Development of a Paper-Based Sensor Compatible with a Mobile Phone for the Detection of Common Iron Formulas Used in Fortified Foods within Resource-Limited Settings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Preparation of Solutions and Samples
2.3. Iron Analysis
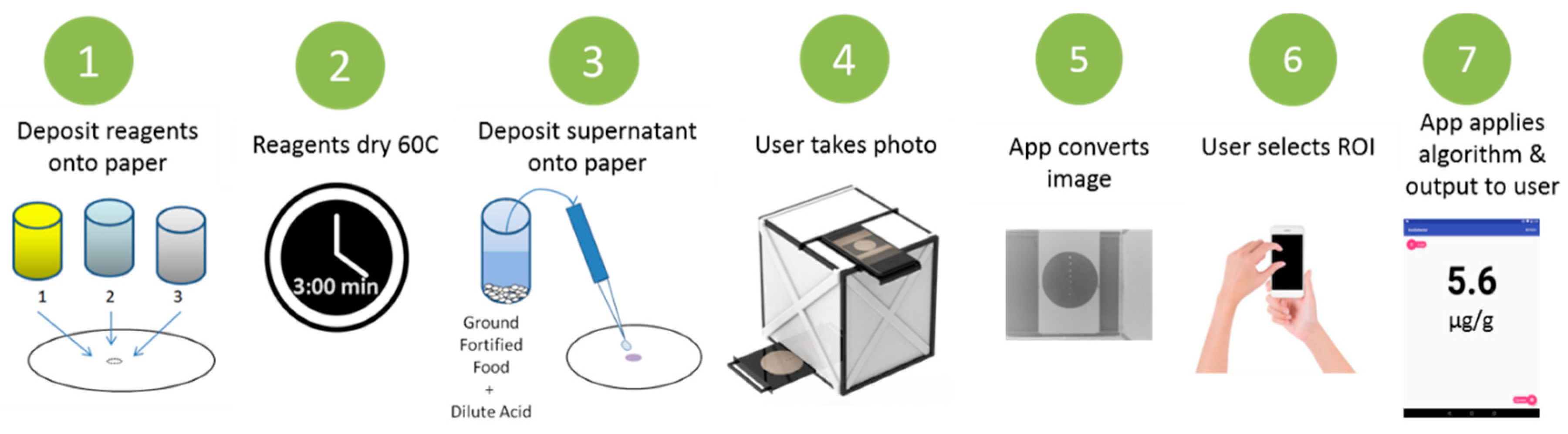
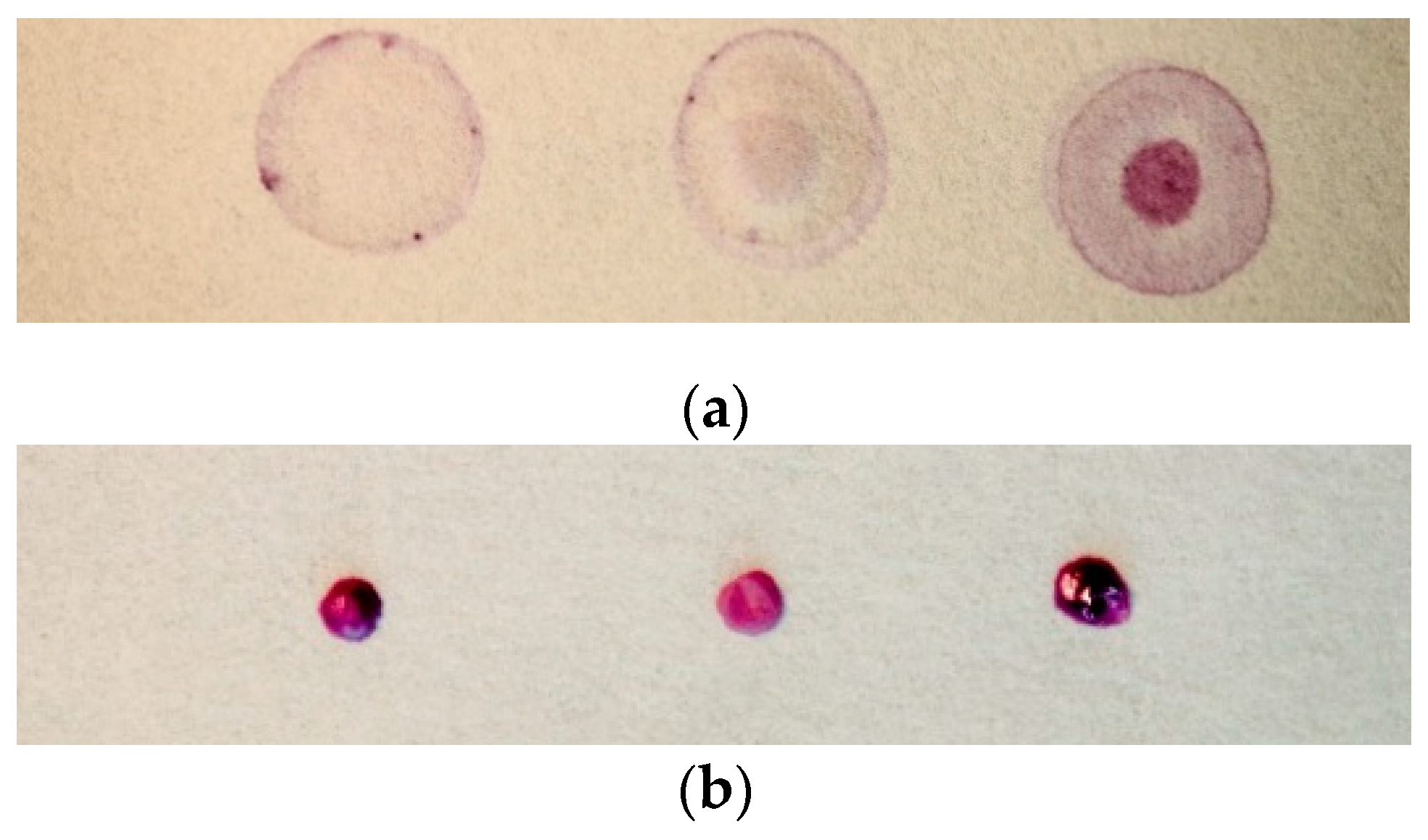
2.4. Fabrication of Paper-Based Assay
2.5. Paper-Based Measurement Procedure
2.6. Interference Studies
2.7. Recovery and Spike Studies
2.8. Calculations and Statistical Analysis
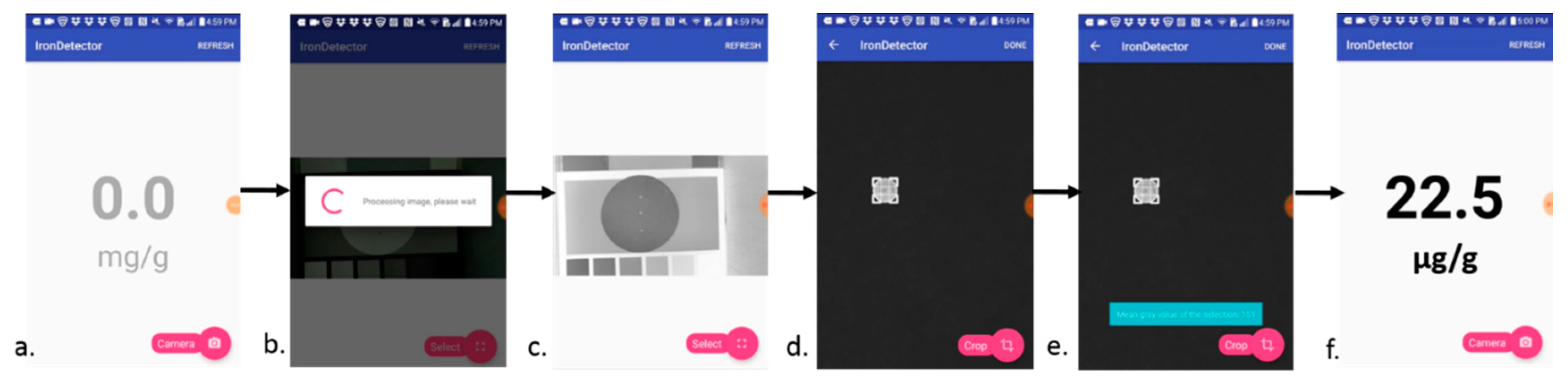
2.9. Development and Evaluation of Mobile Application
3. Results
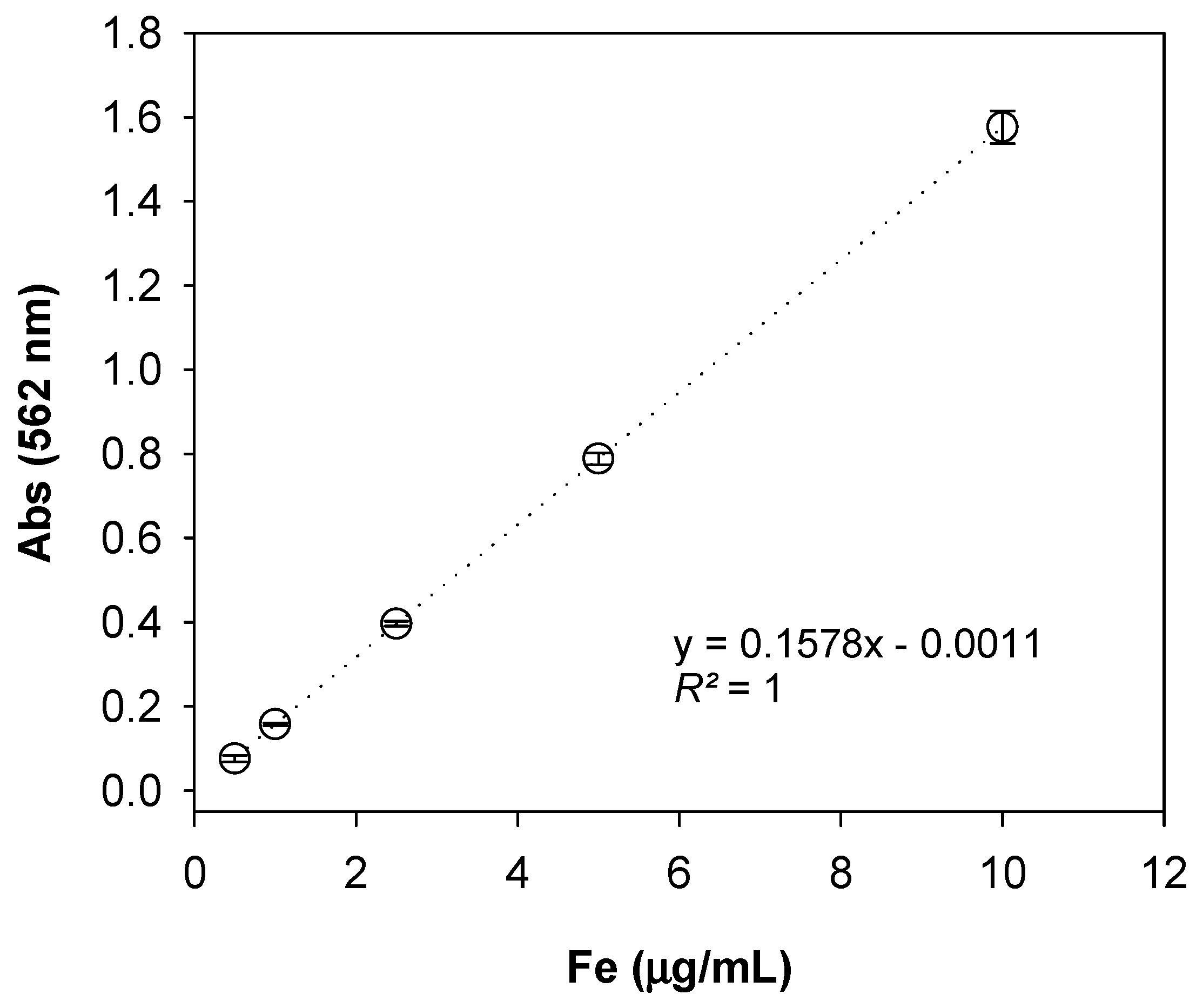
3.1. Iron Measurements of Standard Solutions
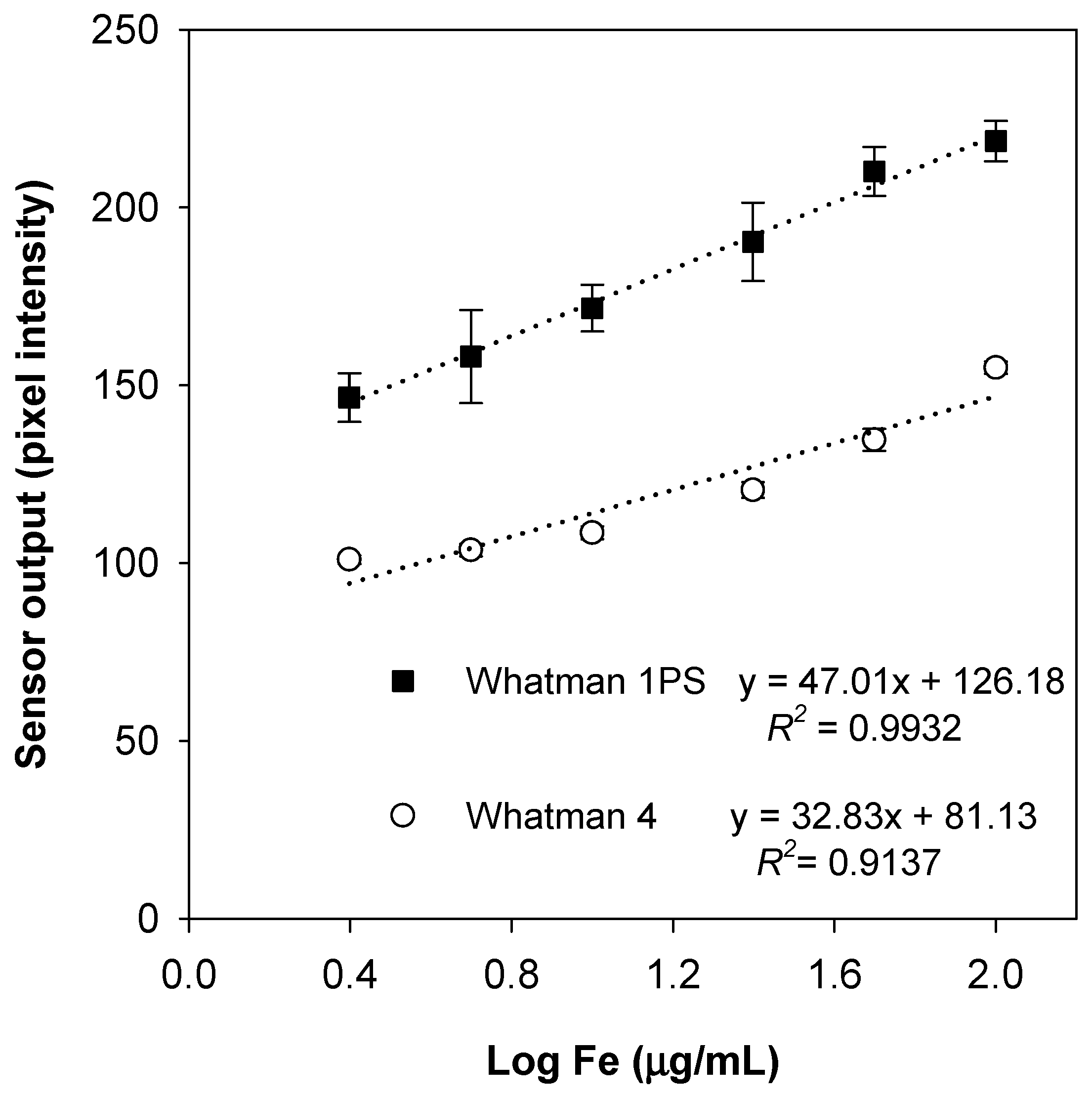
3.2. Analytical Figures of Merit
3.2.1. Range
3.2.2. Sensitivity
3.2.3. Specificity
3.3. Iron Determination in Foods
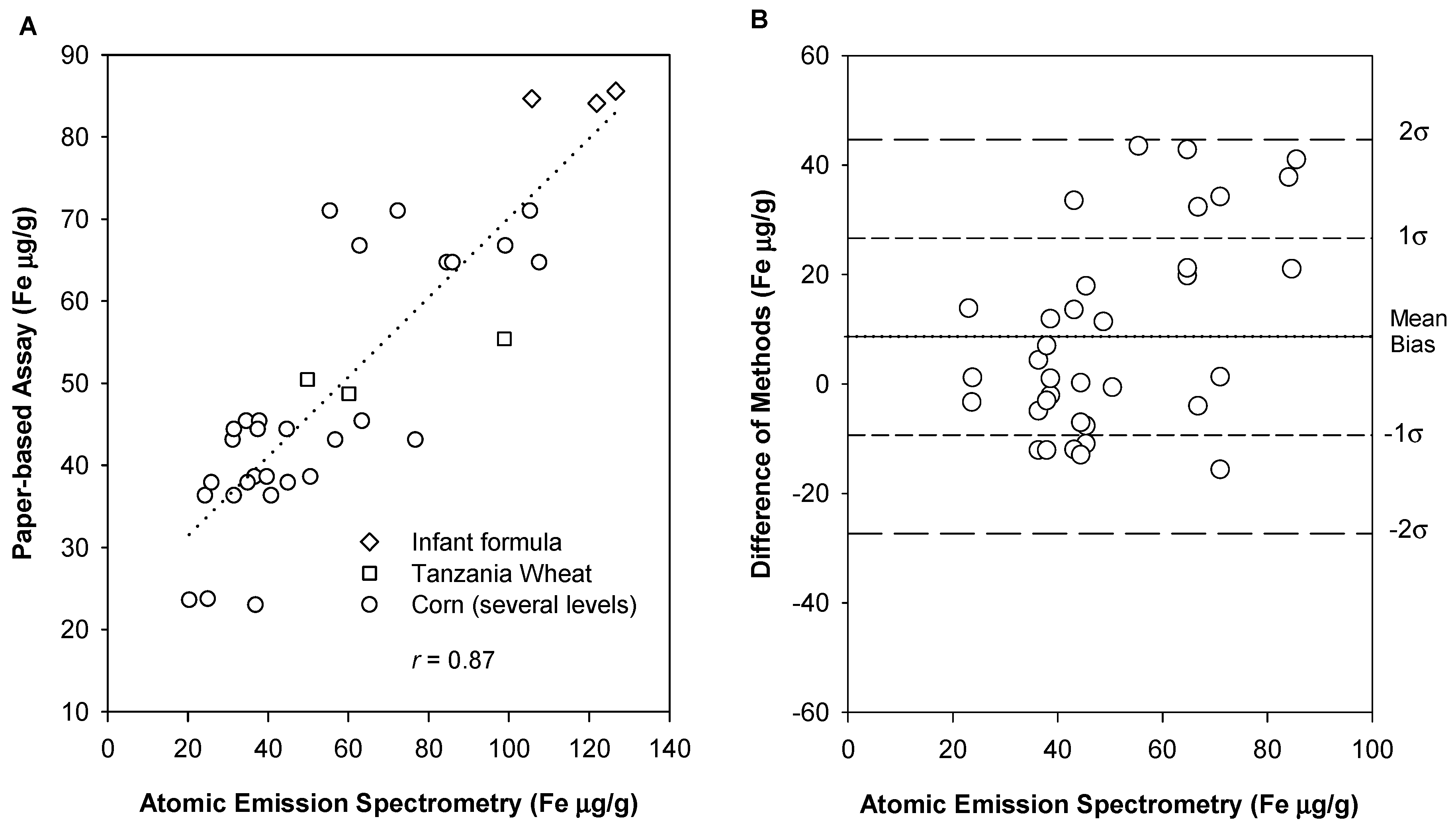
3.4. Accuracy and Determination of Systematic Error
3.5. Precision and Determination of Random Error
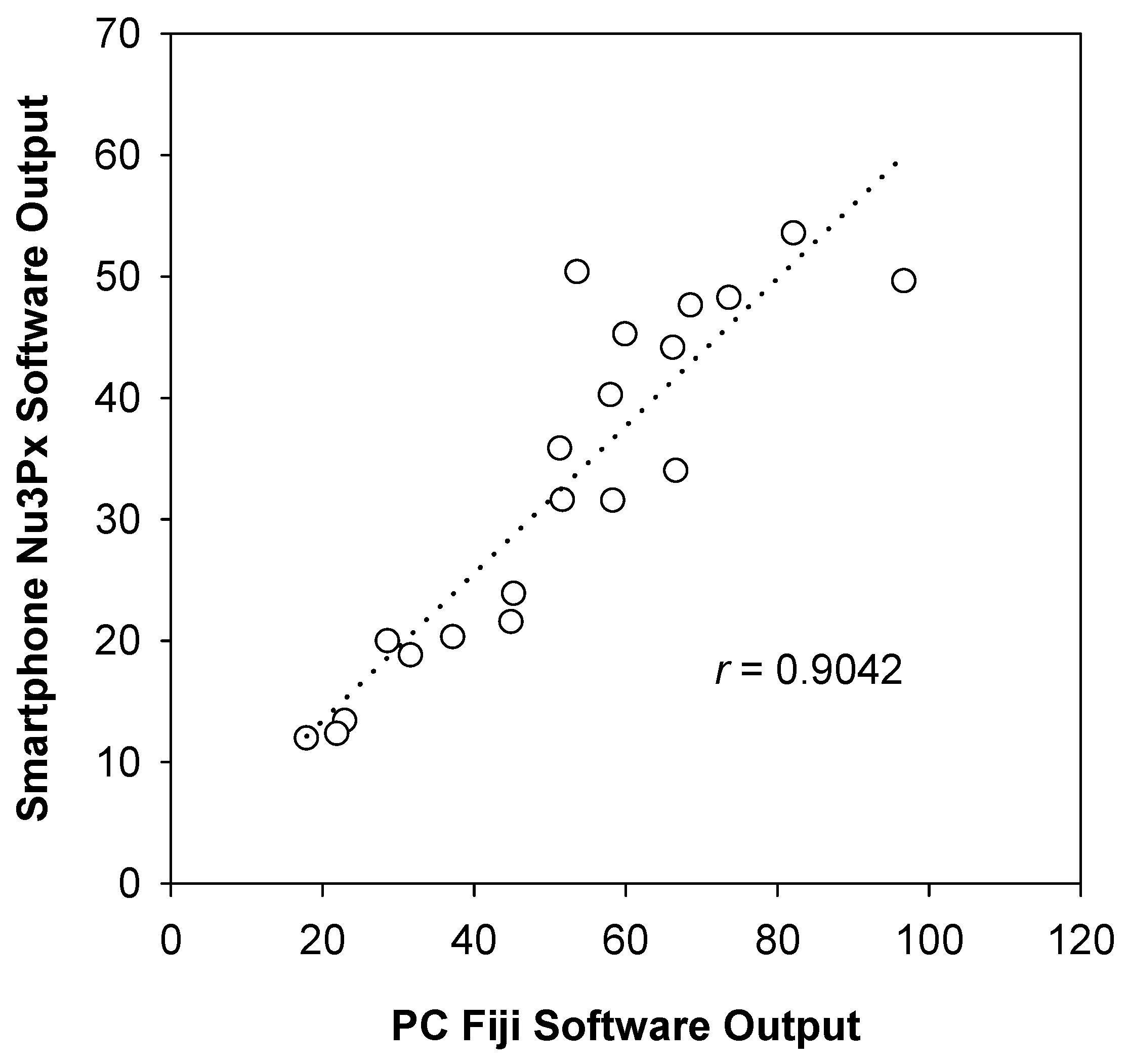
3.6. Pilot Test with Nu3px Mobile App
4. Discussion
4.1. Comparison to Other Methods
4.2. Limitations and Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tondon, R.; Verma, A.; Pandey, P.; Chaudhary, R. Quality evaluation of four hemoglobin screening methods in a blood donor setting along with their comparative cost analysis in an Indian scenario. Asian J. Transfus. Sci. 2009, 3, 66. [Google Scholar] [PubMed]
