The Effect of Beta-Alanine versus Alkaline Agent Supplementation Combined with Branched-Chain Amino Acids and Creatine Malate in Highly-Trained Sprinters and Endurance Athletes: A Randomized Double-Blind Crossover Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
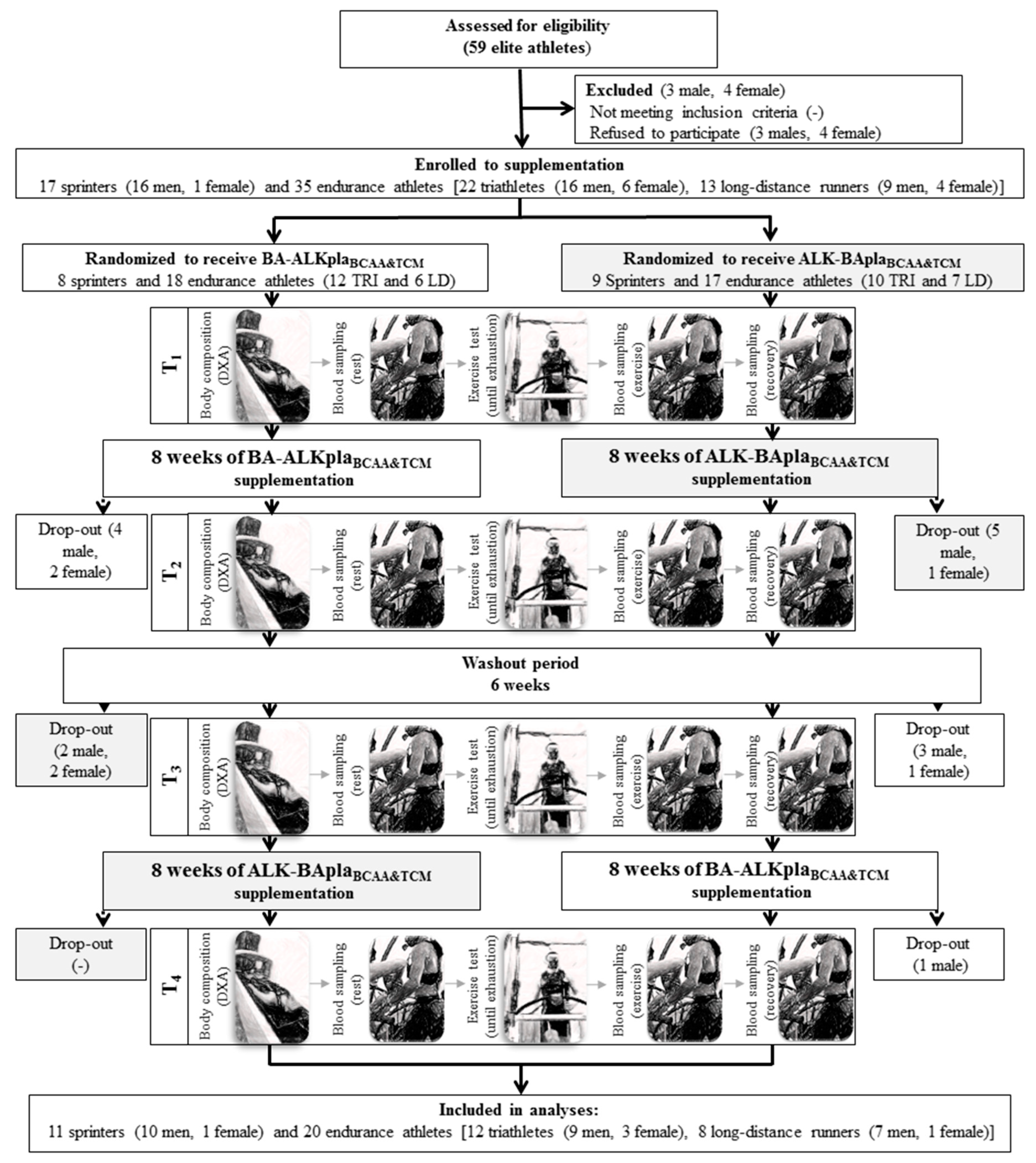
2.2. Experimental Design
2.2.1. Supplementation Characteristics
2.2.2. Study Visits
2.2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
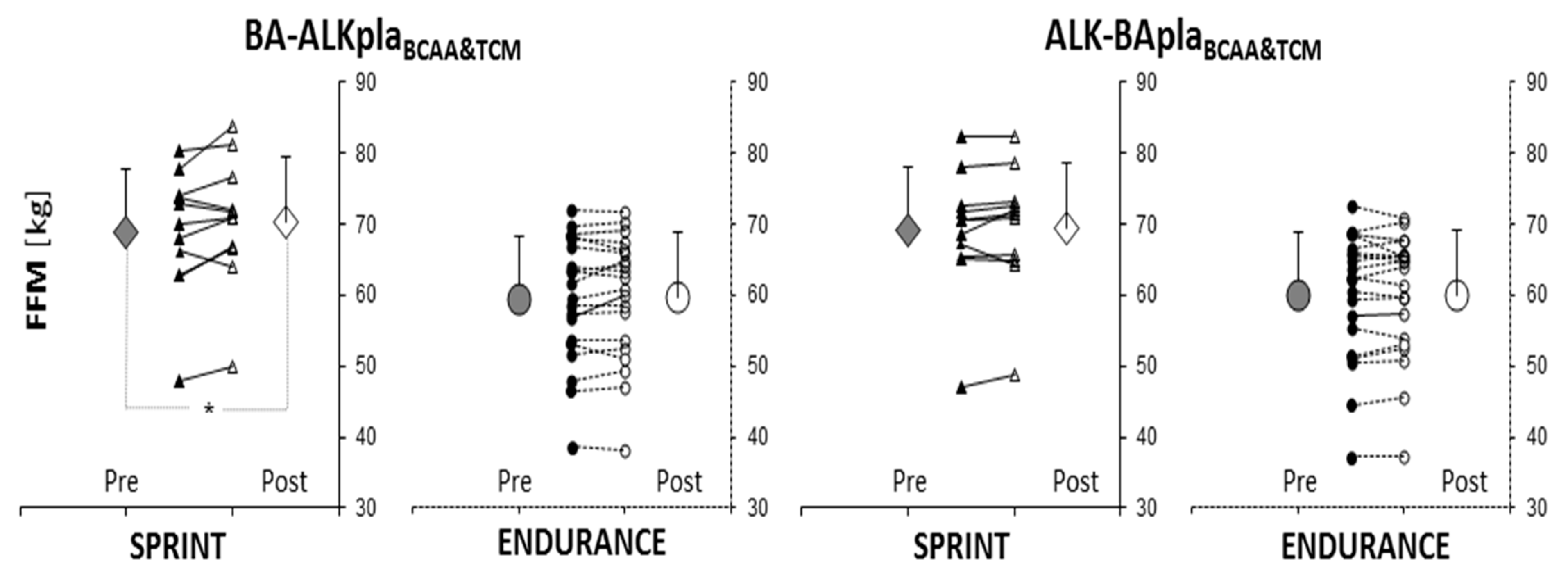
3.2. Body Composition
3.3. Cardiorespiratory Indices before and during the Incremental Exercise Test
3.4. Biochemical Blood Markers
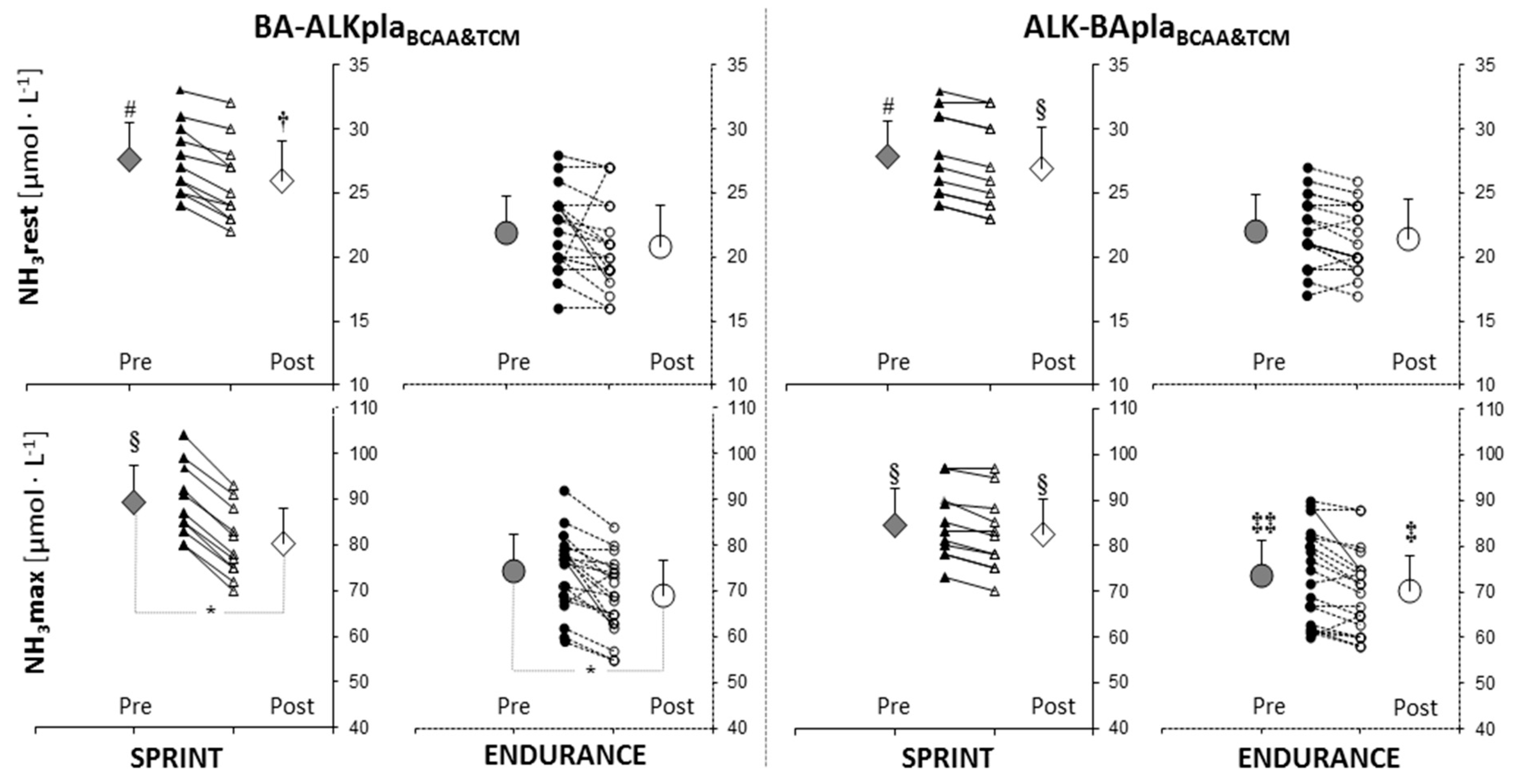
3.4.1. Resting and Post-Exercise Blood Biochemical Marker Concentrations
3.4.2. Blood Biochemical Marker Concentrations during the Post-Exercise Recovery Period
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Negro, M.; Giardina, S.; Marzani, B.; Marzatico, F. Branched-chain amino acid supplementation does not enhance athletic performance but affects muscle recovery and the immune system. J. Sports Med. Phys. Fit. 2008, 48, 347–351. [Google Scholar]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef]
- Holeček, M.; Vodeničarovová, M. Effects of branched-chain amino acids on muscles under hyperammonemic conditions. J. Physiol. Biochem. 2018, 74, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.H.; Shab-Bidar, S.; Mollahosseini, M.; Djafarian, K. Branched-chain amino acid supplementation and exercise-induced muscle damage in exercise recovery: A meta-analysis of randomized clinical trials. Nutrition 2017, 42, 30–36. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.H.; Jeong, W.S.; Lee, H.Y. Effect of BCAA intake during endurance exercises on fatigue substances, muscle damage substances, and energy metabolism substances. J. Exerc. Nutr. Biochem. 2013, 17, 169–180. [Google Scholar] [CrossRef]
- Yamamoto, T.; Azechi, H.; Board, M. Essential role of excessive tryptophan and its neurometabolites in fatigue. Can. J. Neurol. Sci. 2012, 39, 40–47. [Google Scholar] [CrossRef]
- Blomstrand, E. A role for branched-chain amino acids in reducing central fatigue. J. Nutr. 2006, 136, 544S–547S. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 20, 14–20. [Google Scholar] [CrossRef]
- Cooper, R.; Naclerio, F.; Allgrove, J.; Jimenez, A. Creatine supplementation with specific view to exercise/sports performance: An update. J. Int. Soc. Sports Nutr. 2012, 9, 33–43. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Branch, J.D. Effect of Creatine Supplementation on Body Composition and Performance: A Meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 198–226. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Jung, Y.P. Creatine supplementation in exercise, sport, and medicine. J. Exerc. Nutr. Biochem. 2011, 15, 53–69. [Google Scholar] [CrossRef]
- Heibel, A.B.; Perim, P.H.; Oliveira, L.F.; McNaughton, L.R.; Saunders, B. Time to optimize supplementation: Modifying factors influencing the individual responses to extracellular buffering agents. Front. Nutr. 2018, 8, 35. [Google Scholar] [CrossRef]
