Association of Lactase Persistence Genotypes (rs4988235) and Ethnicity with Dairy Intake in a Healthy U.S. Population
Abstract
:1. Introduction
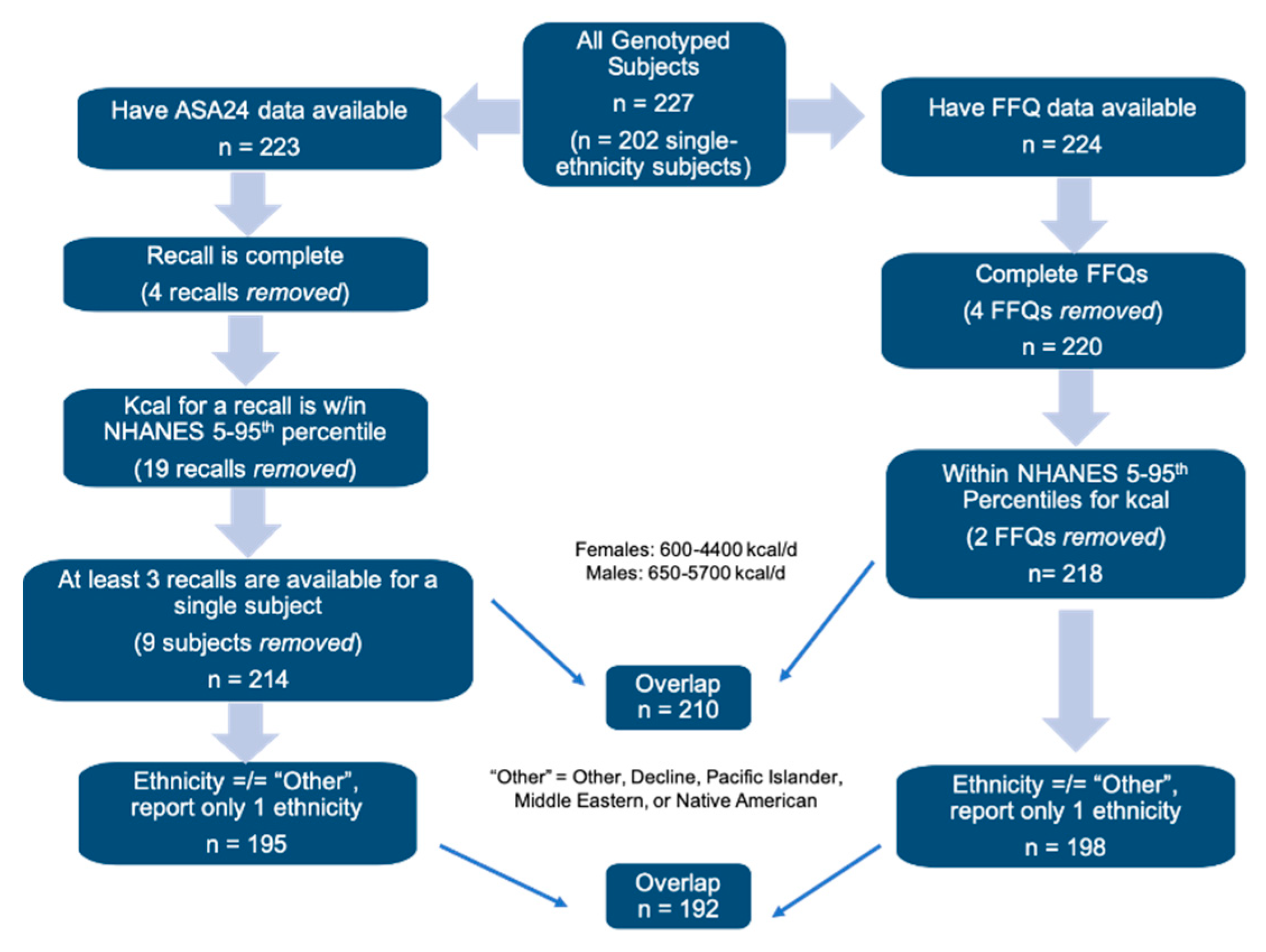
2. Participants and Methods
2.1. Study Population
2.2. Genomic DNA Purification and Genotyping
2.3. Dietary Intake Assessments
2.4. Statistical Analysis
3. Results
3.1. Cohort Characteristics
3.2. Comparison of Habitual (FFQ) and Recent (ASA24) Dairy Intake
3.3. Association of Age, Sex, and BMI with Dairy Intake
3.3.1. Habitual Intake
3.3.2. Recent Intake
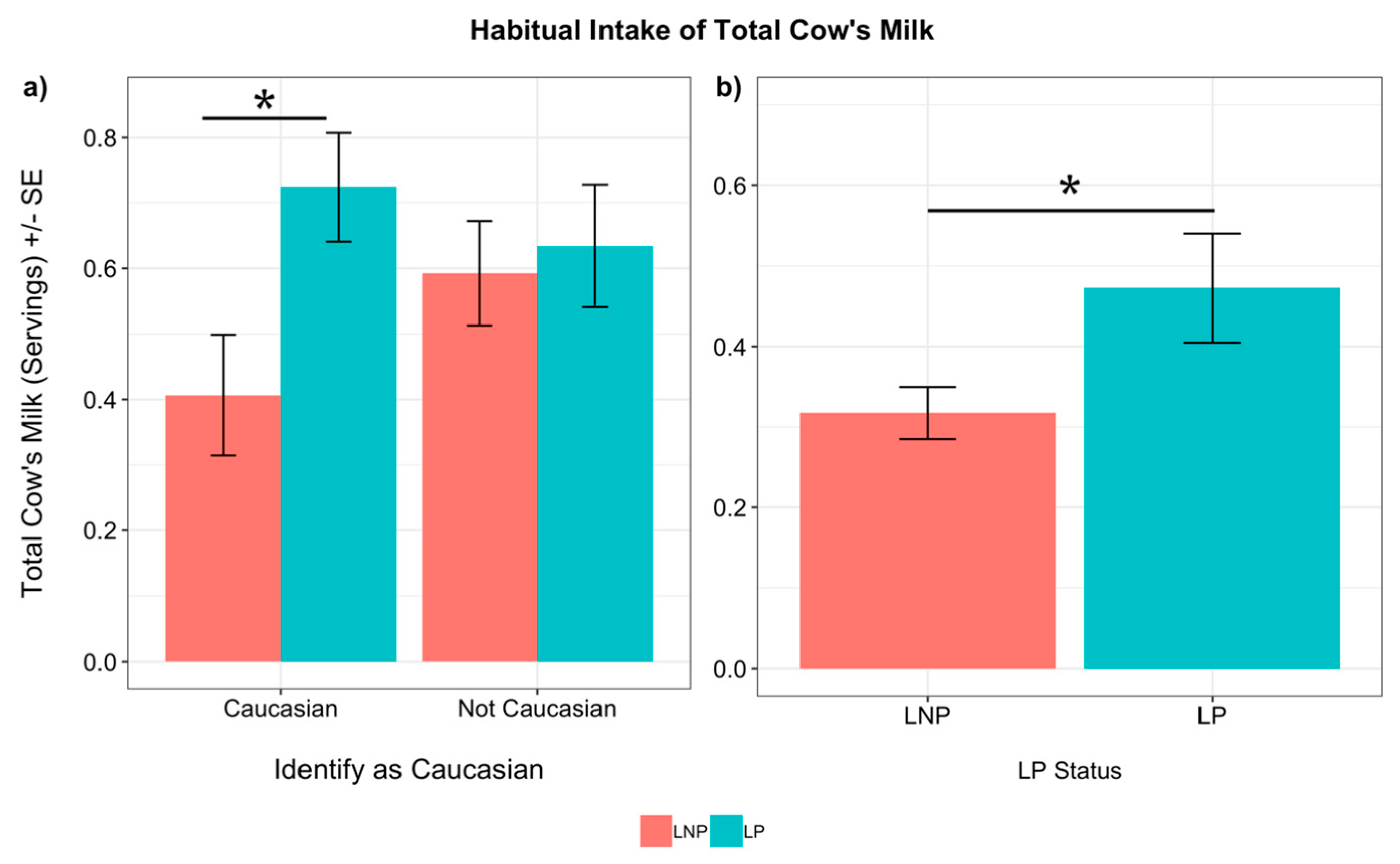
3.4. Genotypes and Reported Consumers of Products in Caucasians
3.5. Genotype and Dairy Intake in Caucasians
3.5.1. Habitual Intake
3.5.2. Recent Intake
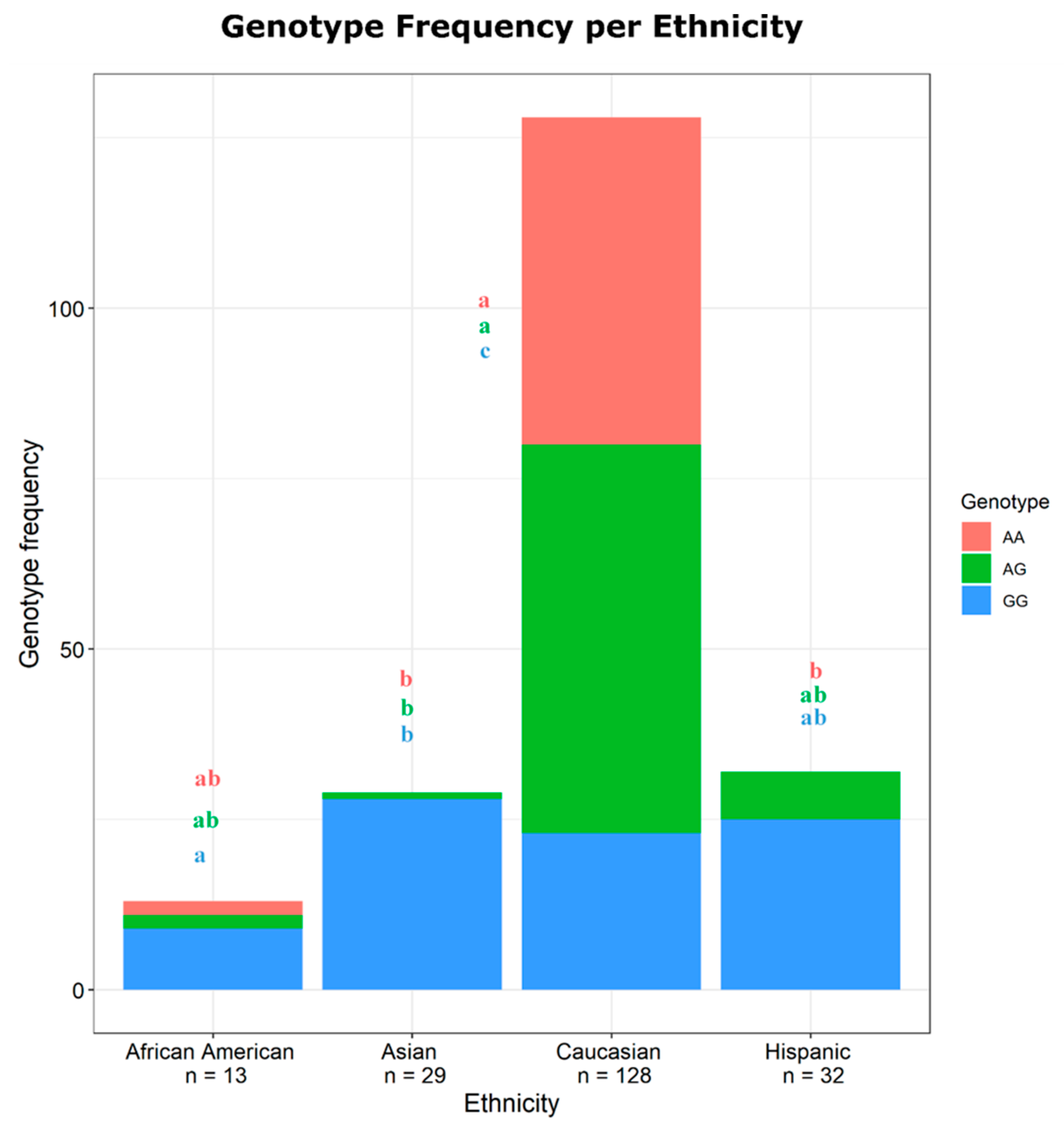
3.6. Genotypes, Ethnicity, and Proportion of Consumers
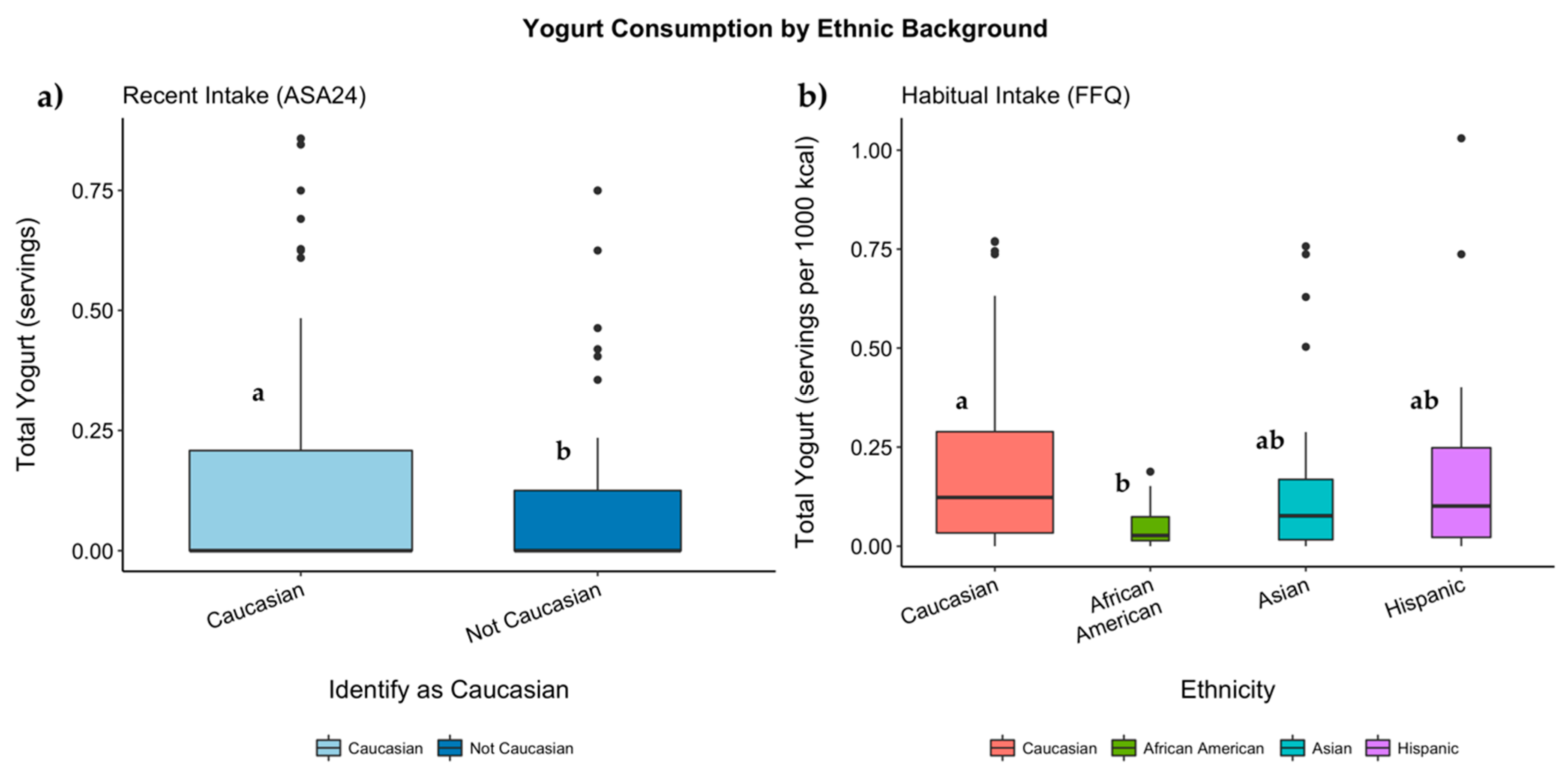
3.7. Genotypes, Ethnicity, and Servings of Dairy Consumed
3.7.1. Habitual Intake
3.7.2. Recent Intake
4. Discussion
4.1. Genotype and Ethnicity in Relation to Consumption of Dairy and Alternative Milk Products
4.2. Limitations
4.3. Health Implications
4.4. Implications for rs4988235 as a Proxy for Dairy Consumption
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ségurel, L.; Bon, C. On the Evolution of Lactase Persistence in Humans. Annu. Rev. Genom. Hum. Genet. Annu. Rev. Genom. Hum. Genet 2017, 8, 297–319. [Google Scholar] [CrossRef] [PubMed]
- Ingram, C.J.E.; Mulcare, C.A.; Itan, Y.; Thomas, M.G.; Swallow, D.M. Lactose digestion and the evolutionary genetics of lactase persistence. Hum. Genet. 2009, 124, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Ingram, C.J.E.; Elamin, M.F.; Mulcare, C.A.; Weale, M.E.; Tarekegn, A.; Raga, T.O.; Bekele, E.; Elamin, F.M.; Thomas, M.G.; Bradman, N.; et al. A novel polymorphism associated with lactose tolerance in Africa: Multiple causes for lactase persistence? Hum. Genet. 