Vitamin D Status and Indices of Mineral Homeostasis in the Population: Differences Between 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Population
2.2. Lab Procedures
2.3. Outcome Variables and Statistical Analysis
3. Results
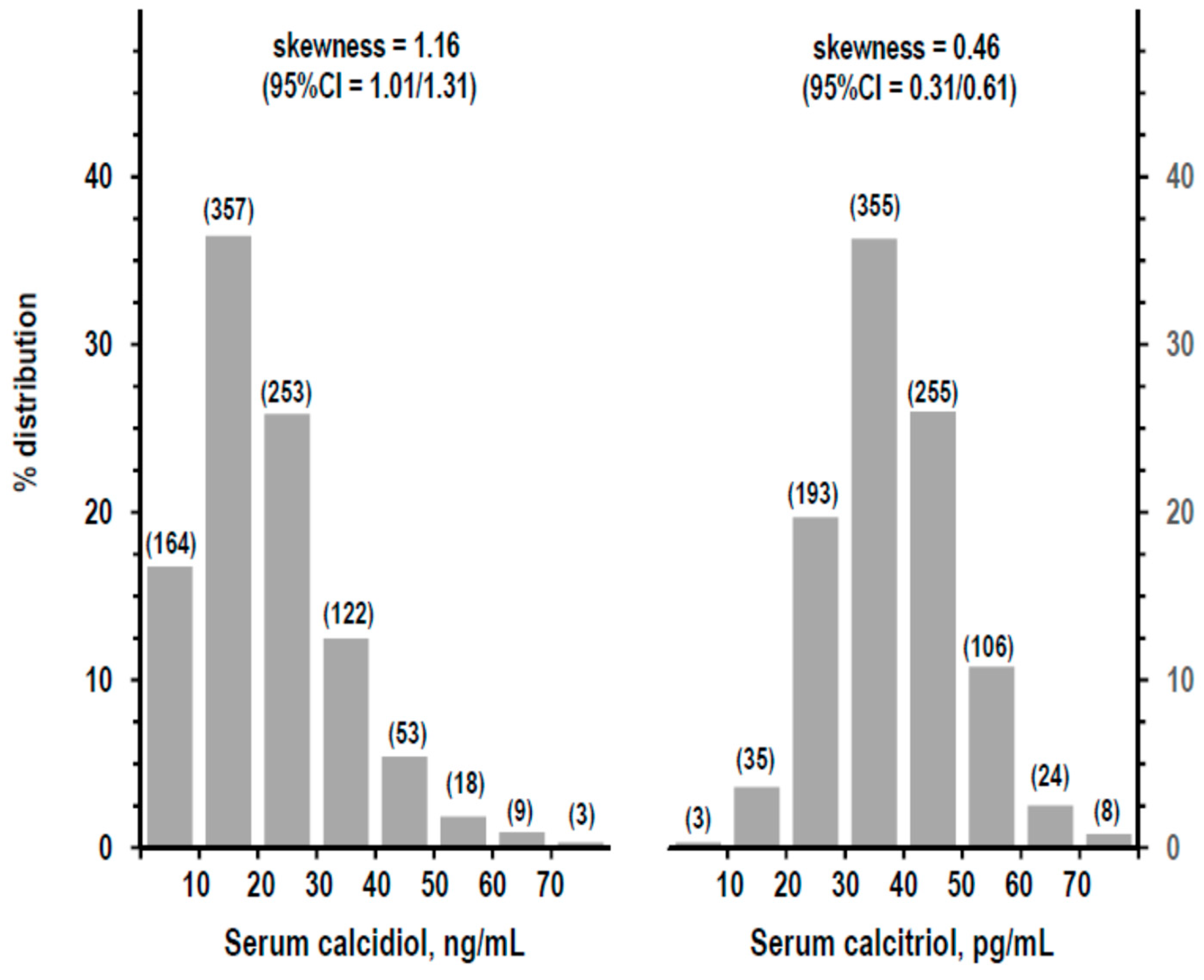
3.1. Descriptive Statistics
3.2. Association of Vitamin D Deficiency with Hypocalcemia and High PTH
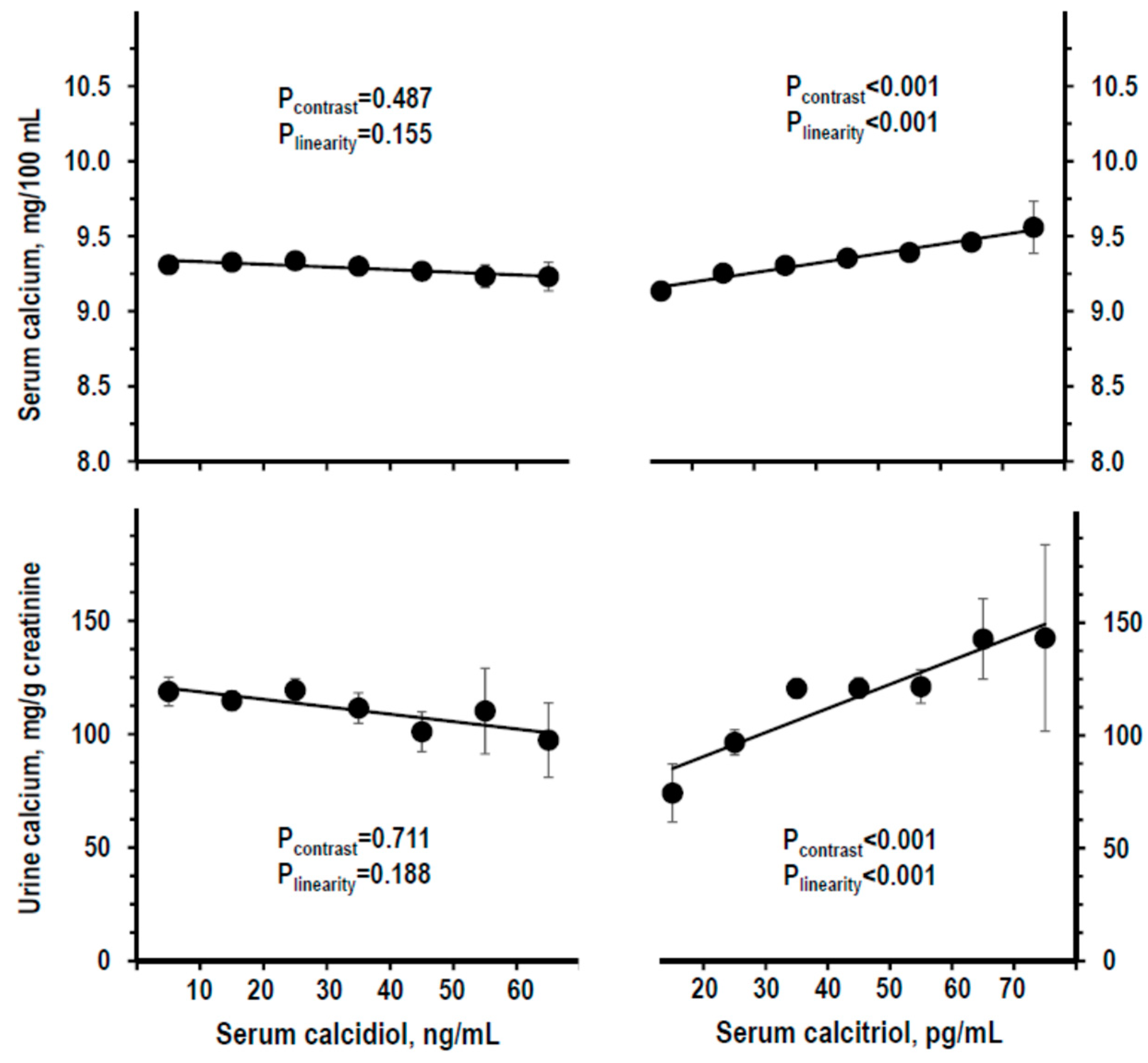
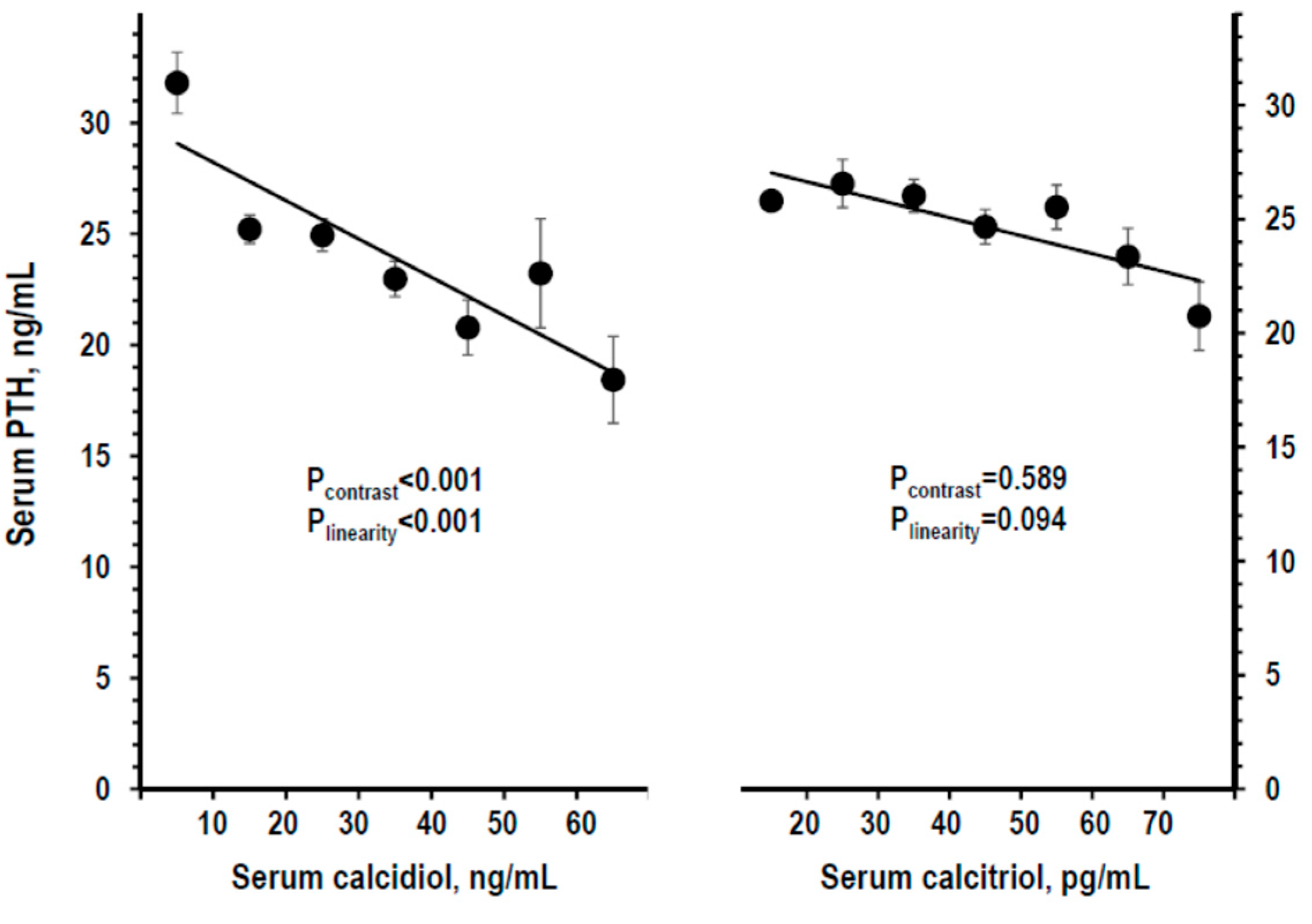