- Lancha, A.H., Jr.; Painelli, V.; Saunders, B.; Artioli, G.G. Nutritional strategies to modulate intracellular and extracellular buffering capacity during high-intensity exercise. Sports Med. 2015, 45, S71–S81. [Google Scholar] [CrossRef]
- Heisler, N. Buffering and H+ ion dynamics in muscle tissues. Respir. Physiol. Neurobiol. 2004, 144, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, P.M. β-Alanine supplementation for athletic performance: An update. J. Strength Cond. Res. 2014, 28, 1751–1770. [Google Scholar] [CrossRef] [PubMed]
- Derave, W.; Ozdemir, M.S.; Harris, R.C.; Pottier, A.; Reyngoudt, H.; Koppo, K.; Wise, J.A.; Achten, E. β-alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters. J. Appl. Physiol. 2007, 103, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Maughan, R.J.; Burke, L.M. Nutrition for power sports: Middle-distance running, track cycling, rowing, canoeing/kayaking, and swimming. J. Sports Sci. 2011, 29, S79–S89. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.R.; Wilborn, C.D.; Sale, C.; Kreider, R.B.; Jäger, R.; Earnest, C.P.; Bannock, L.; et al. International society of sports nutrition position stand: Beta-Alanine. J. Int. Soc. Sports Nutr. 2015, 12, 30. [Google Scholar] [CrossRef]
- Hobson, R.M.; Saunders, B.; Ball, G.; Harris, R.C.; Sale, C. Effects of β-alanine supplementation on exercise performance: A meta-analysis. Amino Acids 2012, 43, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Baguet, A.; Koppo, K.; Pottier, A.; Derave, W. Beta-alanine supplementation reduces acidosis but not oxygen uptake response during high-intensity cycling exercise. Eur. J. Appl. Physiol. 2010, 108, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.E.; Stout, J.R.; Kendall, K.L.; Fukuda, D.H.; Cramer, J.T. Exercise-induced oxidative stress: The effects of β-alanine supplementation in women. Amino Acids 2012, 43, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Requena, B.; Zabala, M.; Padial, P.; Feriche, B. Sodium bicarbonate and sodium citrate: Ergogenic aids? J. Strength Cond. Res. 2005, 19, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.J.; Hopkins, W.G.; Gore, C.J. Effects of acute alkalosis and acidosis on performance: A meta-analysis. Sports Med. 2011, 41, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Percival, M.E.; Martin, B.J.; Gillen, J.B.; Skelly, L.E.; Macinnis, M.J.; Green, A.E.; Tarnopolsky, M.A.; Gibala, M.J. Sodium bicarbonate ingestion augments the increase in PGC-1alpha mRNA expression during recovery from intense interval exercise in human skeletal muscle. J. Appl. Physiol. 2015, 119, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Hollidge-Horvat, M.G.; Parolin, M.L.; Wong, D.; Jones, N.L.; Heigenhauser, G.J. Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E316–E329. [Google Scholar] [CrossRef]
- Sostaric, S.M.; Skinner, S.L.; Brown, M.J.; Sangkabutra, T.; Medved, I.; Medley, T.; Selig, S.E.; Fairweather, I.; Rutar, D.; McKenna, M.J. Alkalosis increases muscle K+ release, but lowers plasma [K+] and delays fatigue during dynamic forearm exercise. J. Physiol. 2006, 570, 185–205. [Google Scholar] [CrossRef]