2007, 120, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Ingram, C.J.E.; Raga, T.O.; Tarekegn, A.; Browning, S.L.; Elamin, M.F.; Bekele, E.; Thomas, M.G.; Weale, M.E.; Bradman, N.; Swallow, D.M. Multiple Rare Variants as a Cause of a Common Phenotype: Several Different Lactase Persistence Associated Alleles in a Single Ethnic Group. J. Mol. Evolut. 2009, 69, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Enattah, N.S.; Sahi, T.; Savilahti, E.; Terwilliger, J.D.; Peltonen, L.; Järvelä, I. Identification of a variant associated with adult-type hypolactasia. Nat. Genet. 2002, 30, 233. [Google Scholar] [CrossRef]
- Szilagyi, A.; Ishayek, N.; Szilagyi, A.; Ishayek, N. Lactose Intolerance, Dairy Avoidance, and Treatment Options. Nutrients 2018, 10, 1994. [Google Scholar] [CrossRef]
- Alharbi, O.; El-Sohemy, A. Lactose Intolerance (LCT-13910C> T) Genotype Is Associated with Plasma 25-Hydroxyvitamin D Concentrations in Caucasians: A Mendelian Randomization Study. J. Nutr. 2017, 147, 1063–1069. [Google Scholar] [CrossRef]
- Travis, R.C.; Appleby, P.N.; Siddiq, A.; Allen, N.E.; Kaaks, R.; Canzian, F.; Feller, S.; Tjønneland, A.; Føns Johnsen, N.; Overvad, K.; et al. Genetic variation in the lactase gene, dairy product intake and risk for prostate cancer in the European prospective investigation into cancer and nutrition. Int. J. Cancer 2013, 132, 1901–1910. [Google Scholar] [CrossRef]
- Bergholdt, H.K.; Nordestgaard, B.G.; Ellervik, C. Milk intake is not associated with low risk of diabetes or overweight-obesity: A Mendelian randomization study in 97,811 Danish individuals. Am. J. Clin. Nutr. 2015, 102, 487–496. [Google Scholar] [CrossRef]
- Corgneau, M.; Scher, J.; Ritie-Pertusa, L.; Le, D.T.L.; Petit, J.; Nikolova, Y.; Banon, S.; Gaiani, C. Recent advances on lactose intolerance: Tolerance thresholds and currently available answers. Crit. Rev. Food. Sci. Nutr. 2017, 57, 3344–3356. [Google Scholar] [CrossRef]
- USDA ARS USDA Food Composition Databases. Available online: https://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=213&nutrient2=&fg=1&subset=0&sort=c&measureby=g (accessed on 27 March 2019).