- Peterson, R.D.; Cunningham, B.T.; Andrade, J.E. A photonic crystal biosensor assay for ferritin utilizing iron-oxide nanoparticles. Biosens. Bioelectron. 2014, 56, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.; Cunningham, B.; Andrade, J. An enhanced photonic crystal biosensor-based immunodiagnostic assay for detection of soluble transferrin receptor as a biomarker of iron deficiency. FASEB J. 2014, 28. [Google Scholar]
- Peterson, R.D.; Chen, W.L.; Cunningham, B.T.; Andrade, J.E. Enhanced sandwich immunoassay using antibody-functionalized magnetic iron-oxide nanoparticles for extraction and detection of soluble transferrin receptor on a photonic crystal biosensor. Biosens. Bioelectron. 2015, 74, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; O’Dell, D.; Finkelstein, J.; Lee, S.; Erickson, D.; Mehta, S. ironPhone: Mobile device-coupled point-of-care diagnostics for assessment of iron status by quantification of serum ferritin. Biosens. Bioelectron. 2018, 99, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, Z.A.; Ahmed, T.; Black, R.E.; Cousens, S.; Dewey, K.; Giugliani, E.; Haider, B.A.; Kirkwood, B.; Morris, S.S.; Sachdev, H. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008, 371, 417–440. [Google Scholar] [CrossRef]
- Bryce, J.; Coitinho, D.; Darnton-Hill, I.; Pelletier, D.; Pinstrup-Andersen, P.; Maternal, G. Child Undernutrition Study Group. Maternal and child undernutrition: Effective action at national level. Lancet 2008, 371, 510–526. [Google Scholar] [CrossRef]
