Exercise-Induced Oxidative Stress, Nitric Oxide and Plasma Amino Acid Profile in Recreational Runners with Vegetarian and Non-Vegetarian Dietary Patterns
Abstract
1. Introduction
2. Materials and Methods
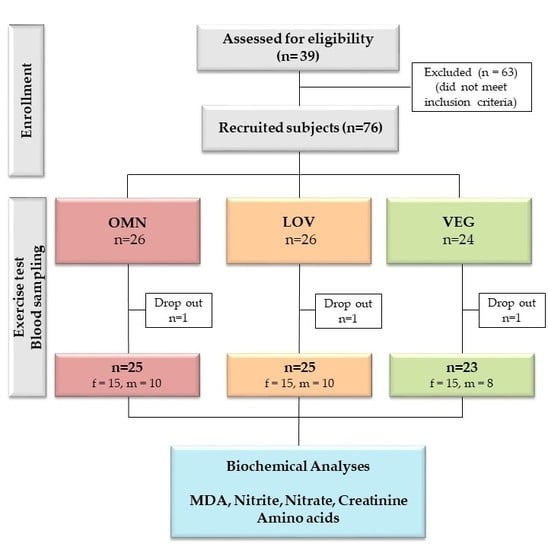
2.1. Study Design, Participants, Physical Exercise and Blood Sampling
2.2. Sample Preparation and Biochemical Analyses
2.3. Gas Chromatographic-Mass Spectrometric Analyses
2.4. Data Analysis and Statistical Methods
3. Results
3.1. Pre-Exercise Concentrations
3.2. Exercise-Induced Changes
3.3. Correlations
3.4. Dietary Intake
3.5. Associations with Exercise Capacity
4. Discussion
4.1. Pre-Exercise Status of Plasma Oxidative Stress, NO Metabolism and Amino Acids Profile
4.2. Exercise-Induced Effects on Oxidative Stress, NO Metabolism and Amino Acids Profile
4.3. Associations with Exercise Capacity
4.4. Strengths and Limitations of the Study
4.5. Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W. Oxidative stress: New approaches to diagnosis and prognosis in atherosclerosis. Am. J. Cardiol. 2003, 91, 12–16. [Google Scholar] [CrossRef]
- Sydow, K.; Schwedhelm, E.; Arakawa, N.; Bode-Böger, S.M.; Tsikas, D.; Hornig, B.; Frölich, J.C.; Böger, R.H. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: Effects of L-arginine and B vitamins. Cardiovasc. Res. 2003, 57, 244–252. [Google Scholar] [CrossRef]
- Tyagi, N.; Sedoris, K.C.; Steed, M.; Ovechkin, A.V.; Moshal, K.S.; Tyagi, S.C. Mechanisms of homocysteine-induced oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2649–H2656. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Dalle-Donne, I.; Tsikas, D.; Rossi, R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit. Rev. Clin. Lab. Sci. 2009, 46, 241–281. [Google Scholar] [CrossRef] [PubMed]
- Scheele, C.; Nielsen, S.; Pedersen, B.K. ROS and myokines promote muscle adaptation to exercise. Trends Endocrinol. Metab. 2009, 20, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Zarse, K.; Oberbach, A.; Kloting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Bluher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Cobley, J.N.; Paschalis, V.; Veskoukis, A.S.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Principles for integrating reactive species into in vivo biological processes: Examples from exercise physiology. Cell. Signal. 2016, 28, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Faude, O.; Fuhrmann, M.; Herrmann, M.; Kindermann, W.; Urhausen, A. Ernährungsanalysen und Vitaminstatus bei deutschen Spitzenathleten. Leistungssport 2005, 35, 4–9. [Google Scholar]
