Abstract
Background and Aims: The association of fatty acids with coronary heart disease (CHD) has been examined, mainly through dietary measurements, and has generated inconsistent results due to measurement error. Large observational studies and randomized controlled trials have shown that plasma phospholipid fatty acids (PL-FA), especially those less likely to be endogenously synthesized, are good biomarkers of dietary fatty acids. Thus, PL-FA profiles may better predict CHD risk with less measurement error. Methods: We performed a matched case-control study of 2428 postmenopausal women nested in the Women’s Health Initiative Observational Study. Plasma PL-FA were measured using gas chromatography and expressed as molar percentage (moL %). Multivariable conditional logistic regression was used to calculate odds ratios (95% CIs) for CHD associated with 1 moL % change in PL-FA. Results: Higher plasma PL long-chain saturated fatty acids (SFA) were associated with increased CHD risk, while higher n-3 polyunsaturated fatty acids (PUFA) were associated with decreased risk. No significant associations were observed for very-long-chain SFA, monounsaturated fatty acids (MUFA), PUFA n-6 or trans fatty acids (TFA). Substituting 1 moL % PUFA n-6 or TFA with an equivalent proportion of PUFA n-3 were associated with lower CHD risk. Conclusions: Higher plasma PL long-chain SFA and lower PUFA n-3 were associated with increased CHD risk. A change in diet by limiting foods that are associated with plasma PL long-chain SFA and TFA while enhancing foods high in PUFA n-3 may be beneficial in CHD among postmenopausal women.
1. Introduction
The relationship between fat intake and heart disease is not entirely clear, particularly among postmenopausal women with relatively low fat consumption [1,2]. Some meta-analyses of observational studies and randomized controlled trials found no association between saturated fatty acids (SFA) and risk of coronary heart disease (CHD) [3,4]. However, these studies lack a consideration of the nutrient substitution framework, under which significant associations have been observed [5,6,7]. Dietary intervention trials have demonstrated that SFA elevate total cholesterol and low-density lipoprotein cholesterol (LDL-C), and substituting SFA with polyunsaturated fatty acids (PUFA) is associated with lower CHD risk [8,9]. Based on the above evidence, several professional organizations have recommended diets limiting SFA and increasing PUFA n-3 [10,11].
Until recently, all SFA were viewed as unhealthy as refined carbohydrates when considering their impact on increasing LDL-C and total cholesterol. Although not all SFA have the same cholesterol-raising effect, no recommendations have been made for specific SFA due to insufficient evidence. Evidence supporting the associations of monounsaturated fatty acids (MUFA) and PUFA n-6 with CHD risk is also less definitive [4,12]. A recent meta-analysis of prospective observational studies and randomized trials concluded that there was no significant association of dietary MUFA and PUFA n-6 with CHD risk, but a higher CHD risk of trans fatty acids (TFA) (pooled RR (95% Cis): 1.16 (1.06–1.27)) and a lower CHD risk of long-chain PUFA n-3 (0.87 (0.78–0.97)) [4]. Several investigators have questioned the interpretation of these results [9,13]. One major concern was that the macronutrient for replacement, which could have had an independent association with CHD outcome, was not identified or accounted for [9,13]. An additional concern was that the dietary assessment methods in these reports were via self-report instruments, which is valid when ranking individuals according to the intake levels, but contributes substantial systematic measurement error when the absolute intake levels are of interest.
To address these concerns, the Multi-Ethnic Study of Atherosclerosis [12,14] and the Women’s Health Initiative Observational Study (WHI-OS) [15] have measured plasma phospholipid fatty acids (PL-FA) as indicators of medium-term dietary FA intake [16,17], and have shown associations between PL-FA and CHD risk. Specifically, one interquartile range increase in PL long-chain PUFA n-3 or SFA 15:0 were associated with a lower CHD risk (HR (95%CIs), 0.40 (0.23–0.69) and 0.76 (0.61–0.93), respectively) [12,14]; 1 moL % increase in PL SFA was associated with a 20% higher CHD risk (95% CIs 1.08–1.32); and 1 moL % increase in PL PUFA n-3 was associated with a 11% lower CHD risk (95% CIs 0.83–0.97) [15]. While plasma PL-FA offer an objective biomarker of dietary FA that could potentially reduce the measurement error from questionnaires or diet recalls, plasma PL-FA reflect both dietary intake and endogenous synthesis. Therefore, caution is needed when interpreting the association with disease outcome.
Large observational studies and dietary intervention trials have demonstrated that PL-FA profiles are good biomarkers of dietary fat, especially for those FA with limited in vivo synthesis—PUFA n-3, n-6, and TFA [16,18]. In addition, randomized trials have shown that substituting dietary PUFA n-6 with PUFA n-3 at 10% of total energy led to a 2–3% plasma substitution [19]. Thus, the substitution of plasma PL-FA by changes in dietary fat types has a potential for evaluating the association of specific dietary FA with CHD risk, with less measurement error. However, the substitution of plasma PL-FA has not been examined so far, and we are still lack evidence from postmenopausal women.
Based upon the previously reported associations between plasma PL-FA and CHD, we sought to examine the following: (1) the association of plasma PL-FA levels, specifically long-chain and very-long-chain plasma PL SFA, with CHD risk; (2) the effect of substituting plasma PUFA n-6 and TFA with PUFA n-3 on CHD risk; and (3) potential food groups that are correlated with plasma PL-FA, using data from the WHI study.
2. Materials and Methods
2.1. Study Population
The WHI-OS is a prospective cohort study that enrolled 93,676 postmenopausal women between the ages of 50 and 79 years in the United States from 1994 to 1998. It is designed to assess the biological, lifestyle, and genetic factors for CHD and other major health events among postmenopausal women. A detailed description of the WHI study design has been published elsewhere [20,21].
A matched case-control design was used for the current study. All CHD cases, including hospitalized myocardial infarction (MI), definite silent MI, and coronary death, were confirmed based on medical records and death certificates. The cases included in the current study were a random sample of all CHD cases identified based on the September 2005 database [22]. A total of 2468 cases were initially selected and those who met the following criteria were excluded: (1) lack of sufficient baseline plasma sample (N = 28) (2) missing baseline dietary measurement (N = 126), and (3) self-reported baseline CVD, which includes angina, MI, coronary artery bypass graft (CABG) surgery, percutaneous transluminal coronary angioplasty (PTCA), carotid artery disease, congestive heart failure, stroke or peripheral vascular disease (N = 765). Potential controls were women from the entire WHI-OS who did not develop CVD during the follow-up (a mean of 4.5 years) and were excluded if meeting the same exclusion criteria as the cases. Cases and controls were matched on age at screening, enrollment date, race/ethnicity (White, Black, Hispanic, other), and hysterectomy status. The sample size for PL-FA assays included 1224 matched case-control pairs and 10% blind duplicates for quality control. We additionally excluded 20 participants due to the lack of plasma PL-FA profile results (N = 11) or missing matched pairs (N = 9), and came up with a final sample size of 1214 matched pairs. A separate approval for using de-identified samples and data for this study was obtained from the Tufts University/Tufts Medical Center Institutional Review Board [15].