- Lamri, A.; Poli, A.; Emery, N.; Bellili, N.; Velho, G.; Lantieri, O.; Balkau, B.; Marre, M.; Fumeron, F. The lactase persistence genotype is associated with body mass index and dairy consumption in the D.E.S.I.R. study. Metabolism 2013, 62, 1323–1329. [Google Scholar] [CrossRef]
- Sacerdote, C.; Guarrera, S.; Smith, G.D.; Grioni, S.; Krogh, V.; Masala, G.; Mattiello, A.; Palli, D.; Panico, S.; Tumino, R.; et al. Lactase Persistence and Bitter Taste Response: Instrumental Variables and Mendelian Randomization in Epidemiologic Studies of Dietary Factors and Cancer Risk. Am. J. Epidemiol. 2007, 166, 576–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corella, D.; Arregui, M.; Coltell, O.; Portolés, O.; Guillem-Sáiz, P.; Carrasco, P.; Sorlí, J.V.; Ortega-Azorín, C.; González, J.I.; Ordovás, J.M. Association of the LCT-13910CT polymorphism with obesity and its modulation by dairy products in a mediterranean population. Obesity 2011, 19, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Coltell, O.; Sorlí, J.V.; Estruch, R.; Martínez-González, M.Á.; Salas-Salvadó, J.; Fitó, M.; Arós, F.; Dashti, H.S.; Lai, C.Q.; et al. Associations of the MCM6-rs3754686 proxy for milk intake in Mediterranean and American populations with cardiovascular biomarkers, disease and mortality: Mendelian randomization. Sci. Rep. 2016, 6, 33188. [Google Scholar] [CrossRef] [Green Version]
- O’Neil, C.E.; Nicklas, T.A.; Keast, D.R.; Fulgoni, V.L. Ethnic disparities among food sources of energy and nutrients of public health concern and nutrients to limit in adults in the United States: NHANES 2003–2006. Food Nutr. Res. 2014, 58, 15784. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, R.S.; Goldman, J.D.; Enns, C.W.; Lacomb, R.P. Fluid Milk Consumption in the United States What We Eat In America, NHANES 2005–2006. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/DBrief/3_milk_consumption_0506.pdf (accessed on 18 July 2019).