- Baranauskas, M.; Stukas, R.; Tubelis, L.; Žagminas, K.; Šurkienė, G.; Švedas, E.; Giedraitis, V.R.; Dobrovolskij, V.; Abaravičius, J.A. Nutritional habits among high-performance endurance athletes. Medicina 2015, 51, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Stewart, K.; Oda, K.; Batech, M.; Herring, R.P.; Fraser, G.E. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, B.J.; Anousheh, R.; Fan, J.; Jaceldo-Siegl, K.; Fraser, G.E. Vegetarian diets and blood pressure among white subjects: Results from the Adventist Health Study-2 (AHS-2). Public Health Nutr. 2012, 15, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Orlich, M.J.; Singh, P.N.; Sabaté, J.; Fan, J.; Sveen, L.; Bennett, H.; Knutsen, S.F.; Beeson, W.L.; Jaceldo-Siegl, K.; Butler, T.L.; et al. Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Intern. Med. 2015, 175, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Goldman, D.M.; Loomis, J.F.; Kahleova, H.; Levin, S.M.; Neabore, S.; Batts, T.C. Plant-Based Diets for Cardiovascular Safety and Performance in Endurance Sports. Nutrients 2019, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Trapp, D.; Knez, W.; Sinclair, W. Could a vegetarian diet reduce exercise-induced oxidative stress? A review of the literature. J. Sports Sci. 2010, 28, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Vanacore, D.; Messina, G.; Lama, S.; Bitti, G.; Ambrosio, P.; Tenore, G.C.; Messina, A.; Monda, V.; Zappavigna, S.; Boccellino, M.; et al. Effect of Restriction Vegan Diet’s on Muscle Mass, Oxidative Status and Myocytes Differentiation: A Pilot Study. J. Cell. Physiol. 2018, 12, 9345–9353. [Google Scholar] [CrossRef] [PubMed]
- Metges, C.C. Contribution of microbial amino acids to amino acid homeostasis of the host. J. Nutr. 2000, 130, 1857S–1864S. [Google Scholar] [CrossRef]
- Cynober, L.A. Plasma amino acid levels with a note on membrane transport: Characteristics, regulation, and metabolic significance. Nutrition 2002, 18, 761–766. [Google Scholar] [CrossRef]
- Volk, O.; Neumann, G. Verhalten ausgewählter Aminosäuren während eines Dreifachlangtriathlons. Dtsch. Z. Sportmed. 2001, 52, 169–174. [Google Scholar]
- Décombaz, J.; Reinhardt, P.; Anantharaman, K.; von Glutz, G.; Poortmans, J.R. Biochemical changes in a 100 km run: Free amino acids, urea, and creatinine. Eur. J. Appl. Physiol. Occup. Physiol. 1979, 41, 61–72. [Google Scholar] [CrossRef]
- Lehmann, M.; Huonker, M.; Dimeo, F.; Heinz, N.; Gastmann, U.; Treis, N.; Steinacker, J.M.; Keul, J.; Kajewski, R.; Häussinger, D. Serum amino acid concentrations in nine athletes before and after the 1993 Colmar ultra triathlon. Int. J. Sports Med. 1995, 16, 155–159. [Google Scholar] [CrossRef]
- Refsum, H.E.; Gjessing, L.R.; Strømme, S.B. Changes in plasma amino acid distribution and urine amino acids excretion during prolonged heavy exercise. Scand. J. Clin. Lab. Investig. 1979, 39, 407–413. [Google Scholar] [CrossRef]
- Blomstrand, E.; Celsing, F.; Newsholme, E.A. Changes in plasma concentrations of aromatic and branched-chain amino acids during sustained exercise in man and their possible role in fatigue. Acta Physiol. Scand. 1988, 133, 115–121. [Google Scholar] [CrossRef]