- Waller, A.W.; Lotton, J.L.; Gaur, S.; Andrade, J.M.; Andrade, J.E. Evaluation of Micronutrient Sensors for Food Matrices in Resource-Limited Settings: A Systematic Narrative Review. J. Food Sci. 2018, 83, 1792–1804. [Google Scholar] [CrossRef]
- Peeling, R.; Holmes, K.; Mabey, D.; Ronald, A. Rapid tests for sexually transmitted infections (STIs): The way forward. Sex. Transm. Infect. 2006, 82, V1–V6. [Google Scholar] [CrossRef]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.; Rodriguez, W.; Bassett, I.V. Evaluating Diagnostic Point-of-Care Tests in Resource-Limited Settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef]
- Sullivan, D.M.; Carpenter, D.E. Methods of Analysis for Nutrition Labeling; AOAC International: Arlington, VA, USA, 1993. [Google Scholar]
- Stookey, L. Ferrozine—A New Spectrophotometric Reagent for Iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Viollier, E.; Inglett, P.; Hunter, K.; Roychoudhury, A.; Van Cappellen, P. The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl. Geochem. 2000, 15, 785–790. [Google Scholar] [CrossRef]
- Carpenter, C.; Clark, E. Evaluation of Methods Used in Meat Iron Analysis and Iron Content of Raw and Cooked Meats. J. Agric. Food Chem. 1995, 43, 1824–1827. [Google Scholar] [CrossRef]
- Lysionek, A.; Zubillaga, M.; Calmanovici, G.; Salgueiro, M.; Sarabia, M.; Barrado, A.; Caro, R.; Weill, R.; Boccio, J. Modification of the Ferrozine technique to analyze iron contents in different foods—Comparative study using an internal standard as reference methodology. Biol. Trace Elem. Res. 1998, 65, 87–94. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- World Health Organization. Recommendations on Wheat and Maize Flour Fortification Meeting Report: Interim Consensus Statemen; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Poitevin, E.; Nicolas, M.; Graveleau, L.; Richoz, J.; Andrey, D.; Monard, F. Improvement of AOAC Official Method 984.27 for the Determination of Nine Nutritional Elements in Food Products by Inductively Coupled Plasma-Atomic Emission Spectroscopy after Microwave Digestion: Single-Laboratory Validation and Ring Trial. J. AOAC Int. 2009, 92, 1484–1518. [Google Scholar]
- Morbioli, G.G.; Mazzu-Nascimento, T.; Stockton, A.M.; Carrilho, E. Technical aspects and challenges of colorimetric detection with microfluidic paper-based analytical devices (µPADs)—A review. Anal. Chim. Acta 2017, 970, 1–22. [Google Scholar] [CrossRef]
- Westgard, J.O.; Barry, P.L.; Quam, E.F.; Ehrmeyer, S.S. Basic Method Validation: Training in Analytical Quality Management for Healthcare Laboratories; Westgard Quality Corporation: Madison, WI, USA, 1999. [Google Scholar]
- Ma, G.; Jin, Y.; Piao, J.; Kok, F.; Guusje, B.; Jacobsen, E. Phytate, calcium, iron, and zinc contents and their molar ratios in foods commonly consumed in China. J. Agric. Food Chem. 2005, 53, 10285–10290. [Google Scholar] [CrossRef]
- Ferretti, R.J.; Levander, O.A. Effect of milling and processing on the selenium content of grains and cereal products. J. Agric. Food Chem. 1974, 22, 1049–1051. [Google Scholar] [CrossRef]
