.B.; Pettersson, K.; Croft, K.D.; Mori, T.A.; Brown, A.J.; Stocker, R. Dietary cosupplementation with vitamin E and coenzyme Q10 inhibits atherosclerosis in apolipoprotein E gene knockout mice. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Meydani, M.; Kwan, P.; Band, M.; Knight, A.; Guo, W.; Goutis, J.; Ordovas, J. Long-term vitamin E supplementation reduces atherosclerosis and mortality in Ldlr−/− mice, but not when fed Western style diet. Atherosclerosis 2014, 233, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Kirac, D.; Negis, Y.; Ozer, N.K. Vitamin E attenuates homocysteine and cholesterol induced damage in rat aorta. Cardiovasc. Pathol. 2013, 22, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.; Hajjar, D.P.; Silverstein, R.L. CD36: A class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J. Clin. Investig. 2001, 108, 785–791. [Google Scholar] [CrossRef]
- Özer, N.K.; Şirikçi, Ö.; Taha, S.; Şan, T.; Moser, U.; Azzi, A. Effect of vitamin E and probucol on dietary cholesterol-induced atherosclerosis in rabbits. Free Radic. Biol. Med. 1998, 24, 226–233. [Google Scholar] [CrossRef]
- Bozaykut, P.; Sozen, E.; Yazgan, B.; Karademir, B.; Kartal-Ozer, N. The role of hypercholesterolemic diet and vitamin E on Nrf2 pathway, endoplasmic reticulum stress and proteasome activity. Free Radic. Biol. Med. 2014, 75, S24. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, B.; Hoseini, Z.; Sahebkar, A.; Mirzaei, H. Anti-atherosclerotic effects of vitamins D and E in suppression of atherogenesis. J. Cell. Physiol. 2017, 232, 2968–2976. [Google Scholar] [CrossRef] [PubMed]
- Cook-Mills, J.M. Isoforms of vitamin E differentially regulate PKC α and inflammation: A review. J. Clin. Cell. Immunol. 2013, 4. [Google Scholar] [CrossRef]
- Mathur, P.; Ding, Z.; Saldeen, T.; Mehta, J.L. Tocopherols in the prevention and treatment of atherosclerosis and related cardiovascular disease. Clin. Cardiol. 2015, 38, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Robles, H.; Rios, A.; Arellano-Mendoza, M.; Escalante, B.A.; Schnoor, M. Antioxidative diet supplementation reverses high-fat diet-induced increases of cardiovascular risk factors in mice. Oxid. Med. Cell. Longev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Yan, C.; Patel, R.; Liu, W.; Dong, E. Vitamins C and E attenuate apoptosis, β-adrenergic receptor desensitisation, and sarcoplasmic reticular Ca2+ ATPase downregulation after myocardial infarction. Free Radic. Biol. Med. 2006, 40, 1827–1842. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.; Smith, H.M.; Hill, M.F. Dietary Supplementation with Vitamin E Ameliorates Cardiac Failure in Type I Diabetic Cardiomyopathy by Suppressing Myocardial Generation of 8-iso-Prostaglandin F2α and Oxidised Glutathione. J. Card. Fail. 2007, 13, 884–892. [Google Scholar] [CrossRef]
- Sozen, E.; Yazgan, B.; Sahin, A.; Ince, U.; Ozer, N.K. High Cholesterol Diet-Induced Changes in Oxysterol and Scavenger Receptor Levels in Heart Tissue. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Li, X.; Zhang, Y.; Gulbins, E.; Zhang, Y. Enhancement of endothelial permeability by free fatty acid through lysosomal cathepsin B-mediated Nlrp3 inflammasome activation. Oncotarget 2016, 7, 73229. [Google Scholar] [CrossRef]
- Ma, P.; Han, L.; Lv, Z.; Chen, W.; Hu, H.; Tu, J.; Zhou, X.; Liu, S.-M. In-hospital free fatty acids levels predict the severity of myocardial ischemia of acute coronary syndrome. BMC Cardiovasc. Disord. 2016, 16, 29. [Google Scholar] [CrossRef]