- Kirkpatrick, S.I.; Dodd, K.W.; Reedy, J.; Krebs-Smith, S.M. Income and Race/Ethnicity Are Associated with Adherence to Food-Based Dietary Guidance among US Adults and Children. J. Acad. Nutr. Diet. 2012, 112, 624–635. [Google Scholar] [CrossRef]
- National Medical Association Lactose Intolerance and African Americans: Implications for the Consumption of Appropriate Intake Levels of Key Nutrients. J. Natl. Med. Assoc. 2009, 101, S5–S23. [CrossRef]
- Dehghan, M.; Mente, A.; Rangarajan, S.; Sheridan, P.; Mohan, V.; Iqbal, R.; Gupta, R.; Lear, S.; Wentzel-Viljoen, E.; Avezum, A.; et al. Articles Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): A prospective cohort study. Lancet 2018, 392, 2288–2297. [Google Scholar] [CrossRef]
- Gijsbers, L.; Ding, E.L.; Malik, V.S.; De Goede, J.; Geleijnse, J.M.; Soedamah-Muthu, S.S. Consumption of dairy foods and diabetes incidence: A dose-response meta-analysis of observational studies. Am. J. Clin. Nutr. 2016, 103, 1111–1135. [Google Scholar] [CrossRef]
- Mazidi, M.; Mikhailidis, D.P.; Sattar, N.; Howard, G.; Graham, I.; Banach, M. Consumption of dairy product and its association with total and cause specific mortality—A population-based cohort study and meta-analysis. Clin. Nutr. 2018. [Google Scholar] [CrossRef]
- Sahni, S.; Tucker, K.L.; Kiel, D.P.; Quach, L.; Casey, V.A.; Hannan, M.T. Milk and yogurt consumption are linked with higher bone mineral density but not with hip fracture: The Framingham Offspring Study. Arch. Osteoporos. 2013, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Huang, T.; Bergholdt, H.K.; Nordestgaard, B.G.; Ellervik, C.; Qi, L.; CHARGE Consortium. Dairy consumption, systolic blood pressure, and risk of hypertension: Mendelian randomization study. BMJ 2017, 356, j1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canadian Dairy Information Centre Global Consumption of Dairy Products: Total Fluid Milk Consumption for Selected Countries. Available online: https://www.dairyinfo.gc.ca/index_e.php?s1=dff-fcil&s2=cons&s3=conscdn&menupos=01.01.11.10 (accessed on 29 March 2019).