- Tokalıoğlu, Ş.; Gürbüz, F. Selective determination of copper and iron in various food samples by the solid phase extraction. Food Chem. 2010, 123, 183–187. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 24.0; IBM Corp: Armonk, NY, USA, 2016. [Google Scholar]
- Burns, D.; Danzer, K.; Townshend, A. Use of the terms “recovery” and "apparent recovery" in analytical procedures—(IUPAC recommendations 2002). Pure Appl. Chem. 2002, 74, 2201–2205. [Google Scholar] [CrossRef]
- Ramasamy, S.; Hurtubise, R. Oxygen sensor via the quenching of room-temperature phosphorescence of perdeuterated phenanthrene adsorbed on Whatman 1PS filter paper. Talanta 1998, 47, 971–979. [Google Scholar] [CrossRef]
- Nery, E.; Kubota, L. Sensing approaches on paper-based devices: A review. Anal. Bioanal. Chem. 2013, 405, 7573–7595. [Google Scholar] [CrossRef]
- Mukaka, M. Statistics Corner: A guide to appropriate use of Correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Busa, L.; Mohammadi, S.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Advances in Microfluidic Paper-Based Analytical Devices for Food and Water Analysis. Micromachines 2016, 7, 86. [Google Scholar] [CrossRef]
- Allen, L.H.; De Benoist, B.; Dary, O.; Hurrell, R. ; World Health Organization. Guidelines on Food Fortification with Micronutrients; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Mentele, M.; Cunningham, J.; Koehler, K.; Volckens, J.; Henry, C. Microfluidic Paper-Based Analytical Device for Particulate Metals. Anal. Chem. 2012, 84, 4474–4480. [Google Scholar] [CrossRef]