- Wróblewski, F.; Ladue, J.S. Lactic dehydrogenase activity in blood. Proc. Soc. Exp. Biol. Med. 1955, 90, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.-H.; Ma, P.; Luo, W.; Xiong, H.; Han, L.; Li, S.-W.; Zhou, X.; Tu, J.-C. Association between serum free fatty acid levels and possible related factors in patients with type 2 diabetes mellitus and acute myocardial infarction. BMC Cardiovasc. Disord. 2014, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, L.; Zhang, H.; Wang, D.; Zhang, M.; Zhang, L. Clinical significance of elevated serum A-FABP and free fatty acid in neonates with hypoxic ischemic brain damage. Exp. Ther. Med. 2016, 12, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.K.; Kumar, A.; Joshi, P.; Arora, J.; Ahanger, A.M. Plasma free Fatty Acid concentrations as a marker for acute myocardial infarction. J. Clin. Diagn. Res. 2013, 7, 2432. [Google Scholar] [CrossRef] [PubMed]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Kokatnur, M.; Oalmann, M.; Johnson, W.; Malcom, G.; Strong, J. Fatty acid composition of human adipose tissue from two anatomical sites in a biracial community. Am. J. Clin. Nutr. 1979, 32, 2198–2205. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, L.G.; Gustafson, J.A.; Han, S.-Y.; Jassal, D.S.; Jones, P.J. High-oleic rapeseed (canola) and flaxseed oils modulate serum lipids and inflammatory biomarkers in hypercholesterolaemic subjects. Br. J. Nutr. 2011, 105, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Huertas, E. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol. Res. 2010, 61, 200–207. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Meng, H.; Matthan, N.R.; Wu, D.; Li, L.; Rodríguez-Morató, J.; Cohen, R.; Galluccio, J.M.; Dolnikowski, G.G.; Lichtenstein, A.H. Comparison of diets enriched in stearic, oleic, and palmitic acids on inflammation, immune response, cardiometabolic risk factors, and fecal bile acid concentrations in mildly hypercholesterolemic postmenopausal women—randomised crossover trial. Am. J. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Sundström, J.; Lind, L.; Vessby, B.; Andrén, B.; Aro, A.; Lithell, H.O. Dyslipidemia and an unfavorable fatty acid profile predict left ventricular hypertrophy 20 years later. Circulation 2001, 103, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018, 378, 34. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, L.; Beneit, N.; Otero, Y.F.; Escribano, Ó.; Díaz-Castroverde, S.; Gómez-Hernández, A.; Benito, M. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovasc. Diabetol. 2015, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.-H.; Chu, P.-M.; Kao, C.-L.; Cheng, Y.-H.; Hung, C.-H.; Tsai, K.-L. Oleic acid activates MMPs up-regulation through SIRT1/PPAR-γ inhibition: A probable linkage between obesity and coronary arterial disease. J. Biochem. 2016, 160, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-H.; Gerhart-Hines, Z.; Dominy, J.E.; Lee, Y.; Kim, S.; Tabata, M.; Xiang, Y.K.; Puigserver, P. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1α complex. J. Biol. Chem. 2013, 288, 7117–7126. [Google Scholar] [CrossRef] [PubMed]
- Thandapilly, S.J.; Raj, P.; Louis, X.L.; Perera, D.; Yamanagedara, P.; Zahradka, P.; Taylor, C.G.; Netticadan, T. Canola oil rich in oleic acid improves diastolic heart function in diet-induced obese rats. J. Physiol. Sci. 2017, 67, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-de-Moraes, I.M.; Gonçalves-de-Albuquerque, C.F.; Kurz, A.R.; Oliveira, F.M.d.J.; Abreu, V.H.P.d.; Torres, R.C.; Carvalho, V.F.; Estato, V.; Bozza, P.T.; Sperandio, M. Omega-9 oleic acid, the main compound of olive oil, mitigates inflammation during experimental sepsis. Oxid. Med. Cell. Longev. 2018. [Google Scholar] [CrossRef]