- Oöpik, V.; Saaremets, I.; Medijainen, L.; Karelson, K.; Janson, T.; Timpmann, S. Effects of sodium citrate ingestion before exercise on endurance performance in well trained college runners. Br. J. Sports Med. 2003, 37, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Durkalec-Michalski, K.; Zawieja, E.E.; Podgórski, T.; Zawieja, B.E.; Michałowska, P.; Łoniewski, I.; Jeszka, J. The Effect of a New Sodium Bicarbonate Loading Regimen on Anaerobic Capacity and Wrestling Performance. Nutrients 2018, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Durkalec-Michalski, K.; Zawieja, E.E.; Podgórski, T.; Łoniewski, I.; Zawieja, B.E.; Warzybok, M.; Jeszka, J. The effect of chronic progressive-dose sodium bicarbonate ingestion on CrossFit-like performance: A double-blind, randomized cross-over trial. PLoS ONE 2018, 13, e0197480. [Google Scholar] [CrossRef] [PubMed]
- Tobias, G.; Benatti, F.B.; de Salles Painelli, V.; Roschel, H.; Gualano, B.; Sale, C.; Harris, R.C.; Lancha, A.H., Jr.; Artioli, G.G. Additive effects of beta-alanine and sodium bicarbonate on upper-body intermittent performance. Amino Acids 2013, 45, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Felippe, L.C.; Lopes-Silva, J.P.; Bertuzzi, R.; McGinley, C.; Lima-Silva, A. Separate and combined effects of caffeine and sodium-bicarbonate intake on judo performance. Int. J. Sports. Physiol. Perform. 2016, 11, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Siegler, J.C.; Hirscher, K. Sodium bicarbonate ingestion and boxing performance. J. Strength Cond. Res. 2010, 24, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Lun, V.; Erdman, K.A.; Fung, T.S.; Reimer, R.A. Dietary supplementation practices in Canadian high-performance athletes. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, A.; Alaranta, A.; Helenius, I.; Vasankari, T. Use of dietary supplements in Olympic athletes is decreasing: A follow-up study between 2002 and 2009. J. Int. Soc. Sports Nutr. 2011, 8, 1. [Google Scholar] [CrossRef]
- Burke, L.M. Practical Issues in Evidence-Based Use of Performance Supplements: Supplement Interactions, Repeated Use and Individual Responses. Sports Med. 2017, 47, 79–100. [Google Scholar] [CrossRef]
- Nana, A.; Slater, G.J.; Hopkins, W.G.; Halson, S.L.; Martin, D.T.; West, N.P.; Burke, L.M. Importance of Standardized DXA Protocol for Assessing Physique Changes in Athletes. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 259–267. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Jeszka, J. The Effect of β-Hydroxy-β-Methylbutyrate on Aerobic Capacity and Body Composition in Trained Athletes. J. Strength Cond. Res. 2016, 9, 2617–2626. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Jeszka, J.; Podgórski, T. The Effect of a 12-Week Beta-hydroxy-beta-methylbutyrate (HMB) Supplementation on Highly-Trained Combat Sports Athletes: A Randomised, Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2017, 14, 753. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, I.P.; Harris, R.C.; Kim, H.J.; Kim, C.K.; Dang, V.H.; Lam, T.Q.; Bui, T.T.; Smith, M.; Wise, J.A. The effects of 10 weeks of resistance training combined with beta-alanine supplementation on whole body strength, force production, muscular endurance and body composition. Amino Acids 2008, 34, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Outlaw, J.J.; Smith-Ryan, A.E.; Buckley, A.L.; Urbina, S.L.; Hayward, S.; Wingfield, H.L.; Campbell, B.; Foster, C.; Taylor, L.W.; Wilborn, C.D. Effects of β-alanine on body composition and performance measures in collegiate women. J. Strength. Cond. Res. 2016, 30, 2627–2637. [Google Scholar] [CrossRef]
- Kresta, J.; Oliver, J.M.; Jagim, A.R.; Fluckey, J.; Riechman, S.; Kelly, K.; Meininger, C.; Mertens-Talcott, S.U.; Rasmussen, C.; Kreider, R.B. Effects of 28 days of beta-alanine and creatine supplementation on muscle carnosine, body composition and exercise performance in recreationally active females. J. Int. Soc. Sports Nutr. 2014, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Walter, A.A.; Graef, J.L.; Kendall, K.L.; Moon, J.R.; Lockwood, C.M.; Fukuda, D.H.; Beck, T.W.; Cramer, J.T.; Stout, J.R. Effects of β-alanine supplementation and high-intensity interval training on endurance performance and body composition in men; a double-blind trial. J. Int. Soc. Sports Nutr. 2009, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.M.; Gray, M.; Stewart, R.W., Jr.; Moyen, N.E.; Kavouras, S.A.; DiBrezzo, R.; Turner, R.; Baum, J.I.; Stone, M.S. Effects of 28-day beta-alanine supplementation on isokinetic exercise performance and body composition in female masters athletes. J. Strength Cond. Res. 2016, 30, 200–207. [Google Scholar] [CrossRef]
- Hoffman, J.; Ratamess, N.; Kang, J.; Mangine, G.; Faigenbaum, A.; Stout, J. Effect of creatine and beta-alanine supplementation on performance and endocrine responses in strength/power athletes. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 430–446. [Google Scholar] [CrossRef]
- Kern, B.D.; Robinson, T.L. Effects of β-alanine supplementation on performance and body composition in collegiate wrestlers and football players. J. Strength Cond. Res. 2011, 25, 1804–1815. [Google Scholar] [CrossRef]
- Zoeller, R.F.; Stout, J.R.; O’kroy, J.A.; Torok, D.J.; Mielke, M. Effects of 28 days of beta-alanine and creatine monohydrate supplementation on aerobic power, ventilatory and lactate thresholds, and time to exhaustion. Amino Acids 2007, 33, 505–510. [Google Scholar] [CrossRef]
- Stout, J.R.; Cramer, J.T.; Zoeller, R.F.; Torok, D.; Costa, P.; Hoffman, J.R.; Harris, R.C.; O’Kroy, J. Effects of beta-alanine supplementation on the onset of neuromuscular fatigue and ventilatory threshold in women. Amino Acids 2007, 32, 381–386. [Google Scholar] [CrossRef]
- Walter, A.A.; Smith, A.E.; Kendall, K.L.; Stout, J.R.; Cramer, J.T. Six weeks of high-intensity interval training with and without beta-alanine supplementation for improving cardiovascular fitness in women. J. Strength Cond. Res. 2010, 24, 1199–1207. [Google Scholar] [CrossRef]