- Sengupta, P.; Ganguly, A.; Bose, A. A phenolic acid based colourimetric ‘naked-eye’chemosensor for the rapid detection of Cu (II) ions. Spectrochim. Acta A. 2018, 198, 204–211. [Google Scholar] [CrossRef]
- Santana-Jiménez, L.; Márquez-Lucero, A.; Osuna, V.; Estrada-Moreno, I.; Dominguez, R. Naked-Eye detection of glucose in saliva with bienzymatic paper-based sensor. Sensors 2018, 18, 1071. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Liu, L.; Wu, X.; Kuang, H.; Xu, C.; Xu, L. A colorimetric paper-based sensor for toltrazuril and its metabolites in feed, chicken, and egg samples. Food Chem. 2019, 276, 707–713. [Google Scholar] [CrossRef]
- Carrio, A.; Sampedro, C.; Sanchez-Lopez, J.; Pimienta, M.; Campoy, P. Automated low-cost smartphone-based lateral flow saliva test reader for drugs-of-abuse detection. Sensors 2015, 15, 29569–29593. [Google Scholar] [CrossRef]
- Lee, S.; Kim, G.; Moon, J. Performance improvement of the one-dot lateral flow immunoassay for aflatoxin B1 by using a smartphone-based reading system. Sensors 2013, 13, 5109–5116. [Google Scholar] [CrossRef]
- Rateni, G.; Dario, P.; Cavallo, F. Smartphone-based food diagnostic technologies: A review. Sensors 2017, 17, 1453. [Google Scholar] [CrossRef]
- Poushter, J. Smartphone ownership and internet usage continues to climb in emerging economies. Pew Res. Cent. 2016, 22, 1–44. [Google Scholar]