2.2. Plasma PL-FA Profiles
Blood samples were collected at baseline and a minimum of 12 h fasting before blood draw was required. All blood samples were maintained at 4 °C for up to an hour until plasma was separated from cells, frozen at −20 °C, and then sent to the central repository stored at −80 °C. Plasma PL-FA profiles were measured by an established gas chromatography method [23] at Tufts University. Peaks of interest were identified by comparison with authentic FA standards (National Institute of Health Fatty Acid Standards A, B, and C, Nu-Check-Prep, Elysian, MN, USA), and expressed as molar percentage (moL %) proportions of FA relative to the internal standard (heptadecanoic acid). Internal and external quality controls were performed to guarantee the validity of the measurements, and detailed information has been published previously [15]. A total of 28 individual plasma PL-FA were measured. We classified these FA into groups based on the number of double bonds—specifically SFA, MUFA, PUFA n-3, PUFA n-6, and TFA. We further classified FA by the length of FA chains: long-chain FA are those with 12–19 carbons and very-long-chain FA are those with 20 or more carbons. Table 1 shows the lipid names, common names, categories, and mean (SD) levels of individual plasma PL-FA that were included in this study.

Table 1.
The lipid names, common names, categories, and mean (SD) levels (moL %) of plasma phospholipid fatty acid profiles measured in the matched case-control study of the Women’s Health Initiative (1994–2005) (N = 2428).
2.3. Covariates and Dietary Data
Standard questionnaires following the same protocol were utilized throughout the study to collect information related to socio-demographics, lifestyle factors, and CHD risk factors [20]. We initially considered the following variables as potential confounders: (1) socio-demographic variables (including age, U.S. region, race/ethnicity, education, and income); (2) lifestyle factors such as recreational physical activity, body mass index (BMI), waist circumference, waist-to-hip ratio, and smoking; and (3) CHD risk factors (family history of MI/diabetes/stroke, anticoagulant/anti-diabetic/lipid lowering medication use, postmenopausal hormone use, and self-reported hypertension/diabetes/hypercholesterolemia/hysterectomy status at baseline).
Information on nutrient intake and food consumption was assessed by food frequency questionnaire at baseline [20]. Potential dietary confounders included alcohol intake (g/day), percent calories from protein/carbohydrates, and total energy intake (kcal/day) [24]. Foods high in fats or that have the potential to influence fat metabolism were assessed for further correlation analysis with plasma PL-FA. For this analysis, we considered the following nine food groups: fish, dairy products, butter, margarine, olive/canola oil, other vegetable oils, red meat, alcohol, and carbohydrates [25,26,27,28].
Age and BMI were treated as continuous variables. Physical activity, measured by recreational physical activity score (MET-h/week) based on a series of questions related to exercise intensity levels [29], was treated as continuous or categorical dichotomized at median. Education was categorized as ≤high school, some college, or postgraduate. Income was categorized as <$20,000, $20,000–74,999, or ≥$75,000 per year. Smoking was categorized as current, past, or never smoker. Family history was defined as first-degree relatives having MI, diabetes, or stroke. Postmenopausal hormone use was categorized into current estrogen + progesterone, current estrogen alone, past users, or never used. Hypertension was defined as self-reported hypertension/taking antihypertensive medication, or systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg. Baseline diabetes and hypercholesterolemia were defined as taking anti-diabetic or cholesterol-lowering medications, respectively.
2.4. Statistical Analysis
We initially examined the baseline distribution of socio-demographics, lifestyle factors, CHD risk factors, and dietary factors by CHD status, as well as by five subtypes of plasma PL-FA in tertiles (Appendix A). Descriptive statistics such as median, mean, standard deviation, frequency, and proportion were used to summarize the aforementioned variables. Depending on the distribution of the variables, we used the paired t-test, Wilcoxon signed rank test, or McNemar test for the comparison between cases and controls.
We employed multivariable conditional logistic regression models to estimate odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for CHD risk in association with a 1 moL % increase in plasma PL-FA. The covariates in the multivariable model were selected based on a hypothesized causal diagram (Appendix B) to adjust for potential confounding, and a backward selection method was used to generate a parsimonious model with the best model fit [30]. The final multivariable model adjusted for matching factors (age, race/ethnicity, enrollment date, and hysterectomy status), income, physical activity, smoking, family history of MI/diabetes, postmenopausal hormone use, self-reported hypertension/diabetes, percent calories from protein/carbohydrates, and total energy intake. We have performed model diagnosis and examined model assumptions. No outlier was observed and all model assumptions held.
Linoleic, α-linolenic, and trans FA cannot be synthesized in vivo. In addition, the elongase and desaturase enzymes in human livers have low activity when regulating the synthesis of long-chain PUFA from their precursors [31]. Therefore, plasma PL PUFA n-3, n-6, and TFA are good biomarkers of corresponding dietary FA, and substitutions of dietary FA can be estimated by plasma substitutions. To estimate the theoretical effect of substituting 1 moL % of plasma PL PUFA n-6 with the same proportion of PUFA n-3, we left out PUFA n-6 in the multivariable model. The relation can be expressed as follows:
where to are regression coefficients. The total of all PL-FA is 100 moL %, so that the coefficient can be interpreted as the effect of substituting 1 moL % of PUFA n-6 with the same proportion of PUFA n-3 while holding other FA constant [24]. This substitution model was similarly applied to TFA substitution analysis.
logit (CHD risk) = β1 * PL PUFA n-3 + β2 * PL SFA + β3 * PL MUFA + β4 * PL TFA
To further examine potential food sources that related to plasma PL-FA levels, we calculated Spearman’s rank correlation coefficients between plasma PL-FA levels and the consumption of nine selected food groups. Considering the cases might have altered metabolic status, thus not representing the overall population, the correlation coefficients were calculated among controls only, adjusting for matching factors and the aforementioned non-dietary confounders.
Multiple imputation (five times) by chained equations [32] was used to impute missing values on the following covariates: income (N = 118), physical activity (N = 28), smoking (N = 28), family history (N = 209, among which 119 were missing MI and 122 were missing diabetes), and self-reported hypertension (N = 47) and diabetes (N = 2).
We conducted the following sensitivity analyses to assess the robustness of findings: (1) comparing the association analysis results from different regression models; (2) examining the association between plasma PL-FA groups and CHD additionally adjusting for anthropometric measures (BMI, waist circumference, waist-to-hip ratio) and chronic weight cycling (three-year BMI change); (3) performing substitution analysis stratified by physical activity levels; and (4) performing the association and substitution analyses among participants with complete information (N = 2181). All analyses were performed using Statistics Analysis Systems software package (version 9.4; SAS Institute, Inc., Cary, NC, USA).
3. Results
The characteristics of the cases and controls can be found in Table 2. The mean (SD) age was 67.8 (6.8) years, and the median time from baseline plasma PL-FA measures to CHD event among cases was 4.5 years. Compared with controls, cases had significantly lower education and income levels, higher BMI (26.9 vs. 25.9 kg/m2) and lower physical activity level (8.3 vs. 10.8 MET-h/week). More cases were smokers, had hypertension or diabetes, or reported a family history of MI and medication use, while fewer were currently using postmenopausal hormones.

Table 2.
Baseline characteristics by cases and controls in the Women’s Health Initiative study (1994–2005) (N = 2428).
3.1. Associations between Increased Plasma PL-FA and CHD Risk
To examine the relationships between plasma PL-FA and CHD risk, we calculated the ORs (95% CIs) of CHD in association with 1 moL % increase in plasma PL-FA (Table 3). In the adjusted multivariable model, we observed higher CHD risk for increased plasma total PL SFA (OR = 1.20 (1.10–1.30)) and long-chain SFA (OR = 1.18 (1.09–1.28)), but not for very-long-chain SFA (OR = 1.00 (0.77–1.30)). We also observed a lower CHD risk associated with plasma PL PUFA n-3 (OR = 0.93 (0.88–0.99)). However, no significant associations were observed for plasma PL MUFA, PUFA n-6, and TFA. The associations between individual plasma PL-FA and CHD have been published elsewhere [15].