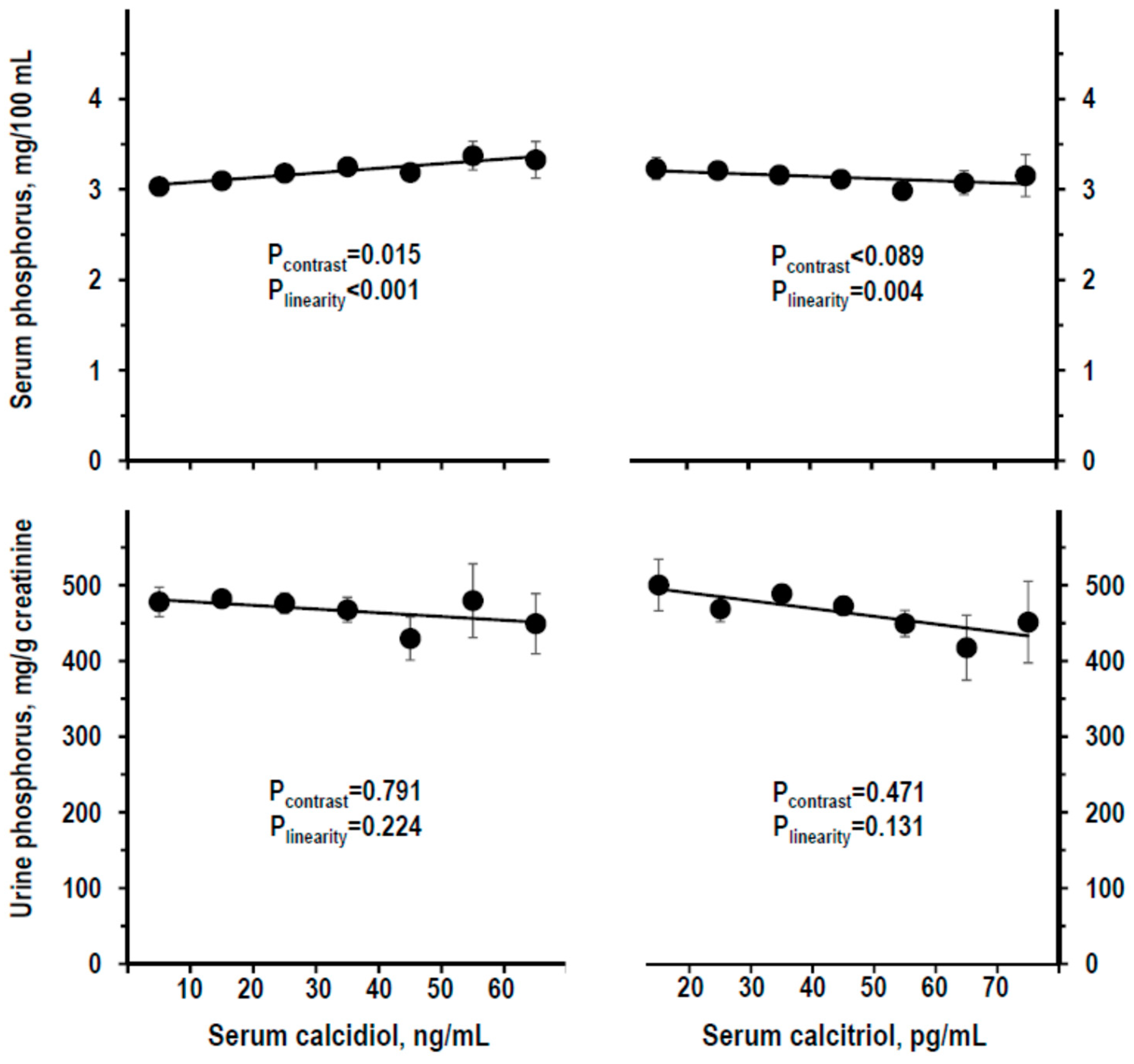
3.3. Indices of Mineral Homeostasis over the Range of Serum Calcidiol and of Serum Calcitriol
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosen, C.J. Vitamin D insufficiency. N. Engl. J. Med. 2011, 364, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Reference Values for Total 25-Hydroxyvitamin D2 and D3. Available online: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/83670 (accessed on 20 June 2019).