- Nicoli, M.C. An Introduction to food shelf life: definitions, basic concepts and regulatory aspects. In Shelf Life Assessment of Food, 1st ed.; CRC press Taylor and Francis group: London, UK, 2012; pp. 1–16. [Google Scholar]
- Cicerale, S.; Conlan, X.A.; Barnett, N.W.; Keast, R.S. Storage of extra virgin olive oil and its effect on the biological activity and concentration of oleocanthal. Food Res. Int. 2013, 50, 597–602. [Google Scholar] [CrossRef]
- Piscopo, A.; Poiana, M. Packaging and storage of olive oil. Olive Germplasm Olive Cultiv. Table Olive Olive Oil Ind. Italy 2012. [Google Scholar] [CrossRef][Green Version]
- Bendini, A.; Cerretani, L.; Salvador, M.; Fregapane, G.; Lercker, G. Stability of the sensory quality of virgin olive oil during storage: An overview. Ital. J. Food Sci. 2009, 21. [Google Scholar]
- Sanmartin, C.; Venturi, F.; Sgherri, C.; Nari, A.; Macaluso, M.; Flamini, G.; Quartacci, M.F.; Taglieri, I.; Andrich, G.; Zinnai, A. The effects of packaging and storage temperature on the shelf-life of extra virgin olive oil. Heliyon 2018, 4, e00888. [Google Scholar] [CrossRef] [PubMed]
- Sanmartin, C.; Venturi, F.; Macaluso, M.; Nari, A.; Quartacci, M.F.; Sgherri, C.; Flamini, G.; Taglieri, I.; Ascrizzi, R.; Andrich, G. Preliminary results about the use of argon and carbon dioxide in the extra virgin olive oil (EVOO) storage to extend oil shelf life: Chemical and sensorial point of view. Eur. J. Lipid Sci. Technol. 2018, 120, 1800156. [Google Scholar] [CrossRef]
- Rotondi, A.; Bendini, A.; Cerretani, L.; Mari, M.; Lercker, G.; Toschi, T.G. Effect of olive ripening degree on the oxidative stability and organoleptic properties of cv. Nostrana di Brisighella extra virgin olive oil. J. Agric. Food Chem. 2004, 52, 3649–3654. [Google Scholar] [CrossRef] [PubMed]
- Dag, A.; Kerem, Z.; Yogev, N.; Zipori, I.; Lavee, S.; Ben-David, E. Influence of time of harvest and maturity index on olive oil yield and quality. Sci. Hortic. 2011, 127, 358–366. [Google Scholar] [CrossRef]
- Zinnai, A.; Venturi, F.; Andrich, L.; Silvestri, S.; Andrich, G. A kinetic method to evaluate the effect of environmental variability on the quality of an extra virgin olive oil. Agrochimica 2014, 58, 35–50. [Google Scholar]
- Vinha, A.F.; Ferreres, F.; Silva, B.M.; Valentao, P.; Gonçalves, A.; Pereira, J.A.; Oliveira, M.B.; Seabra, R.M.; Andrade, P.B. Phenolic profiles of Portuguese olive fruits (Olea europaea L.): Influences of cultivar and geographical origin. Food Chem. 2005, 89, 561–568. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Mitchell, A. Factors influencing phenolic compounds in table olives (Olea europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, B.; Sánchez-Ortiz, A.; Lorenzo, M.L.; Rivas, A. Influence of fruit ripening on agronomic parameters, quality indices, sensory attributes and phenolic compounds of Picudo olive oils. Food Res. Int. 2013, 54, 1860–1867. [Google Scholar] [CrossRef]
- Vekiari, S.; Oreopoulou, V.; Kourkoutas, Y.; Kamoun, N.; Msallem, M.; Psimouli, V.; Arapoglou, D. Characterisation and seasonal variation of the quality of virgin olive oil of the Throumbolia and Koroneiki varieties from Southern Greece. Grasas Aceites 2010, 61, 221–231. [Google Scholar] [CrossRef]