Table 3.
Multivariable adjusted associations (1 moL %) between plasma phospholipid fatty acids and CHD risk in the matched case-control study (N = 2428).
3.2. Plasma PL-FA Substitutions
To further estimate the effect of substituting dietary PUFA n-6 or TFA with PUFA n-3 on CHD risk, we calculated the ORs (95%CIs) of CHD risk from plasma PL-FA substitutions (Table 4). In the initial models, lower CHD risk was observed when 1 moL % of plasma PL PUFA n-6 or TFA were substituted with the same proportion of PUFA n-3. Although these associations were attenuated on adjustment for covariates (model 2), results were still statistically significant at the 0.05 significance level (OR = 0.90 (0.84–0.96) and 0.74 (0.56–0.99), respectively). However, in all models, substituting 1 moL % PL TFA with PUFA n-6 was not associated with CHD risk.

Table 4.
Odds ratios (95% CIs) of CHD associated with 1 moL % substitutions between plasma phospholipid fatty acid groups among participants in the matched case-control study (N = 2428).
3.3. Correlations between Plasma PL-FA and Select Food Groups
Correlations between food groups and plasma PL-FA levels have the potential to provide information regarding food sources that may influence plasma PL-FA concentrations (Appendix C). The strongest correlations that we found were in the PUFA n-3 group: fish and olive/canola oil intakes were positively correlated with plasma PL PUFA n-3 (r = 0.34 and 0.12, respectively; p < 0.0001). We also observed a positive correlation between alcohol intake and plasma PL long-chain SFA (r = 0.13; p < 0.0001). Margarine and red meat intakes were positively correlated with plasma PL PUFA n-6 and TFA (r ranges between 0.11 to 0.15; p < 0.0001).
3.4. Sensitivity Analyses
In the sensitivity analysis of comparing difference regression models, our results were consistent across models thus supporting the robustness of findings (Appendix D). When examining the association between plasma PL-FA and CHD while adjusting for different anthropometric measures, we found very similar results when adjusting for BMI, waist circumference, waist-to-hip ratio, or a three-year BMI change (Appendix E). In the substitution analysis stratified by physical activity levels, we classified participants into two groups: physically active (those with physical activity levels above the median, 9.5 MET-h/week) and physically inactive (those with physical activity levels ≤ 9.5 MET-h/week). As the point estimates varied to a small extent between the physically active and inactive, physical activity is less likely to be an effect modifier than a confounder (Appendix F). When comparing the association and substitution analyses using complete cases versus multiple imputation, we observed very similar results, thus suggesting the validity of the multiple imputation approach (Appendix G and Appendix H).
4. Discussion
This prospective matched case-control study nested in the WHI-OS assessed the association of plasma PL-FA profile with CHD risk among 2428 postmenopausal women. In the adjusted analysis, we found that higher PL SFA, especially long-chain SFA, were associated with increased CHD risk, while higher PL PUFA n-3 were associated with lower CHD risk. No significant associations were found for PL very-long-chain SFA, MUFA, PUFA n-6 and TFA. In the substitution analysis, we found that substituting 1 moL % of plasma PL PUFA n-6 or TFA with the same proportion of PUFA n-3 were associated with lower CHD risk.
4.1. Plasma PL SFA Profiles and CHD Risk
Individual SFA have diverse biological functions determined by the chain length [33]. For example, the effect of raising LDL-C decreases as the chain length increases [33]. Long-chain SFA, especially palmitic (16:0) and stearic (18:0) acids, are the primary dietary FA. Within the human body, long-chain SFA are a major component of cell membranes, and endogenous synthesis contributes a significant portion of SFA in the circulation with palmitic and stearic acids being the primary product [34]. Accumulating evidence has supported the relationship of the aforementioned long-chain FA metabolism with potential CHD risk [33]. The modest positive associations of total and long-chain PL SFA with CHD risk from our study were consistent with some [26,35], but not all [36,37], cohort studies that have assessed either total or individual SFA. The discordance may come from differences in age distributions, sources of blood SFA, and specific SFA included in analyses.
We did not observe significant associations between very-long-chain plasma PL SFA and CHD risk, which differs from a few recent population-based studies showing blood concentrations of very-long-chain SFA were associated with lower risk of cardiometabolic conditions, including CHD [38] and diabetes [39]. The mechanisms underlying these observations are not well established. Compared with long-chain SFA, very-long-chain SFA have lower water solubility and oxidation susceptibility, and they are major components of ceramides and sphingomyelins that affect liver homeostasis, myelin maintenance, and anti-inflammatory response through ceramide synthase expression, therefore showing potential beneficial effects on CHD [40,41]. The discrepancies between our results and other studies may be explained by the following: (1) differences in study populations: participants in other studies were younger and had lower BMI, and research has shown that the association of very-long-chain SFA with lower cardiometabolic risks appeared strongest in participants with normal BMI [39]; and (2) different sources of SFA: most beneficial effects were identified from circulating very-long-chain SFA, while no significant results were found from plasma PL or erythrocyte membranes.
4.2. Plasma PL-FA Substitution in Groups
We observed a lower risk of CHD when substituting plasma PL PUFA n-6 with n-3. The beneficial effects of PUFA n-3 on CHD have been reported in previous studies [12,14,15]. PUFA n-3 have been shown to lower plasma triglyceride levels, to have anti-thrombotic and anti-arrhythmic properties, to reduce macrophage infiltration into the vessel wall, and to reduce the proatherogenic secretion of growth factors and cytokines by monocytes [42].
Although dietary TFA have been shown to increase CHD risk [43], we only observed a significant change in CHD risk when substituting PL TFA with PUFA n-3, while no significant result was observed when TFA were substituted with PUFA n-6. This discrepancy may be explained by the limitation in the gas chromatography methodology we used to measure plasma PL-FA, which did not distinguish TFA isomers elaidic acid (18:1 n-9 t) and vaccenic acid (18:1 n-11 t) [15]. It has been suggested that 18:1 n-11 t, as the predominant TFA in dairy products, may have a weaker association with CHD compared with other TFA resulting from partial hydrogenated vegetable oils, mainly 18:1 n-9 t [44]. Another possibility is that the relatively small proportion of PL TFA, reflecting the dietary characteristics of the cohort of older women, lead to a lack of power to detect an association.
The substitution analysis between different plasma PL-FA may be more informative than the measured plasma PL-FA profiles themselves when examining the association of one type of plasma PL-FA with CHD. The plasma PL-FA were measured in moL % with a summation of 100% for all PL-FA, and 1 moL % increase in one type of plasma PL-FA is accompanied by reciprocal decrease in 1 moL % for another. The plasma PL-FA substitution analysis is also more informative than the substitution analysis of dietary FA and CHD risk, given that there is a lack of accuracy for each type of dietary fat using any diet assessment method [45].
4.3. Potential Food Sources of Plasma PL-FA and Dietary Recommendations
The Spearman correlations we observed between plasma PL-FA and selected food groups were moderate; however, they were consistent and comparable to previous evidence from other observational studies using food frequency questionnaire as dietary measurement [18,46]. In short-term dietary intervention trials and observational studies using multiple dietary measurements, the correlations were stronger, therefore showing the measurement error in food frequency questionnaire might be a cause of our moderate correlations [47,48].