- Blomstrand, E.; Hassmén, P.; Ekblom, B.; Newsholme, E.A. Administration of branched-chain amino acids during sustained exercise—Effects on performance and on plasma concentration of some amino acids. Eur. J. Appl. Physiol. 1991, 63, 83–88. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef]
- Rana, S.K.; Sanders, T.A. Taurine concentrations in the diet, plasma, urine and breast milk of vegans compared with omnivores. Br. J. Nutr. 1986, 56, 17–27. [Google Scholar] [CrossRef]
- Krajcovicová-Kudlácková, M.; Simoncic, R.; Béderová, A.; Babinská, K.; Béder, I. Correlation of carnitine levels to methionine and lysine intake. Physiol. Res. 2000, 49, 399–402. [Google Scholar]
- Abdulla, M.; Andersson, I.; Asp, N.G.; Berthelsen, K.; Birkhed, D.; Dencker, I.; Johansson, C.G.; Jägerstad, M.; Kolar, K.; Nair, B.M.; et al. Nutrient intake and health status of vegans. Chemical analyses of diets using the duplicate portion sampling technique. Am. J. Clin. Nutr. 1981, 34, 2464–2477. [Google Scholar] [CrossRef]
- Abdulla, M.; Aly, K.O.; Andersson, I.; Asp, N.G.; Birkhed, D.; Denker, I.; Johansson, C.G.; Jägerstad, M.; Kolar, K.; Nair, B.M. Nutrient intake and health status of lactovegetarians: Chemical analyses of diets using the duplicate portion sampling technique. Am. J. Clin. Nutr. 1984, 40, 325–338. [Google Scholar] [CrossRef]
- Anderson, G.H.; Blendis, L.M. Plasma neutral amino acid ratios in normal man and in patients with hepatic encephalopathy: Correlations with self-selected protein and energy consumption. Am. J. Clin. Nutr. 1981, 34, 377–385. [Google Scholar] [CrossRef]
- Laidlaw, S.A.; Shultz, T.D.; Cecchino, J.T.; Kopple, J.D. Plasma and urine taurine levels in vegans. Am. J. Clin. Nutr. 1988, 47, 660–663. [Google Scholar] [CrossRef]
- Nebl, J.; Haufe, S.; Eigendorf, J.; Wasserfurth, P.; Tegtbur, U.; Hahn, A. Exercise capacity of vegan, lacto-ovo-vegetarian and omnivorous recreational runners. J. Int. Soc. Sports Nutr. 2019, 16, 23. [Google Scholar] [CrossRef]
- Hanff, E.; Lützow, M.; Kayacelebi, A.A.; Finkel, A.; Maassen, M.; Yanchev, G.R.; Haghikia, A.; Bavendiek, U.; Buck, A.; Lücke, T.; et al. Simultaneous GC-ECNICI-MS measurement of nitrite, nitrate and creatinine in human urine and plasma in clinical settings. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1047, 207–214. [Google Scholar] [CrossRef]
- Hanff, E.; Ruben, S.; Kreuzer, M.; Bollenbach, A.; Kayacelebi, A.A.; Das, A.M.; von Versen-Höynck, F.; von Kaisenberg, C.; Haffner, D.; Ückert, S.; et al. Development and validation of GC-MS methods for the comprehensive analysis of amino acids in plasma and urine and applications to the HELLP syndrome and pediatric kidney transplantation: Evidence of altered methylation, transamidination, and arginase activity. Amino Acids 2019, 51, 529–547. [Google Scholar]