- Bilušić, T.; Žanetić, M.; Ljubenkov, I.; Mekinić, I.G.; Štambuk, S.; Bojović, V.; Soldo, B.; Magiatis, P. Molecular characterisation of Dalmatian cultivars and the influence of the olive fruit harvest period on chemical profile, sensory characteristics and oil oxidative stability. Eur. Food Res. Technol. 2018, 244, 281–289. [Google Scholar] [CrossRef]
- Artajo, L.S.; Romero, M.P.; Motilva, M.J. Transfer of phenolic compounds during olive oil extraction in relation to ripening stage of the fruit. J. Sci. Food Agric. 2006, 86, 518–527. [Google Scholar] [CrossRef]
- Bengana, M.; Bakhouche, A.; Lozano-Sánchez, J.; Amir, Y.; Youyou, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Influence of olive ripeness on chemical properties and phenolic composition of Chemlal extra-virgin olive oil. Food Res. Int. 2013, 54, 1868–1875. [Google Scholar] [CrossRef]
- López-Yerena, A.; Lozano-Castellón, J.; Olmo-Cunillera, A.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Jiménez, B.; Pérez, M.; Vallverdú-Queralt, A. Effects of Organic and Conventional Growing Systems on the Phenolic Profile of Extra-Virgin Olive Oil. Molecules 2019, 24, 1986. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Rapoport, H.F.; Gucci, R. Long-term evaluation of yield components of young olive trees during the onset of fruit production under different irrigation regimes. Irrig. Sci. 2013, 31, 37–47. [Google Scholar] [CrossRef]
- Nari, A.; Taglieri, I.; Pistelli, L.; Ascrizzi, R.; Andrich, G.; Zinnai, A. The effect of ripening degree and irrigation regimes of fruits on the quality of extra-virgin olive oil extracted with or without the addition of carbonic snow. Agrochimica 2018, 62, 79–91. [Google Scholar]
- Clodoveo, M.L.; Hbaieb, R.H. Beyond the traditional virgin olive oil extraction systems: Searching innovative and sustainable plant engineering solutions. Food Res. Int. 2013, 54, 1926–1933. [Google Scholar] [CrossRef]
- Zinnai, A.; Venturi, F.; Sanmartin, C.; Taglieri, I.; Andrich, G. The utilisation of solid carbon dioxide in the extraction of extra-virgin olive oil. Agro Food Ind. Hi-Tech 2015, 26, 24–26. [Google Scholar] [CrossRef]
- Puértolas, E.; de Marañón, I.M. Olive oil pilot-production assisted by pulsed electric field: Impact on extraction yield, chemical parameters and sensory properties. Food Chem. 2015, 167, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Fregapane, G.; Salvador, M. Production of superior quality extra virgin olive oil modulating the content and profile of its minor components. Food Res. Int. 2013, 54, 1907–1914. [Google Scholar] [CrossRef]
- Di Giovacchino, L.; Sestili, S.; Di Vincenzo, D. Influence of olive processing on virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 587–601. [Google Scholar] [CrossRef]
- Ranalli, A.; Pollastri, L.; Contento, S.; Iannucci, E.; Lucera, L. Effect of olive paste kneading process time on the overall quality of virgin olive oil. Eur. J. Lipid Sci. Technol. 2003, 105, 57–67. [Google Scholar] [CrossRef]
- Fadda, C.; Del Caro, A.; Sanguinetti, A.M.; Urgeghe, P.P.; Vacca, V.; Arca, P.; Piga, A. Changes during storage of quality parameters and in vitro antioxidant activity of extra virgin monovarietal oils obtained with two extraction technologies. Food Chem. 2012, 134, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Hadj-Taieb, N.; Grati, N.; Ayadi, M.; Attia, I.; Bensalem, H.; Gargouri, A. Optimisation of olive oil extraction and minor compounds content of Tunisian olive oil using enzymatic formulations during malaxation. Biochem. Eng. J. 2012, 62, 79–85. [Google Scholar] [CrossRef]