- Higgins, M.F.; Wilson, S.; Hill, C.; Price, M.J.; Duncan, M.; Tallis, J. Evaluating the effects of caffeine and sodium bicarbonate, ingested individually or in combination, and a taste-matched placebo on high-intensity cycling capacity in healthy males. Appl. Physiol. Nutr. Metab. 2016, 41, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Silva, J.P.; Da Silva Santos, J.F.; Artioli, G.G.; Loturco, I.; Abbiss, C.; Franchini, E. Sodium bicarbonate ingestion increases glycolytic contribution and improves performance during simulated taekwondo combat. Eur. J. Sports Sci. 2018, 18, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Brisola, G.M.; Miyagi, W.E.; da Silva, H.S.; Zagatto, A.M. Sodium bicarbonate supplementation improved MAOD but is not correlated with 200- and 400-m running performances: A double-blind, crossover, and placebo-controlled study. Appl. Physiol. Nutr. Metab. 2015, 40, 931–937. [Google Scholar] [CrossRef]
- Santalla, A.; Perez, M.; Montilla, M.; Vicente, L.; Davison, R.; Earnest, C.; Lucía, A. Sodium bicarbonate ingestion does not alter the slow component of oxygen uptake kinetics in professional cyclists. J. Sports Sci. 2003, 21, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Peinado, A.B.; Holgado, D.; Luque-Casado, A.; Rojo-Tirado, M.A.; Sanabria, D.; González, C.; Mateo-March, M.; Sánchez-Muñoz, C.; Calderón, F.J.; Zabala, M. Effect of induced alkalosis on performance during a field-simulated BMX cycling competition. J. Sci. Med. Sports 2019, 22, 335–341. [Google Scholar] [CrossRef]
- McGinley, C.; Bishop, D.J. Influence of training intensity on adaptations in acid/base transport proteins, muscle buffer capacity, and repeated-sprint ability in active men. J. Appl. Physiol. 2016, 121, 1290–1305. [Google Scholar] [CrossRef]
- Thomas, C.; Delfour-Peyrethon, R.; Bishop, D.J.; Perrey, S.; Leprêtre, P.M.; Dorel, S.; Hanon, C. Effects of pre-exercise alkalosis on the decrease in VO2 at the end of all-out exercise. Eur. J. Appl. Physiol. 2016, 116, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Kantanista, A.; Kusy, K.; Zarębska, E.; Włodarczyk, M.; Ciekot-Sołtysiak, M.; Zieliński, J. Blood ammonia and lactate responses to incremental exercise in highly-trained male sprinters and triathletes. Biomed. Hum. Kinet. 2016, 8, 32–38. [Google Scholar] [CrossRef]
- Gonçalves, L.C.; Bessa, A.; Freitas-Dias, R.; Luzes, R.; Werneck-de-Castro, J.P.; Bassini, A.; Cameron, L.C. A sportomics strategy to analyze the ability of arginine to modulate both ammonia and lymphocyte levels in blood after high-intensity exercise. J. Int. Soc. Sports Nutr. 2012, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Gorostiaga, E.M.; Asiáin, X.; Izquierdo, M.; Postigo, A.; Aguado, R.; Alonso, J.M.; Ibáñez, J. Vertical jump performance and blood ammonia and lactate levels during typical training sessions in elite 400-m runners. J. Strength Cond. Res. 2010, 24, 1138–1149. [Google Scholar] [CrossRef]
- Bassini-Cameron, A.; Monteiro, A.; Gomes, A.; Werneck-de-Castro, J.P.; Cameron, L. Glutamine protects against increases in blood ammonia in football players in an exercise intensity-dependent way. Br. J. Sports Med. 2008, 42, 260–266. [Google Scholar] [CrossRef]