- Baldiviez, L.M.; Keim, N.L.; Laugero, K.D.; Hwang, D.H.; Huang, L.; Woodhouse, L.R.; Burnett, D.J.; Zerofsky, M.S.; Bonnel, E.L.; Allen, L.H.; et al. Design and implementation of a cross-sectional nutritional phenotyping study in healthy US adults. BMC Nutr. 2017, 3, 79. [Google Scholar] [CrossRef]
- NutritionQuest. Available online: http://nutritionquest.com/ (accessed on 26 January 2019).
- National Cancer Institute ASA24 Reviewing and Cleaning ASA24® Data. Available online: http://epi.grants.cancer.gov/asa24/resources/issues.html (accessed on 22 March 2019).
- Bowman, S.A.; Clemens, J.C.; Friday, J.E.; Thoerig, R.C.; Shimizu, M.; Barrows, B.R.; Moshfegh, A.J. Food Patterns Equivalents Database 2007–2008: Methodology and User Guide. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/fped/FPED_0708.pdf (accessed on 22 March 2019).
- National Cancer Institute Automated Self-Administered 24-Hour (ASA24®) Dietary Assessment Tool. Available online: https://epi.grants.cancer.gov/asa24/ (accessed on 21 March 2019).
- National Cancer Institute ASA24. ASA24® Sample Analysis Files & Data Dictionaries. Available online: https://epi.grants.cancer.gov/asa24/researcher/sample.html (accessed on 22 March 2019).
- Park, Y.; Dodd, K.W.; Kipnis, V.; Thompson, F.E.; Potischman, N.; Schoeller, D.A.; Baer, D.J.; Midthune, D.; Troiano, R.P.; Bowles, H.; et al. Comparison of self-reported dietary intakes from the Automated Self-Administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. Am. J. Clin. Nutr. 2018, 107, 80–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [Green Version]
- Dietary Guidelines for Americans, Appendix 7. Nutritional Goals for Age-Sex Groups Based on Dietary Reference Intakes and Dietary Guidelines Recommendations. Available online: https://health.gov/dietaryguidelines/2015/guidelines/appendix-7/ (accessed on 21 March 2019).
- The 1000 Genomes Project Consortium 1000 Genomes: rs4988235 SNP. Available online: http://phase3browser.1000genomes.org/Homo_sapiens/Variation/Population?db=core;r=2:136608146-136609146;v=rs4988235;vdb=variation;vf=3129823#_ (accessed on 11 April 2019).
- Yang, Q.; Lin, S.L.; Au Yeung, S.L.; Kwok, M.K.; Xu, L.; Leung, G.M.; Schooling, C.M. Genetically predicted milk consumption and bone health, ischemic heart disease and type 2 diabetes: A Mendelian randomization study. Eur. J. Clin. Nutr. 2017, 71, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Enattah, N.S.; Sulkava, R.; Halonen, P.; Kontula, K.; Järvelä, I. Genetic Variant of Lactase-Persistent C/T-13910 Is Associated with Bone Fractures in Very Old Age. J. Am. Geriatr. Soc. 2005, 53, 79–82. [Google Scholar] [CrossRef]
- OECD-FAO Agricultural Outlook-2015 Table, A.20.2-Cheese Projections: Consumption, per Capita. Available online: https://www.oecd-ilibrary.org/agriculture-and-food/oecd-fao-agricultural-outlook-2015_agr_outlook-2015-en (accessed on 25 July 2019).
- Dairy Management Inc DMI Quarterly Retail Snapshot: Milk Q3 2017. Available online: http://www.mmibusinessadvisors.com/wp-content/uploads/2017/12/DMI-Quarterly-Milk-Snapshot-Q3-2017.pdf (accessed on 14 March 2019).
- Stewart, H.; Cessna, J. On Different Trajectories: A Look at Sales of Cow’s Milk and Plant-Based Milk Analogs. Available online: https://www.ers.usda.gov/webdocs/publications/85163/ldp-m-279.pdf?v=42996 (accessed on 14 March 2019).