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; González-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Damasiewicz, M.J.; Magliano, D.J.; Daly, R.M.; Gagnon, C.; Lu, Z.X.; Sikaris, K.A.; Ebeling, P.R.; Chadban, S.J.; Atkins, R.C.; Kerr, P.G.; et al. Serum 25-Hydroxyvitamin D Deficiency and the 5-Year Incidence of CKD. Am. J. Kidney Dis. 2013, 62, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Brannon, P.M.; Rosen, C.J.; Taylor, C.L. Vitamin D Deficiency—Is There Really a Pandemic? N. Engl. J. Med. 2016, 375, 1817–1819. [Google Scholar] [CrossRef]
- Binkley, N.; Krueger, D.; Cowgill, C.S.; Plum, L.; Lake, E.; Hansen, K.E.; DeLuca, H.F.; Drezner, M.K. Assay Variation Confounds the Diagnosis of Hypovitaminosis D: A Call for Standardization. J. Clin. Endocrinol. Metab. 2004, 89, 3152–3157. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Evans, M.K.; Wenger, J.; Zonderman, A.B.; Berg, A.H.; Nalls, M.; Tamez, H.; Zhang, D.; Bhan, I.; Karumanchi, S.A.; et al. Vitamin D–Binding Protein and Vitamin D Status of Black Americans and White Americans. New Engl. J. Med. 2013, 369, 1991–2000. [Google Scholar] [CrossRef]
- Mason, D.; Donabella, D.J.; Nnani, D.; Musteata, F.M.; Mason, D.; Donabella, P.J.; Nnani, D.; Musteata, F.M. Normalized vitamin D metabolite concentrations are better correlated to pharmacological effects than measured concentrations. Future Sci. OA 2015, 1, 83. [Google Scholar] [CrossRef]
- Hannan, F.M.; Thakker, R.V. Investigating hypocalcaemia. BMJ 2013, 346, f2213. [Google Scholar] [CrossRef]
- Caruso, T.J.; Fuzaylov, G. Images in clinical medicine. Severe vitamin D deficiency--rickets. N. Engl. J. Med. 2013, 369, e11. [Google Scholar] [CrossRef]
- Steingrimsdottir, L.; Gunnarsson, O.; Indridason, O.S.; Franzson, L.; Sigurdsson, G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005, 294, 2336–2341. [Google Scholar] [CrossRef]
- Joo, N.S.; Dawson-Hughes, B.; Kim, Y.S.; Oh, K.; Yeum, K.J. Impact of calcium and vitamin D insufficiencies on serum parathyroid hormone and bone mineral density: Analysis of the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHANES IV-3, 2009 and KNHANES V-1, 2010). J. Bone Miner. Res. 2013, 28, 764–770. [Google Scholar] [CrossRef]
- Olmos, J.M.; Hernández, J.L.; García-Velasco, P.; Martínez, J.; Llorca, J.; González-Macías, J. Serum 25-hydroxyvitamin D, parathyroid hormone, calcium intake, and bone mineral density in Spanish adults. Osteoporos. Int. 2016, 27, 105–113. [Google Scholar] [CrossRef]
- Di Castelnuovo, A.; Costanzo, S.; Persichillo, M.; Olivieri, M.; De Curtis, A.; Zito, F.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Distribution of short and lifetime risks for cardiovascular disease in Italians. Eur. J. Prev. Cardiol. 2012, 19, 723–730. [Google Scholar] [CrossRef]
- Iacoviello, L.; De Curtis, A.; Donati, M.B.; De Gaetano, G. Biobanks for cardiovascular epidemiology and prevention. Futur. Cardiol. 2014, 10, 243–254. [Google Scholar] [CrossRef]
- Zeller, T.; Hughes, M.; Tuovinen, T.; Schillert, A.; Conrads-Frank, A.; Ruijter, H.D.; Schnabel, R.B.; Kee, F.; Salomaa, V.; Siebert, U.; et al. BiomarCaRE: Rationale and design of the European BiomarCaRE project including 300,000 participants from 13 European countries. Eur. J. Epidemiol. 2014, 29, 777–790. [Google Scholar] [CrossRef]