- Carvalho-Peixoto, J.; Alves, R.C.; Cameron, L.C. Glutamine and carbohydrate supplements reduce ammonemia increase during endurance field exercise. Appl. Physiol. Nutr. Metab. 2007, 32, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Degoutte, F.; Jouanel, P.; Filaire, E. Energy demands during a judo match and recovery. Br. J. Sports Med. 2003, 37, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, M.; Kusy, K.; Słomińska, E.; Krasiński, Z.; Zieliński, J. Changes in blood concentration of adenosine triphosphate metabolism biomarkers during incremental exercise in highly trained athletes of different sport specializations. J. Strength Cond. Res. 2019, 33, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, J.; Kusy, K. Hypoxanthine: A universal metabolic indicator of the training status in competitive sport. Exerc. Sport Sci. Rev. 2015, 43, 214–221. [Google Scholar] [CrossRef]
- Prado, E.S.; de Rezende Neto, J.M.; de Almeida, R.D.; Dória de Melo, M.G.; Cameron, L.C. Keto analogue and amino acid supplementation affects the ammonaemia response during exercise under ketogenic conditions. Br. J. Nutr. 2011, 28, 1729–1733. [Google Scholar] [CrossRef]
- De Almeida, R.D.; Prado, E.S.; Llosa, C.D.; Magalhães-Neto, A.; Cameron, L.C. Acute supplementation with keto analogues and amino acids in rats during resistance exercise. Br. J. Nutr. 2010, 104, 1438–1442. [Google Scholar] [CrossRef]
- Camerino, S.R.; Lima, R.C.; França, T.C.; Herculano Ede, A.; Rodrigues, D.S.; Gouveia, M.G.; Cameron, L.C.; Prado, E.S. Keto analogue and amino acid supplementation and its effects on ammonemia and performance under thermoneutral conditions. Food Funct. 2016, 7, 872–880. [Google Scholar] [CrossRef]
- Falavigna, G.; Alves de Araújo, J., Jr.; Rogero, M.M.; Pires, I.S.; Pedrosa, R.G.; Martins, E., Jr.; Alves de Castro, I.; Tirapegui, J. Effects of diets supplemented with branched-chain amino acids on the performance and fatigue mechanisms of rats submitted to prolonged physical exercise. Nutrients 2012, 11, 1767–1780. [Google Scholar] [CrossRef]
- Pencharz, P.B.; Elango, R.; Ball, R.O. Determination of the tolerable upper intake level of leucine in adult men. J. Nutr. 2012, 142, 2220S–2224S. [Google Scholar] [CrossRef] [PubMed]
- Koo, G.H.; Woo, J.; Kang, S.; Shin, K.O. Effects of supplementation with BCAA and L-glutamine on blood fatigue factors and cytokines in juvenile athletes submitted to maximal intensity rowing performance. J. Phys. Ther. Sci. 2014, 26, 1241–1246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsueh, C.F.; Wu, H.J.; Tsai, T.S.; Wu, C.L.; Chang, C.K. The effect of branched-chain amino acids, citrulline, and arginine on high-intensity interval performance in young swimmers. Nutrients 2018, 10, 1979. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, J.; Pullinen, T.; Gorostiaga, E.; Postigo, A.; Mero, A. Blood lactate and ammonia in short-term anaerobic work following induced alkalosis. J. Sports Med. Phys. Fit. 1995, 35, 187–193. [Google Scholar]
- Yuan, Y.; Chan, K.M. A review of the literature on the application of blood ammonia measurement in sports science. Res. Q. Exerc. Sport 2000, 71, 145–151. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Smeeton, N.J.; Watt, P.W. Ammonia metabolism, the brain and fatigue; revisiting the link. Prog. Neurobiol. 2010, 91, 200–219. [Google Scholar] [CrossRef]