- Vierhuis, E.; Servili, M.; Baldioli, M.; Schols, H.A.; Voragen, A.G.; Montedoro, G. Effect of enzyme treatment during mechanical extraction of olive oil on phenolic compounds and polysaccharides. J. Agric. Food Chem. 2001, 49, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, M.; Mugelli, M.; Cherubini, C.; Viti, P.; Zanoni, B. Influence of O2 on the quality of virgin olive oil during malaxation. J. Sci. Food Agric. 2006, 86, 2140–2146. [Google Scholar] [CrossRef]
- Zinnai, A.; Venturi, F.; Quartacci, M.; Sanmartin, C.; Favati, F.; Andrich, G. Solid carbon dioxide to promote the extraction of extra-virgin olive oil. Grasas Y Aceites 2016, 67, 1–8. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Eddouzi, J.; Zinnai, A.; Quartacci, M.F.; Zarrouk, M. Olive Leaf Addition Increases Olive Oil Nutraceutical Properties. Molecules 2019, 24, 545. [Google Scholar] [CrossRef] [PubMed]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Zinnai, A. A simplified method to estimate Sc-CO2 extraction of bioactive compounds from different matrices: Chili pepper vs. tomato by-products. Appl. Sci. 2017, 7, 361. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Taglieri, I.; Sgherri, C.; Flamini, G.; Macaluso, M.; Sanmartin, C.; Venturi, F.; Quartacci, M.; Pistelli, L.; Zinnai, A. Nutraceutical Oils Produced by Olives and Citrus Peel of Tuscany Varieties as Sources of Functional Ingredients. Molecules 2019, 24, 65. [Google Scholar] [CrossRef]
- Gargouri, B.; Zribi, A.; Bouaziz, M. Effect of containers on the quality of Chemlali olive oil during storage. J. Food Sci. Technol. 2015, 52, 1948–1959. [Google Scholar] [CrossRef]
- Sgherri, C.; Pinzino, C.; Quartacci, M.F. Reactive oxygen species and photosynthetic functioning: Past and present. In Reactive Oxygen Species in Plants: Boon or Bane–Revisiting the Role of ROS; Wiley Online Library: Hoboken, NJ, USA, 2018; pp. 137–155. [Google Scholar] [CrossRef]
- Limbo, S.; Peri, C.; Piergiovanni, L. Extra-virgin olive oil packaging. In The Extra-Virgin Olive Oil Handbook; Wiley-Blackwell: London, UK, 2014; pp. 179–199. [Google Scholar]
- Pristouri, G.; Badeka, A.; Kontominas, M. Effect of packaging material headspace, oxygen and light transmission, temperature and storage time on quality characteristics of extra virgin olive oil. Food Control 2010, 21, 412–418. [Google Scholar] [CrossRef]

| Main Key Words | Secondary 1 Key Words |
|---|---|
| EVOO2 production EVOO storage Fortified oils EVOO | Olive ripening Olive agronomical practices Packaging Storage conditions |
| Hydroxytyrosol Tyrosol Oleuropein Olive oil polyphenols Oleic acid MUFA3 Olive oil Vitamin E Tocopherols Tocotrienols | Nutraceutical properties Antioxidant Anti-inflammatory Cardiovascular effects Metabolism Bioavailability Clinical trials Preclinical studies |
| Health Status | N.1 | Study | Treatment | Efficacy | Ref. |
|---|---|---|---|---|---|
| Hypercolesterolemia | 4 | Randomized, double-blind, placebo and active comparator (Armolipid Plus) controlled study | Food supplement called Body Lipid, containing monacolin K (10 mg), berberine (500 mg), coenzyme Q10 (2 mg) and HT (5 mg) | + | [60] |
| Randomized, controlled, double-blind, crossover human trial | VOO containing polyphenols 80 mg/kg, or 500 mg/kg, or a mixture from VOO and thyme (500 mg/kg, 1:1) | + | [61] | ||
| Randomized, double-blind crossover, controlled trial | olive oils with different phenolic contents, 80 or 400 ppm | + | [62] | ||