The strongest correlation that we found was between plasma PL PUFA n-3 and fish intake. This result is consistent with both observational study and dietary intervention trials [18,46], showing fatty fish and fish oils are the predominant sources of PUFA n-3. We found a positive correlation between plasma PL long-chain SFA and alcohol intake. This may be because alcohol can increase the activity of acetyl-CoA carboxylase and FA synthase—the key enzymes in SFA 16:0 synthesis [49]. Our analysis also showed that margarine and red meat intake was positively associated with plasma PL TFA levels. Because humans do not synthesize TFA, diet contributes to the occurrence of these FA isomers in the plasma. TFA can be found naturally from ruminant-animal meat (mainly 18:1 n-7 t), dairy fat (mainly 18:1 n-11 t), and unnaturally from industrially hydrogenated vegetable oils (mainly 18:1 n-9 t), such as margarine [50].
Our findings are consistent with the recommendation made in the Scientific Report of the 2015 Dietary Guidelines Advisory Committee on food sources of nutrients among U.S. adults [51]. Foods associated with an increase of plasma PL long-chain SFA and TFA (including alcohol, red meat, and margarine) should be limited. Dietary PUFA n-3, which can be found in fatty fish and canola oil, should be recommended. However, caution is required when attempting to apply dietary recommendations extrapolated from plasma levels of nutrients, which are only modestly correlated with dietary intake.
There are several strengths of this study compared with previous ones. The matched case-control design nested in a prospective cohort study allowed us to assess exposure in cases before the diagnosis of CHD and select controls based on incidence density sampling, thus addressing the temporality between exposure and disease onset. In addition, our study is novel due to the focus on the association of theoretical plasma PL-FA substitutions on CHD risk, which reflect in vivo FA metabolism as well as dietary changes with less measurement error. However, our study had several limitations. We did not detect all plasma PL-FA (such as short- and medium-chain SFA with carbons less than 12 and isomers of TFA), thus limiting our analyses to a selective set of FA. However, these unmeasured FA are present in very small amounts. Additional limitations include: (1) confounding bias due to unmeasured CHD risk factors, such as blood lipids; and (2) limited evidence when extrapolating plasma PL-FA findings to dietary changes.
5. Conclusions
This plasma PL-FA analysis suggests that long-chain SFA may be associated with increased risk of CHD, and substituting PUFA n-6 or TFA with PUFA n-3 may be associated with lower risk of CHD. Further work is needed on distinguishing the specific dietary factors that influence plasma PL-FA levels within the context of the other covariates that may likewise impact outcomes.
Author Contributions
Q.L. contributed to the study conceptualization, analysis, interpretation of data, and drafting of the manuscript. C.B.E. supervised the project, contributed to the study conceptualization, design, interpretation of data, revising the manuscript, and final approval of the manuscript. A.H.L. designed the study, provided the specimen data, and participated in revising the manuscript. N.R.M., J.E.M., B.V.H., L.F.T., M.L.N., L.V.V.H., J.E.R., M.A.A., L.W.M., W.L., L.G.S., and L.W. participated in revising the manuscript, providing critical comments related to model fitting, and data interpretation.
Funding
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Acknowledgments
WHI investigators. Program Office (National Heart, Lung, and Blood Institute, Bethesda, MD, USA): Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford and Nancy Geller. Clinical Coordinating Center (Fred Hutchinson Cancer Research Center, Seattle, WA, USA): Garnet Anderson, Ross Prentice, Andrea LaCroix and Charles Kooperberg. Investigators and Academic Centers (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA): JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC, USA) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA, USA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH, USA) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ, USA) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY, USA) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL, USA) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA, USA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA, USA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC, USA) Sally Shumaker; (University of Nevada, Reno, NV, USA) Robert Brunner. Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC, USA) Mark Espeland.
Conflicts of Interest
There is no disclosure for potential conflict of interests.
Abbreviations
| AA | arachidonic acid |
| CHD | coronary heart disease |
| DHA | docosahexaenoic acid |
| HDL-C | High-density lipoprotein cholesterol |
| LA | linoleic acid |
| LDL-C | low-density lipoprotein cholesterol |
| MI | myocardial infarction |
| MUFA | monounsaturated fatty acids |
| PL-FA | phospholipid fatty acids |
| PUFA | polyunsaturated fatty acids |
| SFA | saturated fatty acids |
| TFA | trans fatty acids |
| WHI-OS | Women’s Health Initiative Observational Study |
Appendix A

Table A1.
Relationship of plasma phospholipid fatty acids a and socio-demographic, lifestyle, and CHD risk factors in the matched case-control study of the Women’s Health Initiative Observational Study (1994–2005) (N = 2428).
Table A1.
Relationship of plasma phospholipid fatty acids a and socio-demographic, lifestyle, and CHD risk factors in the matched case-control study of the Women’s Health Initiative Observational Study (1994–2005) (N = 2428).
| SFA | MUFA | PUFA n-3 | PUFA n-6 | TFA | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | pb | T1 | T2 | T3 | pb | T1 | T2 | T3 | pb | T1 | T2 | T3 | pb | T1 | T2 | T3 | pb | |
| Median, moL % | 44.8 | 46.0 | 47.3 | 10.4 | 11.6 | 13.3 | 3.9 | 4.9 | 6.5 | 34.0 | 36.5 | 38.5 | 0.4 | 0.6 | 1.0 | |||||