- Lopez, R.M.; Casa, D.; McDermott, B.P.; Ganio, M.S.; Armstrong, L.E.; Maresh, C.M. Does creatine supplementation hinder exercise heat tolerance or hydration status? A systematic review with meta-analyses. J. Athl. Train. 2009, 44, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Nana, A.; Slater, G.J.; Hopkins, W.G.; Burke, L.M.; Burke, L.M. Effects of daily activities on dual-energy X-ray absorptiometry measurements of body composition in active people. Med. Sci. Sports Exerc. 2012, 44, 180–189. [Google Scholar] [CrossRef] [PubMed]

| Variable | SPRINTERS | ENDURANCE | Sprinters vs. Endurance * | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | p-Value | ||
| n | 11 | 20 | ||
| Age | (years) | 23.5 ± 3.4 | 22.3 ± 4.4 | 0.13 |
| Body height | (cm) | 184 ± 6 | 178 ± 7 | 0.04 |
| Body mass | (kg) | 77.8 ± 7.8 | 70.4 ± 11.3 | 0.01 |
| Body mass index (BMI) | (kg·m−2) | 23.0 ± 1.1 | 22.1 ± 2.3 | 0.29 |
| Maximal oxygen uptake (VO2max) | (L·min−1) | 4.15 ± 0.53 | 4.46 ± 0.82 | 0.27 |
| Training experience | (years) | 8.6 ± 2.5 | 8.6 ± 1.8 | 0.82 |
| Energy intake | (kcal·day−1) | 2922 ± 335 | 3179 ± 491 | 0.13 |
| (kcal·kg−1) | 37.9 ± 5.3 | 46.6 ± 8.3 | <0.01 | |
| Protein intake | (g·day−1) | 129 ± 29 | 138 ± 34 | 0.48 |
| (g·kg−1) | 1.7 ± 0.3 | 2.0 ± 0.5 | 0.052 | |
| (% of energy) | 17.8 ± 4.0 | 17.3 ± 3.3 | 0.68 | |
| Fat intake | (g·day−1) | 102 ± 18 | 108 ± 28 | 0.50 |
| (g·kg−1) | 1.3 ± 0.3 | 1.6 ± 0.5 | 0.09 | |
| (% of energy) | 31.4 ± 4.0 | 30.4 ± 4.6 | 0.55 | |
| Carbohydrate intake | (g·day−1) | 378 ± 57 | 437 ± 63 | 0.02 |
| (g·kg−1) | 4.9 ± 0.9 | 6.4 ± 1.1 | <0.001 | |
| (% of energy) | 51.8 ± 4.4 | 55.3 ± 5.4 | 0.07 | |
| Variable | Group BA-ALKplaBCAA&TCM | Group ALK-BAplaBCAA&TCM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SPRINT | ENDURANCE | SPRINT | ENDURANCE | |||||||
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | |||
| VErest | (L∙min−1) | Pre | 17.8 ± 2.9 | 15.8–19.8 | 16.1 ± 3.7 | 14.4–17.9 | 18.3 ± 2.2 | 16.8–19.8 | 16.1 ± 5.0 | 13.7–18.4 |
| Post | 18.2 ± 2.2 | 16.7–19.7 | 16.7 ± 4.4 | 14.6–18.7 | 17.5 ± 2.0 | 16.1–18.9 | 16.5 ± 4.5 | 14.4–18.6 | ||
| HR rest | (bpm) | Pre | 88 ± 11 | 81–96 | 74 ± 16 | 67–82 | 90 ± 15 | 80–100 | 77 ± 16 | 69–84 |
| Post | 86 ± 16 | 75–97 | 73 ± 12 | 68–79 | 85 ± 13 | 76–93 | 76 ± 20 | 67–85 | ||
| VO2 rest | (L∙min−1) | Pre | 0.55 ± 0.10 | 0.48–0.62 | 0.50 ± 0.12 | 0.44–0.55 | 0.57 ± 0.08 | 0.52–0.62 | 0.51 ± 0.15 | 0.43–0.58 |
| Post | 0.58 ± 0.07 | 0.53–0.62 | 0.53 ± 0.15 | 0.46–0.60 | 0.59 ± 0.08 | 0.54–0.64 | 0.52 ± 0.13 | 0.45–0.58 | ||
| RER rest | Pre | 0.85 ± 0.08 | 0.79–0.90 | 0.86 ± 0.06 | 0.83–0.89 | 0.83 ± 0.07 | 0.78–0.88 | 0.85 ± 0.08 | 0.81–0.89 | |
| Post | 0.85 ± 0.07 | 0.80–0.89 | 0.83 ± 0.05 | 0.81–0.85 | 0.82 ± 0.03 | 0.80–0.84 | 0.85 ± 0.08 | 0.81–0.89 | ||
| VE VT | (L∙min−1) | Pre | 76.7 ± 19.9 | 63.4–90.1 | 77.0 ± 17.5 | 68.7–85.2 | 76.5 ± 18.7 | 64.0–89.1 | 76.9 ± 18.7 | 68.2–85.7 |
| Post | 77.9 ± 22.1 | 63.0–92.7 | 79.3 ± 11.5 | 73.9–84.7 | 77.8 ± 18.2 | 65.5–90.0 | 81.5 ± 18.6 | 72.8–90.2 | ||