| Observational non-randomized study | Cholesfytol (10 mg Monacolin K and 5 mg HT) | + | [63] | ||
| Obesity | 1 | Randomized, double-blinded, placebo-controlled, crossover | 51.1 mg oleuropein, 9.7 mg hydroxytyrosol | +/− | [64] |
| Metabolic syndrome | 2 | Randomized double-blind placebo-controlled trial | Cholesfytolplus capsule (10.82 mg Monacolins and 9.32 mg HT) | + | [65] |
| Randomized double blind placebo controlled randomized trial | Cholesfytolplus capsule (10.82 mg Monacolins and 9.32 mg HT) | + | [66] | ||
| Hypertension | 2 | Randomized, double-blind, controlled, crossover trial | Phenolic-rich olive leaf extract (136.2 mg Ole and 6.4 mg HT per day) | + | [47] |
| Randomized, double blind, crossover trial | Virgin OO enriched with polyphenols-961 mg/kg | + | [45] | ||
| Arterial stiffness | 1 | Randomized double-blind placebo-controlled trial | Standardized olive fruit extract 250 mg (50 mg HT) or 500 mg (100 mg HT) | + | [67] |
| Healthy volunteers | 9 | Randomized double-blinded, placebo-controlled crossover trial | 15 mg/day of HT | + | [68] |
| Randomized, cross-over, placebo-controlled and double-blind trial group. | 25 mg/day HT (extract of olive mill wastewater called Hytolive) | + | [69] | ||
| Randomized, double-blind, placebo-controlled, cross-over trial | 51 mg Ole and 10 mg HT | + | [70] | ||
| Randomized double-blind, placebo-controlled study | 5 and 25 mg/d HT | − | [44] | ||
| Randomized double-blind placebo-controlled study | Virgin OO enriched with polyphenols—5358 mg/L | + | [71] | ||
| Randomized, double-blind crossover, controlled trial | OO with a low polyphenol content (2.7 mg/kg) or a high phenolic content (366 mg/kg) | + | [72] | ||
| Randomized, double-blind crossover, controlled trial | OO with low (2.7 mg/kg of olive oil), medium (164 mg/kg), or high (366 mg/kg) phenolic content | + | [73] | ||
| Randomized, double-blind crossover, controlled trial | OO with low (2.7 mg/kg), medium (164 mg/kg), or high (366 mg/kg) phenolic content | + | [74] | ||
| Randomized, double-blind crossover, controlled trial | OO with low (0 mg/kg), medium (68 mg/kg) or high (150 mg/kg) phenolic content | + | [75] |
| Health Status | N.1 | Study | Treatment | Efficacy | Ref. |
|---|---|---|---|---|---|
| Healthy subjects | 9 | Prospective cohort study | Vitamin E (as α-tocopherol equivalents) | + | [106] |
| Prospective cohort study | Vitamin E | + | [107,108] | ||
| Prospective cohort study | Vitamin E | + | [110] | ||
| Follow-up | Vitamin E | + | [7] | ||
| Cohort study | Vitamin E supplementation with food intake | + | [112] | ||
| Cohort study | Vitamin E | − | [120] | ||
| Randomized, double-blind, placebo-controlled, cross-over trial | Vitamin E alone, vitamin E + other antioxidants | + | [125] | ||
| Randomized, double-blind, placebo-controlled primary prevention trial | Vitamin E | − | [118] | ||
| Healthy subjects (platelet aggregation induction) | 2 | Randomized, double-blind, placebo-controlled, cross-over trial | α-, γ-, δ-tocopherol | + | [114,115] |
| High cardiovascular risk | 1 | multicenter, parallel group, randomized controlled clinical trial | Vitamin E | − | [124] |
| Patients with evidence of vascular disease or diabetes | 2 | Randomized, double-blind, placebo-controlled, cross-over trial | Vitamin E | − | [122,123] |
| Coronary atherosclerosis | 1 | Double-blind, placebo-controlled study with stratified randomization | Vitamin E | + | [111] |