| Socio-demographics | ||||||||||||||||||||
| Age, y c | 67.8 | 68.1 | 67.5 | 0.34 | 67.4 | 68.1 | 67.9 | 0.16 | 67.4 | 67.7 | 68.3 | <0.01 | 68.0 | 68.0 | 67.4 | 0.09 | 67.6 | 68.0 | 67.8 | 0.64 |
| White, % | 92 | 89 | 88 | 0.41 | 86 | 91 | 92 | <0.01 | 93 | 89 | 86 | <0.01 | 89 | 88 | 91 | 0.04 | 87 | 91 | 90 | 0.02 |
| Income, % | 0.94 | 0.24 | <0.01 | <0.01 | <0.01 | |||||||||||||||
| <$20,000 | 19 | 18 | 18 | 17 | 20 | 18 | 23 | 18 | 14 | 16 | 19 | 20 | 15 | 16 | 25 | |||||
| $20,000–$74,999 | 63 | 63 | 64 | 65 | 63 | 62 | 66 | 65 | 59 | 59 | 66 | 65 | 62 | 66 | 62 | |||||
| >$75,000 | 18 | 18 | 18 | 18 | 17 | 20 | 11 | 17 | 27 | 25 | 16 | 14 | 23 | 18 | 14 | |||||
| Lifestyle factors | ||||||||||||||||||||
| PA, MET-h/week d | 10.5 | 8.3 | 9.8 | <0.01 | 8.6 | 9.5 | 10.5 | <0.01 | 7.5 | 9.0 | 11.3 | <0.01 | 11.1 | 9.0 | 7.8 | <0.01 | 11.3 | 9.5 | 7.5 | <0.01 |
| BMI, kg/m2 d | 25.2 | 26.2 | 27.8 | <0.01 | 27.0 | 26.7 | 25.3 | <0.01 | 27.1 | 26.5 | 25.8 | <0.01 | 26.1 | 26.6 | 26.5 | <0.01 | 26.0 | 26.6 | 26.6 | <0.01 |
| Smoking, % | 0.24 | 0.06 | <0.01 | 0.04 | <0.01 | |||||||||||||||
| Never | 54 | 49 | 49 | 54 | 51 | 47 | 51 | 51 | 51 | 47 | 50 | 54 | 46 | 52 | 54 | |||||
| Past | 40 | 43 | 44 | 40 | 43 | 45 | 39 | 43 | 46 | 46 | 43 | 39 | 46 | 423 | 39 | |||||
| Current | 6 | 8 | 6 | 6 | 7 | 8 | 11 | 6 | 3 | 6 | 7 | 7 | 8 | 5 | 7 | |||||
| CHD risk factors | ||||||||||||||||||||
| Family history, % yes | ||||||||||||||||||||
| MI | 57 | 56 | 58 | 0.75 | 59 | 53 | 55 | 0.37 | 56 | 59 | 56 | 0.41 | 58 | 56 | 57 | 0.75 | 57 | 57 | 57 | 0.93 |
| Diabetes | 31 | 32 | 38 | 0.01 | 36 | 31 | 33 | 0.24 | 35 | 36 | 30 | 0.03 | 33 | 36 | 34 | 0.30 | 33 | 38 | 13 | 0.02 |
| Hormone usage | 0.10 | 0.33 | 0.02 | 0.16 | <0.01 | |||||||||||||||
| Current E + P | 14 | 13 | 17 | 13 | 16 | 15 | 13 | 14 | 17 | 17 | 13 | 14 | 17 | 14 | 13 | |||||
| Current E alone | 20 | 23 | 24 | 23 | 25 | 21 | 20 | 25 | 23 | 23 | 24 | 21 | 25 | 25 | 18 | |||||
| Past Users | 17 | 15 | 14 | 16 | 15 | 17 | 17 | 16 | 13 | 14 | 15 | 18 | 15 | 15 | 16 | |||||
| Never Used | 49 | 48 | 45 | 49 | 45 | 48 | 50 | 45 | 47 | 46 | 48 | 47 | 44 | 45 | 52 | |||||
| Hypertension | <0.01 | <0.01 | 0.85 | 0.90 | 0.01 | |||||||||||||||
| Never | 67 | 54 | 55 | 55 | 57 | 63 | 59 | 57 | 57 | 58 | 58 | 59 | 53 | 59 | 62 | |||||
| Untreated | 8 | 11 | 10 | 9 | 10 | 11 | 10 | 10 | 10 | 11 | 9 | 10 | 10 | 10 | 9 | |||||
| Treated | 25 | 36 | 35 | 36 | 34 | 27 | 31 | 33 | 33 | 32 | 33 | 32 | 37 | 31 | 29 | |||||
| Diabetes, % | 6 | 7 | 10 | 0.01 | 11 | 8 | 5 | <0.01 | 10 | 8 | 6 | 0.01 | 6 | 8 | 10 | 0.01 | 8 | 9 | 7 | 0.62 |
| Hysterectomy, % | 39 | 41 | 43 | 0.19 | 43 | 42 | 38 | 0.13 | 42 | 43 | 39 | 0.28 | 39 | 41 | 43 | 0.25 | 40 | 45 | 38 | 0.01 |
| Dietary Factors | ||||||||||||||||||||
| Alc, g/d d | 0.5 | 1.0 | 1.0 | 0.02 | 0.5 | 1.0 | 1.2 | <0.01 | 0.4 | 1.0 | 1.0 | <0.01 | 1.1 | 1.0 | 0.2 | <0.01 | 1.9 | 0.9 | 0.1 | <0.01 |
| Carb % cal d | 52.3 | 51.2 | 52.6 | 0.55 | 50.9 | 52.0 | 53.4 | <0.01 | 50.0 | 52.0 | 55.0 | <0.01 | 54.7 | 52.3 | 49.7 | <0.01 | 52.4 | 52.1 | 52.1 | 0.96 |
| Protein % cal d | 16.7 | 16.6 | 17.0 | 0.06 | 16.9 | 16.7 | 16.6 | 0.10 | 16.3 | 16.8 | 17.2 | <0.01 | 17.2 | 16.6 | 16.5 | <0.01 | 17.0 | 17.0 | 16.3 | <0.01 |
| Energy, kcal/d d | 1481 | 1500 | 1541 | 0.03 | 1499 | 1495 | 1518 | 0.72 | 1558 | 1535 | 1426 | <0.01 | 1524 | 1471 | 1533 | 0.15 | 1483 | 1542 | 1501 | 0.52 |
a Tertiles of plasma phospholipid fatty acids (moL %) b p trend: Test for linear trends were conducted by treating the median value for each quartile of fatty acids as a continuous variable. c The mean (SD) of continuous variables. d The median (IQR) of continuous variables. Abbreviations: Alc (alcohol), BMI (body mass index). Cal (calories), Carb (carbohydrates), CHD (coronary heart disease), E (Estrogen), MI (myocardial infarction), MET-h (metabolic equivalent-hours), MUFA (mono-unsaturated fatty acids), P (Progesterone), PA (physical activity), PUFA (poly-unsaturated fatty acids), SFA (saturated fatty acids), TFA (trans fatty acids), SD (standard deviation), IQR (interquartile range).
Appendix B
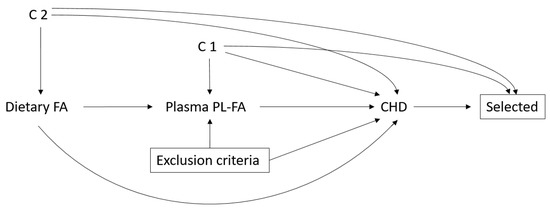
Figure A1.
Hypothesized causal diagram for the matched case-control study. C1 includes age, race/ethnicity, life style factor (BMI, physical activity, and smoking), CHD risk factors (family history of myocardial infarction/diabetes/stroke, medication use, postmenopausal hormone use, self-reported hypertension/diabetes/hypercholesterolemia, and hysterectomy status), and dietary factors (alcohol, percent calories from protein/carbohydrates, and total energy intake). C2 additionally included region, education, and income. A box around a node represents conditioning on that node. Abbreviations: BMI (body mass index), CHD (coronary heart disease), PL-FA (plasma phospholipid fatty acid).
Appendix C
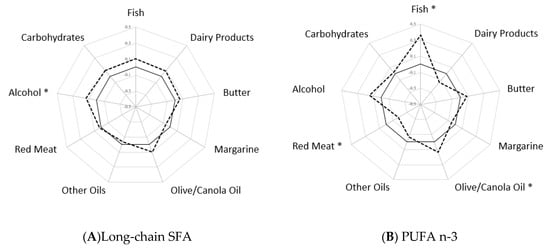
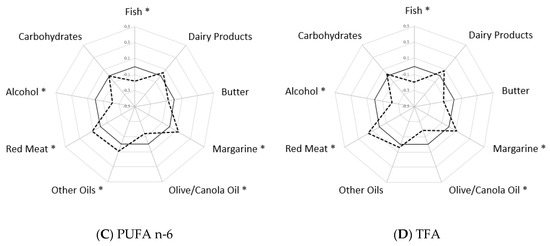
Figure A2.
The adjusted Spearman correlations a between plasma phospholipid fatty acids and select food groups. (A) Correlations between selected food groups and plasma PL long-chain SFA (B) Correlations between selected food groups and plasma PL PUFA n-3 (C) Correlations between selected food groups and plasma PL PUFA n-6 (D) Correlations between selected food groups and plasma PL TFA. a Correlations were adjusted for matching factors (age, race/ethnicity, enrollment date, and hysterectomy status), income, lifestyle factors (physical activity and smoking), and CHD risk factors (family history of myocardial infarction/diabetes, postmenopausal hormone use, and self-reported hypertension/diabetes). Solid line—reference line of 0 Spearman correlations. Dashed line—Spearman correlations among controls. * Significant Spearman correlations with p value < 0.0001. Abbreviations: CHD (coronary heart disease), PL (phospholipid), PUFA (polyunsaturated fatty acids), SFA (saturated fatty acids), TFA (trans fatty acids).