| HRVT | (bpm) | Pre | 156 ± 19 | 144–169 | 157 ± 13 | 151–163 | 159 ± 15 | 148–169 | 158 ± 11 | 153–163 |
| Post | 15 ± 17 | 143–166 | 159 ± 14 | 153–166 | 156 ± 14 | 146–165 | 156 ± 8 | 152–160 | ||
| VO2VT | (L∙min−1) | Pre | 2.86 ± 0.49 | 2.53–3.19 | 3.10 ± 0.66 | 2.80–3.41 | 2.95 ± 0.67 | 2.50–3.39 | 3.12 ± 0.69 | 2.80–3.45 |
| Post | 2.85 ± 0.65 | 2.42–3.29 | 3.11 ± 0.70 | 2.05–3.59 | 2.90 ± 0.48 | 2.56–3.24 | 3.23 ± 0.72 | 2.89–3.56 | ||
| RERVT | Pre | 0.88 ± 0.06 | 0.84–0.92 | 0.90 ± 0.04 | 0.88–0.92 | 0.90 ± 0.04 | 0.87–0.92 | 0.99 ± 0.44 | 0.78–1.20 | |
| Post | 0.89 ± 0.07 | 0.84–0.94 | 0.90 ± 0.03 | 0.89–0.92 | 0.91 ± 0.03 | 0.88–0.93 | 0.89 ± 0.03 | 0.88–0.91 | ||
| VEMAX | (L∙min−1) | Pre | 152 ± 26 | 134–169 | 159 ± 23 | 149–170 | 154 ± 24 | 137–170 | 160 ± 25 | 148–172 |
| Post | 153 ± 24 | 135–170 | 158 ± 23 | 148–169 | 152 ± 22 | 137–167 | 158 ± 26 | 146–170 | ||
| HRMAX | (bpm) | Pre | 192 ± 9 | 186–198 | 194 ± 10 | 189–198 | 193 ± 9 | 187–200 | 193 ± 10 | 188–198 |
| Post | 193 ± 11 | 185–201 | 193 ± 10 | 188–197 | 192 ± 8 | 186–197 | 193 ± 11 | 188–198 | ||
| VO2MAX | (L∙min−1) | Pre | 4.16 ± 0.60 | 3.76–4.56 | 4.45 ± 0.76 | 4.00–4.80 | 4.18 ± 0.53 | 3.82–4.53 | 4.55 ± 0.85 | 4.16–4.95 |
| Post | 4.19 ± 0.54 | 3.80–4.57 | 4.45 ± 0.77 | 4.09–4.81 | 4.23 ± 0.51 | 3.89–4.57 | 4.51 ± 0.78 | 4.15–4.88 | ||
| RERMAX | Pre | 1.06 ± 0.03 | 1.03–1.08 | 1.09 ± 0.06 | 1.07–1.12 | 1.05 ± 0.03 | 1.04–1.07 | 1.09 ± 0.07 | 1.05–1.12 | |
| Post | 1.08 ± 0.04 | 1.05–1.10 | 1.09 ± 0.04 | 1.07–1.11 | 1.08 ± 0.02 | 1.06–1.10 | 1.07 ± 0.06 | 1.04–1.09 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durkalec-Michalski, K.; Kusy, K.; Ciekot-Sołtysiak, M.; Zieliński, J. The Effect of Beta-Alanine versus Alkaline Agent Supplementation Combined with Branched-Chain Amino Acids and Creatine Malate in Highly-Trained Sprinters and Endurance Athletes: A Randomized Double-Blind Crossover Study. Nutrients 2019, 11, 1961. https://doi.org/10.3390/nu11091961
Durkalec-Michalski K, Kusy K, Ciekot-Sołtysiak M, Zieliński J. The Effect of Beta-Alanine versus Alkaline Agent Supplementation Combined with Branched-Chain Amino Acids and Creatine Malate in Highly-Trained Sprinters and Endurance Athletes: A Randomized Double-Blind Crossover Study. Nutrients. 2019; 11(9):1961. https://doi.org/10.3390/nu11091961
Chicago/Turabian StyleDurkalec-Michalski, Krzysztof, Krzysztof Kusy, Monika Ciekot-Sołtysiak, and Jacek Zieliński. 2019. "The Effect of Beta-Alanine versus Alkaline Agent Supplementation Combined with Branched-Chain Amino Acids and Creatine Malate in Highly-Trained Sprinters and Endurance Athletes: A Randomized Double-Blind Crossover Study" Nutrients 11, no. 9: 1961. https://doi.org/10.3390/nu11091961
APA StyleDurkalec-Michalski, K., Kusy, K., Ciekot-Sołtysiak, M., & Zieliński, J. (2019). The Effect of Beta-Alanine versus Alkaline Agent Supplementation Combined with Branched-Chain Amino Acids and Creatine Malate in Highly-Trained Sprinters and Endurance Athletes: A Randomized Double-Blind Crossover Study. Nutrients, 11(9), 1961. https://doi.org/10.3390/nu11091961