| Patients surviving after recent myocardial infarction (3 months) | 1 | Multicenter, open-label design, in which patients were randomly allocated | Vitamin E | − | [121] |
| Postmenopausal women | 1 | Prospective cohort study Follow-up | Vitamin E | + | [109] |
| Hemodialysis patients with pre-existing cardiovascular disease | 1 | Randomized, double-blind, placebo-controlled, cross-over trial | Vitamin E | + | [113] |
| Type 2 diabetes | 1 | Randomized, double-blind, placebo-controlled, cross-over trial | Tocotrienols + tocopherols | + | [117] |
| Metabolic syndrome | 1 | Randomized, double-blind, placebo-controlled, cross-over trial | γ-tocopherol, α-tocopherol | + | [116] |
| Health Status | N.1 | Study | Treatment | Efficacy | Ref. |
|---|---|---|---|---|---|
| CVD risk subjects | 1 | 32 g/day of EVOO | + | [164] | |
| Hypercholesterolemic patients | 1 | Randomized crossover study | Experimental diet enriched with oleic acid | + | [162] |
| Patients with left ventricular hypertrophy risk | 1 | Longitudinal cohort | - | [163] | |
| Healthy subjects | 5 | Randomized control trial | Milk enriched with oleic acid and/or PUFA | + | [160] |
| Control non-randomized | Milk enriched with oleic acid and/or PUFA | +/− | [160] | ||
| Hypercholesterolemic patients | 1 | Randomized control study | Milk enriched with oleic acid and/or PUFA | + | [160] |
| Metabolic syndrome subjects | 1 | Randomized control study | Milk enriched with oleic acid and/or PUFA | + | [160] |
| Peripheral vascular disease patients | 1 | Randomized control study | Milk enriched with oleic acid and/or PUFA | + | [160] |
| Myocardial infarction patients | 1 | Randomized control study | Milk enriched with oleic acid and/or PUFA | + | [160] |
| Packaging Material | Barrier Against Gases | Light Protection | Absence of Metals | Interaction FCM/oil |
|---|---|---|---|---|
| Glass |  |  |  |  |
| Glass + additives anti-UV |  |  |  |  |
| Aluminium/Aluminium alloys tin-plate |  |  |  |  |
| Chromium tin-free steel |  |  |  |  |
| Tin-plate + resins coating |  |  |  |  |
| Polyethylene |  |  |  |  |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flori, L.; Donnini, S.; Calderone, V.; Zinnai, A.; Taglieri, I.; Venturi, F.; Testai, L. The Nutraceutical Value of Olive Oil and Its Bioactive Constituents on the Cardiovascular System. Focusing on Main Strategies to Slow Down Its Quality Decay during Production and Storage. Nutrients 2019, 11, 1962. https://doi.org/10.3390/nu11091962
Flori L, Donnini S, Calderone V, Zinnai A, Taglieri I, Venturi F, Testai L. The Nutraceutical Value of Olive Oil and Its Bioactive Constituents on the Cardiovascular System. Focusing on Main Strategies to Slow Down Its Quality Decay during Production and Storage. Nutrients. 2019; 11(9):1962. https://doi.org/10.3390/nu11091962
Chicago/Turabian StyleFlori, Lorenzo, Sandra Donnini, Vincenzo Calderone, Angela Zinnai, Isabella Taglieri, Francesca Venturi, and Lara Testai. 2019. "The Nutraceutical Value of Olive Oil and Its Bioactive Constituents on the Cardiovascular System. Focusing on Main Strategies to Slow Down Its Quality Decay during Production and Storage" Nutrients 11, no. 9: 1962. https://doi.org/10.3390/nu11091962
APA StyleFlori, L., Donnini, S., Calderone, V., Zinnai, A., Taglieri, I., Venturi, F., & Testai, L. (2019). The Nutraceutical Value of Olive Oil and Its Bioactive Constituents on the Cardiovascular System. Focusing on Main Strategies to Slow Down Its Quality Decay during Production and Storage. Nutrients, 11(9), 1962. https://doi.org/10.3390/nu11091962