Appendix D

Table A2.
Associations between plasma phospholipid fatty acids (1 moL %) and CHD risk adjusting for different confounders in the matched case-control study (N = 2428).
Table A2.
Associations between plasma phospholipid fatty acids (1 moL %) and CHD risk adjusting for different confounders in the matched case-control study (N = 2428).
| Plasma Phospholipid Fatty Acids | Crude Model a | Fully Adjusted Model b | Parsimonious Model c | Adjusted Model from a Prior Study [15] d |
|---|---|---|---|---|
| SFA | 1.19 (1.11, 1.28) | 1.23 (1.13, 1.34) | 1.20 (1.10, 1.30) | 1.20 (1.08, 1.32) |
| Long-chain SFA | 1.17 (1.09, 1.25) | 1.21 (1.12, 1.32) | 1.18 (1.09, 1.28) | NR |
| Very-long-chain SFA | 1.00 (0.80, 1.26) | 1.00 (0.76, 1.30) | 1.00 (0.77, 1.30) | NR |
| MUFA | 0.96 (0.91, 1.01) | 0.99 (0.93, 1.05) | 0.98 (0.93, 1.04) | 0.97 (0.91, 1.04) |
| PUFA n-3 | 0.89 (0.84, 0.94) | 0.94 (0.88, 0.99) | 0.93 (0.88, 0.99) | 0.89 (0.83, 0.97) |
| PUFA n-6 | 1.03 (0.99, 1.06) | 0.98 (0.95, 1.03) | 1.00 (0.96, 1.03) | 1.02 (0.97, 1.07) |
| TFA | 1.06 (0.84, 1.33) | 0.97 (0.74, 1.27) | 1.01 (0.78, 1.31) | 1.00 (0.81, 1.24) |
a Crude model adjusted for matching factors (age, race/ethnicity, enrollment date, and hysterectomy status). b Fully adjusted model additionally included socio-demographic variables (region, education, and income), lifestyle factors (physical activity, BMI, smoking), and CHD risk factors (family history of MI/diabetes/stroke, medication use, postmenopausal hormone use, and self-reported hypertension/diabetes/hypercholesterolemia), and dietary factors (dietary alcohol intake, percent calories from protein/carbohydrates, and total energy intake). c Parsimonious model included matching factors, income, lifestyle factors (physical activity and smoking), CHD risk factors (family history of myocardial infarction/diabetes, postmenopausal hormone use, and self-reported hypertension/diabetes), and dietary factors (percent calories from protein/carbohydrates, and total energy intake). d Adjusted model from the prior study included matching factors, education, BMI, lifestyle factors (physical activity, BMI, smoking), CHD risk factors (systolic blood pressure, family history of cardiovascular disease/stroke/MI/diabetes, medication use, and postmenopausal hormone use), and dietary factors (carbohydrate, protein, and alcohol intake). Abbreviations: BMI (body mass index), CHD (coronary heart disease), MI (myocardial infarction), MUFA (monounsaturated fatty acids), NR (not reported), PUFA (polyunsaturated fatty acids), SFA (saturated fatty acids), TFA (trans fatty acids).
Appendix E

Table A3.
Associations (1 moL %) between plasma phospholipid fatty acids and CHD risk when adjusting for different anthropometric measures in the matched case-control study (N = 2428).
Table A3.
Associations (1 moL %) between plasma phospholipid fatty acids and CHD risk when adjusting for different anthropometric measures in the matched case-control study (N = 2428).
| Plasma Phospholipid Fatty Acids | BMI Adjusted Model a OR (95% CIs) | Waist Circumference Adjusted Model a OR (95% CIs) | Waist: Hip Adjusted Model a OR (95% CIs) | BMI Change Adjusted Model a,b OR (95% CIs) |
|---|---|---|---|---|
| SFA | 1.20 (1.10, 1.30) | 1.17 (1.08, 1.28) | 1.17 (1.08, 1.28) | 1.26 (1.13, 1.39) |
| Long-chain SFA | 1.18 (1.09, 1.28) | 1.16 (1.07, 1.26) | 1.16 (1.07, 1.26) | 1.23 (1.12, 1.36) |
| Very-long-chain SFA | 1.00 (0.77, 1.30) | 1.01 (0.78, 1.32) | 0.99 (0.76, 1.29) | 1.04 (0.75, 1.44) |
| MUFA | 0.99 (0.93, 1.05) | 0.99 (0.94, 1.05) | 1.00 (0.94, 1.05) | 0.97 (0.90, 1.04) |
| PUFA n-3 | 0.93 (0.88, 0.99) | 0.93 (0.88, 0.99) | 0.94 (0.88, 1.00) | 0.93 (0.87, 1.00) |
| PUFA n-6 | 1.00 (0.96, 1.03) | 1.00 (0.96, 1.04) | 0.99 (0.96, 1.03) | 0.99 (0.95, 1.04) |
| TFA | 1.00 (0.78, 1.30) | 1.04 (0.80, 1.35) | 1.00 (0.77, 1.31) | 1.01 (0.74, 1.39) |
a Multivariable model included matching factors (age, race/ethnicity, enrollment date, and hysterectomy status), income), lifestyle factors (physical activity and smoking), CHD risk factors (family history of myocardial infarction /diabetes, postmenopausal hormone use, and self-reported hypertension /diabetes), and dietary factors (percent calories from protein/carbohydrates, and total energy intake). b BMI change indicated the difference between two BMI measures, one at baseline and one at year 3. Abbreviations: BMI (body mass index), CHD (coronary heart disease), CIs (confidence intervals), MUFA (mono-unsaturated fatty acids), PUFA (polyunsaturated fatty acids), OR (odds ratio), SFA (saturated fatty acids), TFA (trans fatty acids).
Appendix F

Table A4.
Odds ratios a (95% CIs) of CHD associated with 1 moL % substitutions between plasma phospholipid fatty acid groups stratified by physical activity levels in the matched case-control study (N = 2428).
Table A4.
Odds ratios a (95% CIs) of CHD associated with 1 moL % substitutions between plasma phospholipid fatty acid groups stratified by physical activity levels in the matched case-control study (N = 2428).
| Plasma Phospholipid Fatty Acids | Overall | Physically Active | Physically Inactive |
|---|---|---|---|
| PUFA n-6 ↓ PUFA n-3 ↑ (1 moL %) | 0.90 (0.84, 0.96) | 0.95 (0.84, 1.06) | 0.88 (0.76, 1.02) |
| TFA ↓ PUFA n-3 ↑ (1 moL %) | 0.74 (0.56, 0.99) | 0.88 (0.50, 1.54) | 1.04 (0.60, 1.82) |
| TFA ↓ PUFA n-6↑ (1 moL %) | 0.82 (0.61, 1.11) | 0.92 (0.52, 1.65) | 1.19 (0.67, 2.11) |
a Multivariable model adjusted for matching factors (age, race/ethnicity, enrollment date, and hysterectomy status), income, lifestyle factors (physical activity and smoking), CHD risk factors (family history of myocardial infarction/diabetes, postmenopausal hormone use, and self-reported hypertension/diabetes), and dietary factors (percent calories from protein/carbohydrates and total energy intake). Abbreviations: CHD (coronary heart disease), CIs (confidence intervals), PUFA (polyunsaturated fatty acids), TFA (trans fatty acids).
Appendix G

Table A5.
Multivariable adjusted associations a (1 moL %) between plasma phospholipid fatty acids and CHD risk among participants with complete information versus using multiple imputation in the matched case-control study.