- Kumar, S.; Thakur, N.; Parab, H.J.; Pandey, S.P.; Shinde, R.N.; Pandey, A.K.; Kumar, S.D.; Reddy, A.V. A visual strip sensor for determination of iron. Anal. Chim. Acta. 2014, 851, 87–94. [Google Scholar] [CrossRef]
- Filho Kelte, I.; Butik, M.; Jaski, A.; Quináia, S. Fast method to determine the elements in maize flour: Reduction in preparation time and reagent consumption. Braz. J. Food Technol. 2018, 21. [Google Scholar] [CrossRef][Green Version]
| Interferent (µg/g) | Mean Measurement ± SD (µg Fe/g) | Difference (µg Fe/g) |
|---|---|---|
| Unfortified (0) | 0.95 ± 0.23 | N/A |
| Fe (40) + no interferent | 48.45 ± 1.60 | N/A |
| Fe (40) + Zn (7.0) | 44.34 ± 5.73 | −4.11 |
| Fe (40) + Se (1.0) | 57.19 ± 6.42 | 8.74 |
| Fe (40) + Cu (2.0) | 49.28 ± 5.13 | 0.83 |
| Fe (40) + Co (11) | 47.02 ± 8.35 | −1.43 |
| Avg. Interference | 1.01 |
| Sensor | Matrix | LOD | Working Range | Equipment Needed | Time | Estimated Cost of Materials (USD) |
|---|---|---|---|---|---|---|
| Current work | Fortified foods dispersed in diluted acid | 3.691 µg/mL or 18.5 ng (dried) | 5.0–100 µg/mL | Reactive paper + Smartphone + Light-tight box | 5 min | $0.29 |
| Original in vitro Ferrozine assay [13] | Several matrices dispersed in diluted acid | 0.5 µg/mL | 0.5–10 µg/mL | Liquid reagents + Spectro-photometer | 20 min | $3.55 |
| Trace metal paper-based sensor [31] | Metal containing aerosols | 1500 ng | 1.5–10 µg | Strip + Image Scanner | Sample collected over 8-h | $0.013 (estimated by authors) |