- McCarthy, K.S.; Parker, M.; Ameerally, A.; Drake, S.L.; Drake, M.A. Drivers of choice for fluid milk versus plant-based alternatives: What are consumer perceptions of fluid milk? J. Dairy Sci. 2017, 100, 6125–6138. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Gary, T.L.; Caballero, B.H.; Lawrence, R.S.; Cheskin, L.J.; Wang, Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 1914–1925. [Google Scholar] [CrossRef] [Green Version]
- Keith, J.N.; Nicholls, J.; Reed, A.; Kafer, K.; Gregory, D.M. The Prevalence of Self-reported Lactose Intolerance and the Consumption of Dairy Foods Among African American Adults Are Less Than Expected. J. Natl. Med. Assoc. 2011, 103, 36–45. [Google Scholar] [CrossRef]
- Nicklas, T.A.; Qu, H.; Hughes, S.O.; He, M.; Wagner, S.E.; Foushee, H.R.; Shewchuk, R.M. Self-perceived lactose intolerance results in lower intakes of calcium and dairy foods and is associated with hypertension and diabetes in adults. Am. J. Clin. Nutr. 2011, 94, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, T.; Venema, K.; Priebe, M.G.; Welling, G.W.; Brummer, R.-J.M.; Vonk, R.J. The role of colonic metabolism in lactose intolerance. Eur. J. Clin. Investig. 2008, 38, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.E.; Kirkpatrick, S.I.; Subar, A.F.; Reedy, J.; Schap, T.E.; Wilson, M.M.; Krebs-Smith, S.M. The National Cancer Institute’s Dietary Assessment Primer: A Resource for Diet Research. J. Acad. Nutr. Diet. 2015, 115, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Nilsson, L.M.; Esberg, A.; Jansson, J.-H.; Winkvist, A. Dairy intake revisited–associations between dairy intake and lifestyle related cardio-metabolic risk factors in a high milk consuming population. Nutr. J. 2018, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Tishkoff, S.A.; Reed, F.A.; Ranciaro, A.; Voight, B.F.; Babbitt, C.C.; Silverman, J.S.; Powell, K.; Mortensen, H.M.; Hirbo, J.B.; Osman, M.; et al. Convergent adaptation of human lactase persistence in Africa and Europe. Nat. Genet. 2007, 39, 31–40. [Google Scholar] [CrossRef]
- The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojeda-Granados, C.; Panduro, A.; Rebello Pinho, J.R.; Ramos-Lopez, O.; Gleyzer, K.; Malta, F.D.M.; Gonzalez-Aldaco, K.; Roman, S. Association of Lactase Persistence Genotypes with High Intake of Dairy Saturated Fat and High Prevalence of Lactase Non-Persistence among the Mexican Population. J. Nutrigenet. Nutrigenom. 2016, 9, 83–94. [Google Scholar] [CrossRef]
- Charati, H.; Peng, M.-S.; Chen, W.; Yang, X.-Y.; Jabbari Ori, R.; Aghajanpour-Mir, M.; Esmailizadeh, A.; Zhang, Y.-P. The evolutionary genetics of lactase persistence in seven ethnic groups across the Iranian plateau. Hum. Genom. 2019, 13, 7. [Google Scholar] [CrossRef]
- Dietary Guidelines for Americans 2015–2020 Dietary Guidelines for Americans. Available online: https://www.dietaryguidelines.gov/current-dietary-guidelines/2015-2020-dietary-guidelines (accessed on 22 March 2019).
- USDA Food and Nutrition Service HEI-2015 Components and Scoring Standards. Available online: https://fns-prod.azureedge.net/sites/default/files/healthy_eating_index/HEI-2015 Components and Scoring Standards_2.pdf (accessed on 24 July 2019).
- Chen, G.-C.; Szeto, I.M.Y.; Chen, L.-H.; Han, S.-F.; Li, Y.-J.; van Hekezen, R.; Qin, L.-Q. Dairy products consumption and metabolic syndrome in adults: Systematic review and meta-analysis of observational studies. Sci. Rep. 2015, 5, 14606. [Google Scholar] [CrossRef]
- Kurian, A.K.; Cardarelli, K.M. Racial and Ethnic Differences in Cardiovascular Disease Risk Factors: A Systematic Review. Ethn. Dis. 2007, 17, 143–152. [Google Scholar]
- American Diabetes Association Are You at Risk? Age, Race, Gender & Family History. Available online: http://www.diabetes.org/are-you-at-risk/lower-your-risk/nonmodifiables.html (accessed on 29 March 2019).
- Centers for Disease Control and Prevention Adult Obesity Facts. Available online: http://www.healthaffairs.org/doi/10.1377/hlthaff.28.5.w822 (accessed on 22 July 2019).