Table A5.
Multivariable adjusted associations a (1 moL %) between plasma phospholipid fatty acids and CHD risk among participants with complete information versus using multiple imputation in the matched case-control study.
| Plasma Phospholipid Fatty Acids | Complete Analysis (N = 2181) | Multiple Imputation (N = 2428) |
|---|---|---|
| OR (95% CIs) | OR (95% CIs) | |
| SFA | 1.21 (1.08, 1.35) | 1.20 (1.10, 1.30) |
| Long-chain SFA b | 1.19 (1.07, 1.33) | 1.18 (1.09, 1.28) |
| Very-long-chain SFA c | 0.99 (0.70, 1.41) | 1.00 (0.77, 1.30) |
| MUFA | 0.98 (0.91, 1.06) | 0.98 (0.93, 1.04) |
| PUFA n-3 | 0.93 (0.86, 1.01) | 0.93 (0.88, 0.99) |
| PUFA n-6 | 1.00 (0.95, 1.05) | 1.00 (0.96, 1.03) |
| TFA | 0.97 (0.69, 1.38) | 1.01 (0.78, 1.31) |
a Multivariable model adjusted for matching factors (age, race/ethnicity, enrollment date, and hysterectomy status), income, lifestyle factors (physical activity and smoking), CHD risk factors (family history of myocardial infarction/diabetes, postmenopausal hormone use, and self-reported hypertension/diabetes), and dietary factors (percent calories from protein/carbohydrates and total energy intake). b Long-chain SFA included lauric acid (12:0), myristic acid (14:0), pentadecylic acid (15:0), palmitic acid (16:0), and stearic acid (18:0). c Very-long-chain SFA included arachidic acid (20:0), behenic acid (22:0), and lignoceric acid (24:0). Abbreviations: CHD (coronary heart disease), CIs (confidence intervals), MUFA (mono-unsaturated fatty acids), PUFA (polyunsaturated fatty acids), OR (odds ratio), SFA (saturated fatty acids), TFA (trans fatty acids).
Appendix H

Table A6.
Odds ratios a (95% CIs) of CHD associated with 1 moL % substitutions between plasma phospholipid fatty acid groups among participants with complete information versus using multiple imputation in the matched case-control study.
Table A6.
Odds ratios a (95% CIs) of CHD associated with 1 moL % substitutions between plasma phospholipid fatty acid groups among participants with complete information versus using multiple imputation in the matched case-control study.
| Plasma Phospholipid Fatty Acids | Complete Analysis (N = 2181) | Multiple Imputation (N = 2428) |
|---|---|---|
| OR (95% CIs) | OR (95% CIs) | |
| PUFA n-6 ↓ PUFA n-3 ↑ (1 moL %) | 0.90 (0.85, 0.97) | 0.90 (0.84, 0.96) |
| TFA ↓ PUFA n-3 ↑ (1 moL %) | 0.75 (0.57, 1.00) | 0.74 (0.56, 0.99) |
| TFA ↓ PUFA n-6↑ (1 moL %) | 0.85 (0.62, 1.15) | 0.82 (0.61, 1.11) |
a Multivariable model adjusted for matching factors (age, race/ethnicity, enrollment date, and hysterectomy status), income, lifestyle factors (physical activity and smoking), CHD risk factors (family history of myocardial infarction/diabetes, postmenopausal hormone use, and self-reported hypertension/diabetes), and dietary factors (percent calories from protein/carbohydrates and total energy intake).Abbreviations: CHD (coronary heart disease), CIs (confidence intervals), PUFA (polyunsaturated fatty acids), OR (odds ratio), TFA (trans fatty acids).
References
- Li, Z.; Otvos, J.D.; Lamon-Fava, S.; Carrasco, W.V.; Lichtenstein, A.H.; McNamara, J.R.; Ordovás, J.M.; Schaefer, E.J. Men and women differ in lipoprotein response to dietary saturated fat and cholesterol restriction. J. Nutr. 2003, 133, 3428–3433. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Zamora, D.; Leelarthaepin, B.; Majchrzak-Hong, S.F.; Faurot, K.R.; Suchindran, C.M.; Ringel, A.; Davis, J.M.; Hibbeln, J.R. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: Evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013, 346, e8707. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Bälter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Mozaffarian, D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Katan, M.B. Effect of a diet enriched with monounsaturated or polyunsaturated fatty acids on levels of low-density and high-density lipoprotein cholesterol in healthy women and men. N. Engl. J. Med. 1989, 321, 436–441. [Google Scholar] [CrossRef]
- Appel, L.J.; Sacks, F.M.; Carey, V.J.; Obarzanek, E.; Swain, J.F.; Miller, E.R.; Conlin, P.R.; Erlinger, T.P.; Rosner, B.A.; Laranjo, N.M.; et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the OmniHeart randomized trial. JAMA 2005, 294, 2455–2464. [Google Scholar] [CrossRef]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Miller, N.H.; Hubbard, V.S.; Lee, I.-M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2960–2984. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias: The task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 253, 281–344. [Google Scholar]
- De Oliveira Otto, M.C.; Wu, J.H.; Baylin, A.; Vaidya, D.; Rich, S.S.; Tsai, M.Y.; Jacobs, D.R.; Mozaffarian, D. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2013, 2, e000506. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J. Diet-heart: A problematic revisit. Am. J. Clin. Nutr. 2010, 91, 497–499. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Otto, M.C.; Nettleton, J.A.; Lemaitre, R.N.; Steffen, L.M.; Kromhout, D.; Rich, S.S.; Tsai, M.Y.; Jacobs, D.R.; Mozaffarian, D. Biomarkers of dairy fatty acids and risk of cardiovascular disease in the multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. 2013, 2, e000092. [Google Scholar] [CrossRef] [PubMed]
- Matthan, N.R.; Ooi, E.M.; Van Horn, L.; Neuhouser, M.L.; Woodman, R.; Lichtenstein, A.H. Plasma Phospholipid Fatty Acid Biomarkers of Dietary Fat Quality and Endogenous Metabolism Predict Coronary Heart Disease Risk: A Nested Case-Control Study Within the Women’s Health Initiative Observational Study. J. Am. Heart Assoc. 2014, 3, e000764. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef] [PubMed]
- Arab, L. Biomarkers of fat and fatty acid intake. J. Nutr. 2003, 133 (Suppl. 3), 925S–932S. [Google Scholar] [CrossRef]
- Saadatian-Elahi, M.; Slimani, N.; Chajes, V.; Jenab, M.; Goudable, J.; Biessy, C.; Ferrari, P.; Byrnes, G.; Autier, P.; Peeters, P.H.; et al. Plasma phospholipid fatty acid profiles and their association with food intakes: Results from a cross-sectional study within the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2009, 89, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, V.K.; Pu, S.; Jenkins, D.A.; Lamarche, B.; Kris-Etherton, P.M.; West, S.G.; Fleming, J.A.; Liu, X.; McCrea, C.E.; Jones, P.J. Plasma fatty acid changes following consumption of dietary oils containing n-3, n-6, and n-9 fatty acids at different proportions: Preliminary findings of the Canola Oil Multicenter Intervention Trial (COMIT). Trials 2014, 15, 136. [Google Scholar] [CrossRef] [PubMed]
- The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control. Clin. Trials 1998, 19, 61–109. [Google Scholar] [CrossRef]
- Hays, J.; Hunt, J.R.; Hubbell, F.A.; Anderson, G.L.; Limacher, M.; Allen, C.; Rossouw, J.E. The Women’s Health Initiative recruitment methods and results. Ann. Epidemiol. 2003, 13, S18–S77. [Google Scholar] [CrossRef]