- Valcour, A.; Zierold, C.; Podgorski, A.L.; Olson, G.T.; Wall, J.V.; DeLuca, H.F.; Bonelli, F. A novel, fully-automated, chemiluminescent assay for the detection of 1,25-dihydroxyvitamin D in biological samples. J. Steroid Biochem. Mol. Boil. 2016, 164, 120–126. [Google Scholar] [CrossRef]
- Phinney, K.W.; Bedner, M.; Tai, S.S.-C.; Vamathevan, V.V.; Sander, L.C.; Sharpless, K.E.; Wise, S.A.; Yen, J.H.; Schleicher, R.L.; Chaudhary-Webb, M.; et al. Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal. Chem. 2012, 84, 956–962. [Google Scholar] [CrossRef]
- De la Hunty, A.; Wallace, A.M.; Gibson, S.; Viljakainen, H.; Lamberg-Allardt, C.; Ashwell, M. UK Food Standards Agency Workshop Consensus Report: The choice of method for measuring 25-hydroxyvitamin D to estimate vitamin D status for the UK National Diet and Nutrition Survey. Br. J. Nutr. 2010, 104, 612–619. [Google Scholar] [CrossRef]
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008, 87, 1087S–1091S. [Google Scholar] [CrossRef]
- Schleicher, R.L.; Encisco, S.E.; Chaudhary-Webb, M.; Paliakov, E.; McCoy, L.F.; Pfeiffer, C.M. Isotope dilution ultra performance liquid chromatography-tandem mass spectrometry method for simultaneous measurement of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 in human serum. Clin. Chim. Acta 2011, 412, 1594–1599. [Google Scholar] [CrossRef]
- Pauli, D.; Seyfarth, M.; Dibbelt, L. The Abbott Architect c8000: Analytical performance and productivity characteristics of a new analyzer applied to general chemistry testing. Clin. Lab. 2005, 51, 31–41. [Google Scholar]
- Myers, G.L.; Miller, W.G.; Coresh, J.; Fleming, J.; Greenberg, N.; Greene, T.; Hostetter, T.; Levey, A.S.; Panteghini, M.; Welch, M.; et al. National Kidney Disease Education Program Laboratory Working Group. Recommendations for improving serum creatinine measurement: A report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin. Chem. 2006, 52, 5–18. [Google Scholar] [CrossRef]
- Dominguez, J.R.; Kestenbaum, B.; Chonchol, M.; Block, G.; Laughlin, G.A.; Lewis, C.E.; Katz, R.; Barrett-Connor, E.; Steve Cummings, S.; Eric, S.; et al. Relationships between serum and urine phosphorus with all-cause and cardiovascular mortality: The osteoporotic fractures in men (MrOS) Study. Am. J. Kidney Dis. 2013, 61, 555–563. [Google Scholar] [CrossRef]
- Gokce, C. Use of random urine samples to estimate total urinary calcium and phosphate excretion. Arch. Intern. Med. 1991, 151, 1587–1588. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.M.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D Deficiency in Adults: When to Test and How to Treat. Mayo Clin. Proc. 2010, 85, 752–758. [Google Scholar] [CrossRef]
- Reference Values for Serum 1,25-Dihydroxyvitamin D. Available online: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/8822 (accessed on 20 June 2019).
- Reference Values for Serum Total Calcium. Available online: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/601514 (accessed on 20 June 2019).
- Reference Values for Serum Parathyroid Hormone. Available online: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/28379 (accessed on 20 June 2019).