- Heaney, R.P. Calcium, Dairy Products and Osteoporosis. J. Am. Coll. Nutr. 2000, 19, 83S–99S. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.; Siris, E.S.; Wehren, L.E.; Miller, P.D.; Abbott, T.A.; Berger, M.L.; Santora, A.C.; Sherwood, L.M. Osteoporosis and Fracture Risk in Women of Different Ethnic Groups. J. Bone Miner. Res. 2004, 20, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Story, M.; Harris, L.J. Food Preferences, Beliefs, and Practices of Southeast Asian Refugee Adolescents. J. Sch. Health 1988, 58, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Fisberg, M.; Machado, R. History of yogurt and current patterns of consumption. Nutr. Rev. 2015, 73, 4–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| All Subjects (n = 227) | FFQ Subjects (n = 218) | ASA24 Subjects (n = 214) | rs4988235 Genotype (All Genotyped Subjects, n = 227) | |||||
|---|---|---|---|---|---|---|---|---|
| AA (n = 53) | AG (n = 75) | GG (n = 99) | p1 | |||||
| Metadata mean (SD) or n (%) | Age (years) | 39.17 (13.96) | 39.09 (13.73) | 39.12 (13.82) | 41.77 (13.67) a,b | 41.07 (12.91) a | 36.33 (14.49) b | 0.010 |
| BMI kg/m2 | 26.36 (4.65) | 26.86 (4.63) | 26.74 (4.60) | 26.66 (5.42) | 26.12 (4.10) | 26.37 (4.63) | 0.930 | |
| Height (cm) | 168.86 (9.91) | 169.21 (9.77) | 168.84 (9.73) | 171.72 (9.24) a | 170.85 (9.87) a | 165.82 (9.52) b | 0.000 | |
| Weight (kg) | 77.04 (17.12) | 77.33 (17.26) | 76.62 (17.00) | 80.82 (21.14) | 77.95 (14.67) | 74.32 (16.14) | 0.113 | |
| Males | 94 (41.41%) | 92 (42.20%) | 87 (40.65%) | 23 (43.40%) | 32 (42.67%) | 39 (39.39%) | 0.860 | |
| Ethnicity n (%) | African American | 13 (5.73%) | 13 (5.96%) | 11 (5.14%) | 2 (3.77%) | 2 (2.67%) | 9 (9.09%) | 0.010 |
| Asian | 29 (12.78%) | 27 (12.39%) | 27 (12.62%) | 0 (0.00%) | 1 (1.33%) | 28 (28.28%) | - | |
| Caucasian | 128 (56.39%) | 126 (57.80%) | 125 (58.41%) | 48 (90.57%) | 57 (76.00%) | 23 (23.23%) | - | |
| Hispanic | 32 (14.10%) | 32 (14.68%) | 32 (14.95%) | 0 (0.00%) | 7 (9.33%) | 25 (25.25%) | - | |
| Other | 13 (5.73%) | 11 (5.05%) | 11 (5.14%) | 0 (0.00%) | 4 (5.33%) | 9 (9.09%) | - | |
| Multi | 12 (5.29%) | 9 (4.13%) | 8 (3.74%) | 3 (5.66%) | 4 (5.33%) | 5 (5.05%) | - | |
| Genotype n (%) | AA | 53 (23.35%) | 52 (23.85%) | 51 (23.83%) | - | - | - | - |
| AG | 75 (33.04%) | 71 (32.57%) | 72 (33.64%) | - | - | - | - | |
| GG | 99 (43.61%) | 95 (43.58%) | 91 (42.52%) | - | - | - | - | |
| Genotype | Ethnicity 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | African-American | Asian | Caucasian | Hispanic | Multi | Other | |
| Habitual Intake (FFQ, n = 218) | |||||||||
| Total Dairy (n = 218) | 52 (100%) | 71 (100%) | 95 (100%) | 13 (100%) | 27 (100%) | 126 (100%) | 32 (100%) | 9 (100%) | 11 (100%) |
| Total Cheese (n = 218) | 52 (100%) | 71 (100%) | 95 (100%) | 13 (100%) | 27 (100%) | 126 (100%) | 32 (100%) | 9 (100%) | 11 (100%) |
| Total Yogurt (n = 157) | 43 (83%) | 49 (69%) | 65 (68%) | 5 (38%) | 19 (70%) | 95 (75%) | 23 (72%) | 7 (78%) | 8 (73%) |
| Total Cow’s Milk (n = 218) | 52 (100%) | 71 (100%) | 95 (100%) | 13 (100%) | 27 (100%) | 126 (100%) | 32 (100%) | 9 (100%) | 11 (100%) |
| Fluid Cow’s Milk (n = 145) | 38 (73%) | 44 (62%) | 63 (66%) | 8 (62%) | 19 (70%) | 80 (63%) | 22 (69%) | 7 (78%) | 9 (82%) |
| Alternative Milk (n = 58) | 13 (25%) | 22 (31%) | 23 (24%) | 5 (38%) | 5 (19%) | 36 (29%) | 8 (25%) | 2 (22%) | 2 (18%) |
| Recent Intake (ASA24, n = 214) | |||||||||
| Total Dairy (n = 214) | 51 (100%) | 72 (100%) | 91 (100%) | 11 (100%) | 27 (100%) | 125 (100%) | 32 (100%) | 8 (100%) | 11 (100%) |
| Total Cheese (n = 202) | 50 (98%) | 70 (97%) | 82 (90%) | 9 (82%) | 23 (85%) | 121 (97%) | 31 (97%) | 8 (100%) | 10 (91%) |
| Total Yogurt (n = 79) | 21 (41%) | 27 (38%) | 31 (34%) | 2 (18%) | 8 (30%) | 51 (41%) | 9 (28%) | 4 (50%) | 5 (45%) |
| Total Cow’s Milk (n = 174) | 44 (86%) | 60 (83%) | 70 (77%) | 9 (82%) | 21 (78%) | 103 (82%) | 26 (81%) | 6 (75%) | 9 (82%) |
| Fluid Cow’s Milk (n = 94) | 24 (47%) | 34 (47%) | 36 (40%) | 4 (36%) | 14 (52%) | 61 (49%) | 10 (31%) | 2 (25%) | 3 (27%) |
| Alternative Milk (n = 48) | 11 (22%) | 18 (25%) | 19 (21%) | 2 (18%) | 3 (11%) | 29 (23%) | 9 (28%) | 2 (25%) | 3 (27%) |
| Habitual Intake (FFQ) | Recent Intake (ASA24) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | p1 | p2 | AA | AG | GG | p1 | p2 | |
| Servings·day | ||||||||||
| Total Dairy | 1.86 (1.06) | 1.68 (1.07) | 1.62 (1.01) | 0.279 | 0.196 | 1.57 (1) a,b | 1.64 (1) a | 1.25 (0.76) b | 0.019 | 0.005 |
| Total Cheese | 0.87 (0.50) | 0.80 (0.47) | 0.74 (0.54) | 0.105 | 0.043 | 0.81 (0.60) a,b | 0.91 (0.69) a | 0.66 (0.56) b | 0.030 | 0.012 |
| Total Yogurt | 0.19 (0.21) | 0.17 (0.18) | 0.20 (0.30) | 0.661 | 0.715 | 0.14 (0.21) | 0.10 (0.16) | 0.11 (0.20) | 0.317 | 0.262 |
| Total Milk | 0.74 (0.84) | 0.67 (0.75) | 0.62 (0.69) | 0.632 | 0.402 | 0.58 (0.76) | 0.58 (0.54) | 0.43 (0.49) | 0.117 | 0.056 |