- Curb, J.D.; McTiernan, A.; Heckbert, S.R.; Kooperberg, C.; Stanford, J.; Nevitt, M.; Johnson, K.C.; Proulx-Burns, L.; Pastore, L.; Criqui, M.; et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann. Epidemiol. 2003, 13, S122–S128. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Matthan, N.R.; Jalbert, S.M.; Resteghini, N.A.; Schaefer, E.J.; Ausman, L.M. Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am. J. Clin. Nutr. 2006, 84, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Stampfer, M.J.; Rimm, E.; Ascherio, A.; Rosner, B.A.; Spiegelman, D.; Willett, W.C. Dietary fat and coronary heart disease: A comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am. J. Epidemiol. 1999, 149, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Pottala, J.V.; Sands, S.A.; Jones, P.G. Comparison of the effects of fish and fish-oil capsules on the n–3 fatty acid content of blood cells and plasma phospholipids. Am. J. Clin. Nutr. 2007, 86, 1621–1625. [Google Scholar] [CrossRef][Green Version]
- Khaw, K.-T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: The EPIC-Norfolk prospective study. PLoS Med. 2012, 9, e1001255. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Rossouw, J.E.; Roberts, M.B.; Liu, S.; Johnson, K.C.; Shikany, J.M.; Manson, J.E.; Tinker, L.F.; Eaton, C.B. Theoretical effects of substituting butter with margarine on risk of cardiovascular disease. Epidemiology 2017, 28, 145. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Fong, J.; Bemert, J.T., Jr.; Browner, W.S. Relation of smoking and alcohol consumption to serum fatty acids. Am. J. Epidemiol. 1996, 144, 325–334. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef]
- Bursac, Z.; Gauss, C.H.; Williams, D.K.; Hosmer, D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008, 3, 17. [Google Scholar] [CrossRef]
- Emken, E.A.; Adlof, R.O.; Gulley, R.M. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim. Biophys. Acta 1994, 1213, 277–288. [Google Scholar] [CrossRef]
- Resche-Rigon, M.; White, I.R. Multiple imputation by chained equations for systematically and sporadically missing multilevel data. Stat. Methods Med. Res. 2018, 27, 1634–1649. [Google Scholar] [CrossRef] [PubMed]
- Flock, M.R.; Kris-Etherton, P.M. Diverse physiological effects of long-chain saturated fatty acids: Implications for cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; King, I.B.; Sotoodehnia, N.; Knopp, R.H.; Mozaffarian, D.; McKnight, B.; Rice, K.; Friedlander, Y.; Lumley, T.S.; Raghunathan, T.E.; et al. Endogenous red blood cell membrane fatty acids and sudden cardiac arrest. Metab. Clin. Exp. 2010, 59, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Shipley, M.; Armitage, J.; Collins, R.; Harris, W. Plasma phospholipid fatty acids and CHD in older men: Whitehall study of London civil servants. Br. J. Nutr. 2009, 102, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Lemaitre, R.N.; Imamura, F.; King, I.B.; Song, X.; Spiegelman, D.; Siscovick, D.S.; Mozaffarian, D. Fatty acids in the de novo lipogenesis pathway and risk of coronary heart disease: The Cardiovascular Health Study. Am. J. Clin. Nutr. 2011, 94, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Folsom, A.R.; Eckfeldt, J.H. Plasma fatty acid composition and incidence of coronary heart disease in middle aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Nutr. Metab. Cardiovasc. Dis. 2003, 13, 256–266. [Google Scholar] [CrossRef]
- Malik, V.S.; Chiuve, S.E.; Campos, H.; Rimm, E.B.; Mozaffarian, D.; Hu, F.B.; Sun, Q. Circulating very-long chain saturated fatty acids and incident coronary heart disease in US men and women. Circulation 2015, 132, 260–268. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Fretts, A.M.; Sitlani, C.M.; Biggs, M.L.; Mukamal, K.; King, I.B.; Song, X.; Djoussé, L.; Siscovick, D.S.; McKnight, B.; et al. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: The Cardiovascular Health Study. Am. J. Clin. Nutr. 2015, 101, 1047–1054. [Google Scholar] [CrossRef]
- Kihara, A. Very long-chain fatty acids: Elongation, physiology and related disorders. J. Biochem. 2012, 152, 387–395. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cho, Y.; Shin, M.J. Dietary Very Long Chain Saturated Fatty Acids and Metabolic Factors: Findings from the Korea National Health and Nutrition Examination Survey 2013. Clin. Nutr. Res. 2015, 4, 182–189. [Google Scholar] [CrossRef]
- Demaison, L.; Moreau, D. Dietary n-3 polyunsaturated fatty acids and coronary heart disease-related mortality: A possible mechanism of action. Cell. Mol. Life Sci. 2002, 59, 463–477. [Google Scholar] [CrossRef]
- Bendsen, N.T.; Christensen, R.; Bartels, E.M.; Astrup, A. Consumption of industrial and ruminant trans fatty acids and risk of coronary heart disease: A systematic review and meta-analysis of cohort studies. Eur. J. Clin. Nutr. 2011, 65, 773–783. [Google Scholar] [CrossRef]
- Gebauer, S.K.; Chardigny, J.-M.; Jakobsen, M.U.; Lamarche, B.; Lock, A.L.; Proctor, S.D.; Baer, D.J. Effects of ruminant trans fatty acids on cardiovascular disease and cancer: A comprehensive review of epidemiological, clinical, and mechanistic studies. Adv. Nutr. 2011, 2, 332–354. [Google Scholar] [CrossRef]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef]
- Warensjo Lemming, E.; Nalsen, C.; Becker, W.; Ridefelt, P.; Mattisson, I.; Lindroos, A.K. Relative validation of the dietary intake of fatty acids among adults in the Swedish National Dietary Survey using plasma phospholipid fatty acid composition. J. Nutr. Sci. 2015, 4, e25. [Google Scholar] [CrossRef]
- Kobayashi, M.; Sasaki, S.; Kawabata, T.; Hasegawa, K.; Akabane, M.; Tsugane, S. Single measurement of serum phospholipid fatty acid as a biomarker of specific fatty acid intake in middle-aged Japanese men. Eur. J. Clin. Nutr. 2001, 55, 643–650. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Nissensohn, M.; Overby, N.C.; Fekete, K. Dietary methods and biomarkers of omega 3 fatty acids: A systematic review. Bri. J. Nutr. 2012, 107 (Suppl. 2), S64–S76. [Google Scholar] [CrossRef]
- Fusconi, E.; Pala, V.; Riboli, E.; Vineis, P.; Sacerdote, C.; Del Pezzo, M.; De Magistris, M.S.; Palli, D.; Masala, G.; Sieri, S.; et al. Relationship between plasma fatty acid composition and diet over previous years in the Italian centers of the European Prospective Investigation into Cancer and Nutrition (EPIC). Tumori J. 2003, 89, 624–635. [Google Scholar] [CrossRef]
- US Department of Agriculture. National Nutrient Database for Standard Reference, Release 28; US Department of Agriculture: Washington, DC, USA, 2015.
- Frost, H.R.; Andrew, A.S.; Karagas, M.R.; Moore, J.H. A screening-testing approach for detecting gene-environment interactions using sequential penalized and unpenalized multiple logistic regression. In Proceedings of the Pacific Symposium on Biocomputing, Waimea, HI, USA, 4–8 January 2015; pp. 183–194. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).