- Pilz, S.; Gaksch, M.; Kienreich, K.; Grübler, M.; Verheyen, N.; Fahrleitner-Pammer, A.; Treiber, G.; Drechsler, C.; Hartaigh, B.Ó.; Obermayer-Pietsch, B.; et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: A randomized controlled trial. Hypertension 2015, 65, 1195–1201. [Google Scholar] [CrossRef]
- Ritter, C.S.; Armbrecht, H.J.; Slatopolsky, E.; Brown, A.J. 25-Hydroxyvitamin D3 suppresses parathyroid hormone synthesis and secretion by bovine parathyroid cells. Kidney Int. 2006, 70, 654–659. [Google Scholar] [CrossRef]
- Shieh, A.; Ma, C.; Chun, R.F.; Witzel, S.; Rafison, B.; Contreras, H.T.M.; Wittwer-Schegg, J.; Swinkels, L.; Huijs, T.; Hewison, M.; et al. Effects of Cholecalciferol vs Calcifediol on Total and Free 25-Hydroxyvitamin D and Parathyroid Hormone. J. Clin. Endocrinol. Metab. 2017, 102, 1133–1140. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Brandi, M.L.; Cusano, N.E.; Mannstadt, M.; Rejnmark, L.; Rizzoli, R.; Rubin, M.R.; Winer, K.K.; Liberman, U.A.; Potts, J.T., Jr. Management of Hypoparathyroidism: Present and Future. J. Clin. Endocrinol. Metab. 2016, 101, 2313–2324. [Google Scholar] [CrossRef]
- Kumar, R.; Tebben, P.J.; Thompson, J.R. Vitamin D and the Kidney. Arch. Biochem. Biophys. 2012, 523, 77–86. [Google Scholar] [CrossRef]
| Hypocalcemia a | High Serum PTH b | |||||
|---|---|---|---|---|---|---|
| Prevalence | Odds Ratio (95%CI) | ROCAUC (95%CI) | Prevalence | Odds Ratio (95%CI) | ROCAUC (95%CI) | |
| Serum calcidiol | ||||||
| with severe deficiency <10 ng/mL n = 164 | 1.8% | 1.69 (0.40/7.14) | 0.556ns (0.35/0.76) | 5.5% | 8.81 (2.35/32.9) | 0.748 ** (0.61/0.89) |
| with mild-to-moderate deficiency 10–19 ng/mL n = 357 | 0.3% | 0.25 (0.03/2.19) | 0.363ns (0.17/0.56) | 1.1% | 1.72 (0.38/7.73) | 0.567 ns (0.35/0.78) |
| without deficiency ≥20 ng/mL n = 458 | 1.1% | 1 (ref) | 0.7% | 1 (ref) | ||
| Serum calcitriol | ||||||
| with deficiency <18 pg/mL n = 30 | 13.3% | 29.05 (7.37/114.5) | 0.709 * (0.50/0.92) | 0.0% | 0.97 (0.96/0.98) | 0.484 ns (0.34/0.62) |
| without deficiency ≥18 pg/mL n = 949 | 0.5% | 1 (ref) | 1.7% | 1 (ref) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, M.; Bilancio, G.; Guarino, E.; Cavallo, P.; Lombardi, C.; Costanzo, S.; De Curtis, A.; Di Castelnuovo, A.; Iacoviello, L. Vitamin D Status and Indices of Mineral Homeostasis in the Population: Differences Between 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D. Nutrients 2019, 11, 1777. https://doi.org/10.3390/nu11081777
Cirillo M, Bilancio G, Guarino E, Cavallo P, Lombardi C, Costanzo S, De Curtis A, Di Castelnuovo A, Iacoviello L. Vitamin D Status and Indices of Mineral Homeostasis in the Population: Differences Between 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D. Nutrients. 2019; 11(8):1777. https://doi.org/10.3390/nu11081777
Chicago/Turabian StyleCirillo, Massimo, Giancarlo Bilancio, Ermanno Guarino, Pierpaolo Cavallo, Cinzia Lombardi, Simona Costanzo, Amalia De Curtis, Augusto Di Castelnuovo, and Licia Iacoviello. 2019. "Vitamin D Status and Indices of Mineral Homeostasis in the Population: Differences Between 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D" Nutrients 11, no. 8: 1777. https://doi.org/10.3390/nu11081777
APA StyleCirillo, M., Bilancio, G., Guarino, E., Cavallo, P., Lombardi, C., Costanzo, S., De Curtis, A., Di Castelnuovo, A., & Iacoviello, L. (2019). Vitamin D Status and Indices of Mineral Homeostasis in the Population: Differences Between 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D. Nutrients, 11(8), 1777. https://doi.org/10.3390/nu11081777