| Fluid Milk | 0.37 (0.72) | 0.35 (0.66) | 0.30 (0.52) | 0.841 | 0.809 | 0.33 (0.73) | 0.27 (0.42) | 0.22 (0.45) | 0.479 | 0.236 |
| Alt. Milk | 0.08 (0.20) | 0.13 (0.45) | 0.09 (0.27) | 0.621 | 0.516 | 0.07 (0.19) | 0.23 (0.64) | 0.11 (0.29) | 0.656 | 0.636 |
| Servings/1000-kcal·day | ||||||||||
| Total Dairy | 0.93 (0.48) | 0.85 (0.38) | 0.81 (0.37) | 0.297 | 0.250 | 0.73 (0.42) | 0.74 (0.37) | 0.62 (0.35) | 0.075 | 0.023 |
| Total Cheese | 0.44 (0.23) | 0.41 (0.20) | 0.37 (0.22) | 0.114 | 0.051 | 0.38 (0.26) | 0.41 (0.29) | 0.32 (0.25) | 0.147 | 0.059 |
| Total Yogurt | 0.10 (0.10) | 0.09 (0.11) | 0.11 (0.20) | 0.637 | 0.873 | 0.07 (0.10) | 0.04 (0.07) | 0.05 (0.10) | 0.351 | 0.314 |
| Total Milk | 0.37 (0.42) | 0.33 (0.31) | 0.30 (0.30) | 0.715 | 0.496 | 0.27 (0.32) | 0.26 (0.24) | 0.22 (0.25) | 0.231 | 0.107 |
| Fluid Milk | 0.19 (0.40) | 0.16 (0.27) | 0.14 (0.22) | 0.877 | 0.816 | 0.15 (0.32) | 0.12 (0.18) | 0.12 (0.24) | 0.527 | 0.273 |
| Alt. Milk | 0.04 (0.11) | 0.06 (0.19) | 0.04 (0.13) | 0.696 | 0.593 | 0.03 (0.08) | 0.09 (0.25) | 0.06 (0.17) | 0.680 | 0.668 |
| Habitual Intake (FFQ) | Recent Intake (ASA24) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| African-American | Asian | Caucasian | Hispanic | p1 | p2 | African-American | Asian | Caucasian | Hispanic | p1 | p2 | |
| Servings·day | ||||||||||||
| Total Dairy | 1.41 (0.41) | 1.43 (1.08) | 1.72 (1.06) | 1.68 (0.87) | 0.242 | 0.226 | 1.22 (0.91) a,b | 1.01 (0.62) a | 1.6 (0.98) b | 1.35 (0.76) a,b | 0.014 | 0.005 |
| Total Cheese | 0.91 (0.42) a | 0.53 (0.5) b | 0.82 (0.47) a | 0.79 (0.49) a | 0.002 | 0.036 | 0.73 (0.70) a,b | 0.39 (0.41) a | 0.85 (0.61) b | 0.76 (0.60) b | 0.000 | 0.002 |
| Total Yogurt | 0.05 (0.06) | 0.17 (0.22) | 0.18 (0.19) | 0.18 (0.22) | 0.052 | 0.123 | 0.04 (0.09) | 0.10 (0.19) | 0.13 (0.20) | 0.09 (0.19) | 0.139 | 0.042 |
| Total Milk | 0.43 (0.21) | 0.63 (0.75) | 0.67 (0.79) | 0.64 (0.53) | 0.785 | 0.805 | 0.43 (0.45) | 0.46 (0.45) | 0.57 (0.64) | 0.44 (0.58) | 0.613 | 0.181 |
| Fluid Milk | 0.12 (0.16) | 0.41 (0.67) | 0.33 (0.68) | 0.32 (0.47) | 0.548 | 0.386 | 0.28 (0.47) | 0.26 (0.39) | 0.30 (0.57) | 0.21 (0.58) | 0.289 | 0.239 |
| Alt. Milk | 0.13 (0.24) | 0.11 (0.37) | 0.10 (0.36) | 0.08 (0.27) | 0.586 | 0.651 | 0.08 (0.18) | 0.07 (0.21) | 0.15 (0.50) | 0.19 (0.40) | 0.375 | 0.739 |
| Servings/1000-kcal·day | ||||||||||||
| Total Dairy | 0.69 (0.28) | 0.73 (0.27) | 0.9 (0.44) | 0.78 (0.24) | 0.153 | 0.040 | 0.58 (0.45) | 0.53 (0.31) | 0.74 (0.38) | 0.66 (0.36) | 0.044 | 0.015 |
| Total Cheese | 0.45 (0.25) a | 0.27 (0.18) b | 0.43 (0.23) a | 0.36 (0.11) a,b | 0.000 | 0.004 | 0.34 (0.31) a,b | 0.21 (0.19) a | 0.40 (0.27) b | 0.34 (0.22) b | 0.006 | 0.009 |
| Total Yogurt | 0.02 (0.03) a | 0.10 (0.13) a,b | 0.10 (0.10) b | 0.09 (0.10) a,b | 0.019 | 0.094 | 0.01 (0.03) | 0.06 (0.11) | 0.06 (0.09) | 0.04 (0.10) | 0.145 | 0.055 |
| Total Milk | 0.21 (0.14) | 0.32 (0.27) | 0.34 (0.37) | 0.3 (0.23) | 0.741 | 0.961 | 0.21 (0.21) | 0.24 (0.24) | 0.26 (0.27) | 0.25 (0.34) | 0.746 | 0.299 |
| Fluid Milk | 0.06 (0.10) | 0.17 (0.24) | 0.16 (0.33) | 0.15 (0.22) | 0.546 | 0.466 | 0.13 (0.21) | 0.13 (0.20) | 0.14 (0.24) | 0.13 (0.31) | 0.315 | 0.311 |
| Alt. Milk | 0.04 (0.07) | 0.05 (0.13) | 0.05 (0.16) | 0.05 (0.18) | 0.644 | 0.678 | 0.03 (0.08) | 0.04 (0.13) | 0.06 (0.19) | 0.11 (0.26) | 0.382 | 0.750 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, E.L.; Huang, L.; Bouzid, Y.Y.; Kirschke, C.P.; Durbin-Johnson, B.; Baldiviez, L.M.; Bonnel, E.L.; Keim, N.L.; Korf, I.; Stephensen, C.B.; et al. Association of Lactase Persistence Genotypes (rs4988235) and Ethnicity with Dairy Intake in a Healthy U.S. Population. Nutrients 2019, 11, 1860. https://doi.org/10.3390/nu11081860
Chin EL, Huang L, Bouzid YY, Kirschke CP, Durbin-Johnson B, Baldiviez LM, Bonnel EL, Keim NL, Korf I, Stephensen CB, et al. Association of Lactase Persistence Genotypes (rs4988235) and Ethnicity with Dairy Intake in a Healthy U.S. Population. Nutrients. 2019; 11(8):1860. https://doi.org/10.3390/nu11081860
Chicago/Turabian StyleChin, Elizabeth L., Liping Huang, Yasmine Y. Bouzid, Catherine P. Kirschke, Blythe Durbin-Johnson, Lacey M. Baldiviez, Ellen L. Bonnel, Nancy L. Keim, Ian Korf, Charles B. Stephensen, and et al. 2019. "Association of Lactase Persistence Genotypes (rs4988235) and Ethnicity with Dairy Intake in a Healthy U.S. Population" Nutrients 11, no. 8: 1860. https://doi.org/10.3390/nu11081860