- Van Hall, G.; Saltin, B.; Wagenmakers, A.J. Muscle protein degradation and amino acid metabolism during prolonged knee-extensor exercise in humans. Clin. Sci. 1999, 97, 557–567. [Google Scholar] [CrossRef]
- Tsikas, D.; Wu, G. Homoarginine, arginine, and relatives: Analysis, metabolism, transport, physiology, and pathology. Amino Acids 2015, 47, 1697–1702. [Google Scholar] [CrossRef]
- Nebl, J.; Schuchardt, J.P.; Ströhle, A.; Wasserfurth, P.; Haufe, S.; Eigendorf, J.; Tegtbur, U.; Hahn, A. Micronutrient Status of Recreational Runners with Vegetarian or Non-Vegetarian Dietary Patterns. Nutrients 2019, 11, 1146. [Google Scholar] [CrossRef]
- McConell, G.K.; Rattigan, S.; Lee-Young, R.S.; Wadley, G.D.; Merry, T.L. Skeletal muscle nitric oxide signaling and exercise: A focus on glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E301–E307. [Google Scholar] [CrossRef]
- Lansley, K.E.; Winyard, P.G.; Bailey, S.J.; Vanhatalo, A.; Wilkerson, D.P.; Blackwell, J.R.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef]
| OMN (n = 25) | LOV (n = 25) | VEG (n = 23) | p Value | |
|---|---|---|---|---|
| Age (years) | 27.2 ± 4.1 | 27.6 ± 4.4 | 27.3 ± 4.3 | 0.917a |
| Gender (n, males/females) | 10/15 | 10/15 | 8/15 | 0.913b |
| BMI (kg/m2) | 22.3 ± 1.8 | 21.5 ± 1.9 | 21.9 ± 2.2 | 0.412a |
| Weekly training frequency | 3.0 ± 0.9 | 3.2 ± 0.9 | 3.0 ± 0.9 | 0.757a |
| Weekly running (h) | 2.7 ± 1.1 | 3.3 ± 1.3 | 2.7 ± 1.4 | 0.122a |
| Parameters | OMN | p Value OMN vs. LOV | LOV | p Value LOV vs. VEG | VEG | p Value OMN vs. VEG | p Value |
|---|---|---|---|---|---|---|---|
| Antioxidants (mg) | |||||||
| Vitamin C | 143 ± 153 | n.s. | 148 ± 145 | n.s. | 218 ± 138 | n.s. | 0.037 |
| Vitamin E | 12.0 ± 6.36 | n.s. | 13.1 ± 11.0 | 0.040 | 19.7 ± 12.0 | n.s. | 0.021 |
| Amino acids (g) | |||||||
| Arg | 4.57 ± 2.60 | - | 3.75 ± 2.21 | - | 4.53 ± 2.72 | - | 0.442 |
| Thr | 3.60 ± 2.40 | - | 2.95 ± 1.86 | - | 2.47 ± 1.25 | - | 0.185 |
| Val | 4.93 ± 3.04 | - | 4.38 ± 2.73 | - | 3.51 ± 1.87 | - | 0.213 |
| Leu+Ile | 11.4 ± 7.10 | - | 10.2 ± 6.60 | - | 8.05 ± 4.51 | - | 0.200 |
| Met | 1.90 ± 1.24 | n.s. | 1.56 ± 1.17 | n.s. | 0.99 ± 0.61 | 0.004 | 0.005 |
| Phe | 4.02 ± 2.23 | - | 3.68 ± 2.24 | - | 3.29 ± 1.80 | - | 0.507 |
| Tyr | 3.27 ± 1.89 | - | 3.04 ± 2.08 | - | 2.29 ± 1.33 | - | 0.134 |
| Lys | 5.64 ± 3.85 | - | 4.57 ± 3.21 | - | 3.36 ± 1.97 | - | 0.062 |
| Trp | 1.07 ± 0.64 | - | 0.91 ± 0.54 | - | 0.81 ± 0.39 | - | 0.346 |
| Fatty acids | |||||||
| ALA (g) | 1.43 ± 1.58 | - | 1.85 ± 2.27 | - | 2.16 ± 1.65 | - | 0.115 |
| PUFA (EN%) | 4.87 ± 2.30 | - | 4.76 ± 2.34 | - | 6.26 ± 2.94 | - | 0.085 |
| Parameters | OMN | LOV | VEG | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δ (%) | Pre | Post | Δ (%) | Pre | Post | Δ (%) | |
| Oxidative Stress/NO Metabolism | (n = 25) | (n = 25) | (n = 23) | ||||||