| Visual strip sensor [39] | Ground water and fruit juices | 0.02 µg/mL | 0.02–2.0 µg/mL | Visual Strip + Spectrophotometer | 15 min | N/A |
| Atomic emission spectroscopy [40] | Any | 0.5 µg/mL | 0.5–4.0 µg/mL | Flame Atomic Absorption Spectrometer | 1 h 30 min | $25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waller, A.W.; Toc, M.; Rigsby, D.J.; Gaytán-Martínez, M.; Andrade, J.E. Development of a Paper-Based Sensor Compatible with a Mobile Phone for the Detection of Common Iron Formulas Used in Fortified Foods within Resource-Limited Settings. Nutrients 2019, 11, 1673. https://doi.org/10.3390/nu11071673
Waller AW, Toc M, Rigsby DJ, Gaytán-Martínez M, Andrade JE. Development of a Paper-Based Sensor Compatible with a Mobile Phone for the Detection of Common Iron Formulas Used in Fortified Foods within Resource-Limited Settings. Nutrients. 2019; 11(7):1673. https://doi.org/10.3390/nu11071673
Chicago/Turabian StyleWaller, Anna W., Marco Toc, Dylan J. Rigsby, Marcela Gaytán-Martínez, and Juan E. Andrade. 2019. "Development of a Paper-Based Sensor Compatible with a Mobile Phone for the Detection of Common Iron Formulas Used in Fortified Foods within Resource-Limited Settings" Nutrients 11, no. 7: 1673. https://doi.org/10.3390/nu11071673
APA StyleWaller, A. W., Toc, M., Rigsby, D. J., Gaytán-Martínez, M., & Andrade, J. E. (2019). Development of a Paper-Based Sensor Compatible with a Mobile Phone for the Detection of Common Iron Formulas Used in Fortified Foods within Resource-Limited Settings. Nutrients, 11(7), 1673. https://doi.org/10.3390/nu11071673