| MDA | 0.52 ± 0.09 | 0.56 ± 0.10 | +9.2 | 0.50 ± 0.07 | 0.62 ± 0.15 † | +24 | 0.57 ± 0.13 | 0.68 ± 0.15 † | +15 |
| Nitrate | 70.7 ± 15.9 | 70.5 ± 17.8 | −0.3 | 91.9 ± 43.8 | 94.9 ± 50.8 | +3.3 | 120 ± 146 | 102 ± 41.9 | −5.7 |
| Nitrite | 1.93 ± 0.26 | 1.85 ± 0.21 | −4.4 | 2.67 ± 0.61 | 2.56 ± 0.53 | −4.2 | 2.50 ± 1.09 | 2.36 ± 0.42 | −18 |
| Kidney function | |||||||||
| Creatinine | 94.1 ± 20.7 | 93.4 ± 17.3 | −0.7 | 90.0 ± 32.8 | 92.4 ± 40.2 | +2.7 | 86.4 ± 37.6 | 84.7 ± 20.7 | −2.1 |
| Amino acids | (n = 24) | (n = 25) | (n = 22) | ||||||
| Ala | 411 ± 100 | 530 ± 112 ‡ | +28 | 400 ± 85.9 | 485 ± 111 ‡ | +21 | 439 ± 104 | 557 ± 117 ‡ | +28 |
| Thr | 194 ± 75.0 | 167 ± 63.4 ‡ | −15 | 161 ± 47.9 | 148 ± 66.6 † | −8.0 | 158 ± 41.7 | 157 ± 62.9* | −0.1 |
| Gly | 249 ± 68.4 | 231 ± 57.6 * | −7.4 | 248 ± 77.5 | 227 ± 63.8 † | −8.3 | 324 ± 79.5 | 312 ± 70.8 | −4.2 |
| Val | 373 ± 150 | 332 ± 104 † | −12 | 332 ± 116 | 302 ± 99.9 * | −8.9 | 298 ± 106 | 284 ± 75.8 | −4.2 |
| Ser | 204 ± 152 | 235 ± 220 | +16 | 168 ± 59.9 | 147 ± 78.6 * | −17 | 185 ± 91.5 | 150 ± 22.3 * | −19 |
| Sar | 2.24 ± 0.99 | 2.22 ± 1.00 | −1.6 | 1.84 ± 0.55 | 2.05 ± 0.64 † | +11 | 1.66 ± 0.46 | 1.95 ± 0.51 † | +17 |
| Leu +Ile | 300 ± 125 | 251 ± 76.5 † | −17 | 231 ± 102 | 209 ± 74.7 * | −9.4 | 227 ± 75.9 | 254 ± 127 | +12 |
| GAA | 3.79 ± 1.24 | 3.25 ± 0.89 | −13 | 2.63 ± 0.64 | 3.01 ± 0.94 | +9.9 | 3.42 ± 0.74 | 3.88 ± 1.02 | +12 |
| Asp+Asn | 104 ± 28.9 | 94.7 ± 32.2 | −9.7 | 75.8 ± 17.0 | 66.1 ± 18.7 † | −13 | 107 ± 22.9 | 97.0 ± 17.3 * | −9 |
| Pro | 213 ± 75.3 | 188 ± 59.9 † | −12 | 220 ± 73.2 | 199 ± 66.6 † | −9.6 | 214 ± 59.1 | 209 ± 67.9 | −0.8 |
| Met | 65.5 ± 13.0 | 62.6 ± 12.7 * | −4.4 | 60.2 ± 7.03 | 57.7 ± 7.88 | −4.2 | 65.5 ± 6.94 | 65.4 ± 6.78 | +0.2 |
| Glu+Gln | 802 ± 236 | 787 ± 235 | −1.6 | 682 ± 106 | 654 ± 116 | −4.1 | 766 ± 107 | 773 ± 117 | +1.6 |
| Orn+Cit | 59.8 ± 21.2 | 50.1 ± 15.8 ‡ | −17 | 55.1 ± 13.7 | 48.0 ± 12.1 ‡ | −13 | 59.0 ± 19.6 | 56.2 ± 18.3 | −3.8 |
| Phe | 80.6 ± 20.6 | 72.0 ± 17.5 † | −12 | 68.1 ± 17.4 | 61.8 ± 13.9 † | −9.2 | 73.7 ± 16.6 | 70.7 ± 12.9 | −3.3 |
| Tyr | 75.3 ± 34.1 | 66.2 ± 25.3 † | −13 | 65.9 ± 25.4 | 60.6 ± 21.2 † | −9.4 | 58.3 ± 18.0 | 58.1 ± 15.8 | −0.4 |
| Lys | 206 ± 69.1 | 184 ± 51.0 † | −12 | 109 ± 37.5 | 120 ± 39.9 * | +9.3 | 153 ± 47.2 | 149 ± 33.4 | −1.9 |
| Arg | 91.8 ± 29.7 | 77.4 ± 21.0 † | −12 | 69.1 ± 19.1 | 66.5 ± 18.2 * | −6.0 | 93.5 ± 25.9 | 90.3 ± 23.6 | −2.9 |
| hArg | 1.89 ± 0.93 | 1.64 ± 0.93 | −11 | 1.09 ± 0.39 | 1.10 ± 0.38 | +1.2 | 1.51 ± 0.81 | 1.58 ± 0.75 | +5 |
| Trp | 34.5 ± 11.6 | 45.8 ± 68.8 | +33 | 22.9 ± 9.28 | 17.2 ± 5.43 ‡ | −25 | 22.7 ± 4.70 | 18.6 ± 5.45 | −18 |
| GAA/hArg | 2.26 ± 0.91 | 2.53 ± 1.22 | +11 | 2.62 ± 1.25 | 2.98 ± 1.49 * | +12 | 2.79 ± 1.34 | 2.86 ± 1.21 | +2.5 |
| GABR | 1.57 ± 0.33 | 1.58 ± 0.30 | +6.3 | 1.27 ± 0.27 | 1.41 ± 0.31 | +17 | 1.65 ± 0.34 | 1.67 ± 0.32 | +1.9 |
| Parameter at the Respective Time | MDA (r, p) | Nitrate (r, p) | Nitrite (r, p) | |||
|---|---|---|---|---|---|---|
| Pre-Exercise | ||||||
| Ala | n.s. | n.s. | 0.290 | 0.013 | n.s. | n.s. |
| Gly | n.s. | n.s. | 0.391 | 0.001 | n.s. | n.s. |
| GAA | −0.247 | 0.041 | n.s. | n.s. | −0.251 | 0.038 |
| Lys | n.s. | n.s. | n.s. | n.s. | −0.361 | 0.002 |
| hArg | n.s. | n.s. | n.s. | n.s. | −0.469 | <0.001 |
| Trp | n.s. | n.s. | n.s. | n.s. | 0.237 | 0.048 |
| GABR | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Post-exercise | ||||||
| Gly | n.s. | n.s. | 0.495 | <0.001 | n.s. | n.s. |
| Met | n.s. | n.s. | 0.242 | 0.040 | n.s. | n.s. |
| Orn+Cit | n.s. | n.s. | 0.264 | 0.025 | n.s. | n.s. |
| Ser | n.s. | n.s. | n.s. | n.s. | −0.274 | 0.030 |
| Leu+Ile | n.s. | n.s. | n.s. | n.s. | −0.286 | 0.015 |
| Asp+Asn | n.s. | n.s. | n.s. | n.s. | −0.308 | 0.008 |
| Lys | n.s. | n.s. | n.s. | n.s. | −0.442 | <0.001 |
| Trp | −0.246 | 0.045 | n.s. | n.s. | −0.299 | 0.014 |
| GABR | 0.275 | 0.029 | n.s. | n.s. | n.s. | n.s. |
| Pre-Exercise | Post-Exercise | |||
|---|---|---|---|---|
| Amino Acids | r | p | r | p |
| Arg | 0.317 | 0.007 | 0.282 | 0.025 |
| Thr | 0.256 | 0.030 | 0.190 | 0.109 |
| Val | 0.560 | <0.001 | 0.571 | <0.001 |
| Leu+Ile | 0.459 | <0.001 | 0.421 | <0.001 |
| Met | 0.157 | 0.188 | 0.145 | 0.224 |
| Phe | 0.351 | 0.003 | 0.321 | 0.006 |
| Tyr | 0.341 | 0.003 | 0.380 | 0.001 |
| Lys | 0.335 | 0.004 | 0.342 | 0.003 |
| Trp | 0.129 | 0.286 | 0.324 | 0.007 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nebl, J.; Drabert, K.; Haufe, S.; Wasserfurth, P.; Eigendorf, J.; Tegtbur, U.; Hahn, A.; Tsikas, D. Exercise-Induced Oxidative Stress, Nitric Oxide and Plasma Amino Acid Profile in Recreational Runners with Vegetarian and Non-Vegetarian Dietary Patterns. Nutrients 2019, 11, 1875. https://doi.org/10.3390/nu11081875
Nebl J, Drabert K, Haufe S, Wasserfurth P, Eigendorf J, Tegtbur U, Hahn A, Tsikas D. Exercise-Induced Oxidative Stress, Nitric Oxide and Plasma Amino Acid Profile in Recreational Runners with Vegetarian and Non-Vegetarian Dietary Patterns. Nutrients. 2019; 11(8):1875. https://doi.org/10.3390/nu11081875
Chicago/Turabian StyleNebl, Josefine, Kathrin Drabert, Sven Haufe, Paulina Wasserfurth, Julian Eigendorf, Uwe Tegtbur, Andreas Hahn, and Dimitrios Tsikas. 2019. "Exercise-Induced Oxidative Stress, Nitric Oxide and Plasma Amino Acid Profile in Recreational Runners with Vegetarian and Non-Vegetarian Dietary Patterns" Nutrients 11, no. 8: 1875. https://doi.org/10.3390/nu11081875
APA StyleNebl, J., Drabert, K., Haufe, S., Wasserfurth, P., Eigendorf, J., Tegtbur, U., Hahn, A., & Tsikas, D. (2019). Exercise-Induced Oxidative Stress, Nitric Oxide and Plasma Amino Acid Profile in Recreational Runners with Vegetarian and Non-Vegetarian Dietary Patterns. Nutrients, 11(8), 1875. https://doi.org/10.3390/nu11081875