Diversity of Gut Microbiota Affecting Serum Level of Undercarboxylated Osteocalcin in Patients with Crohn’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Participants
2.3. Analysis of Gut Microbiota (Meta-16S rRNA Gene Sequence Analysis)
2.4. Measurement of Vit K
2.5. Endpoints and Examination Items
2.6. Statistical Considerations
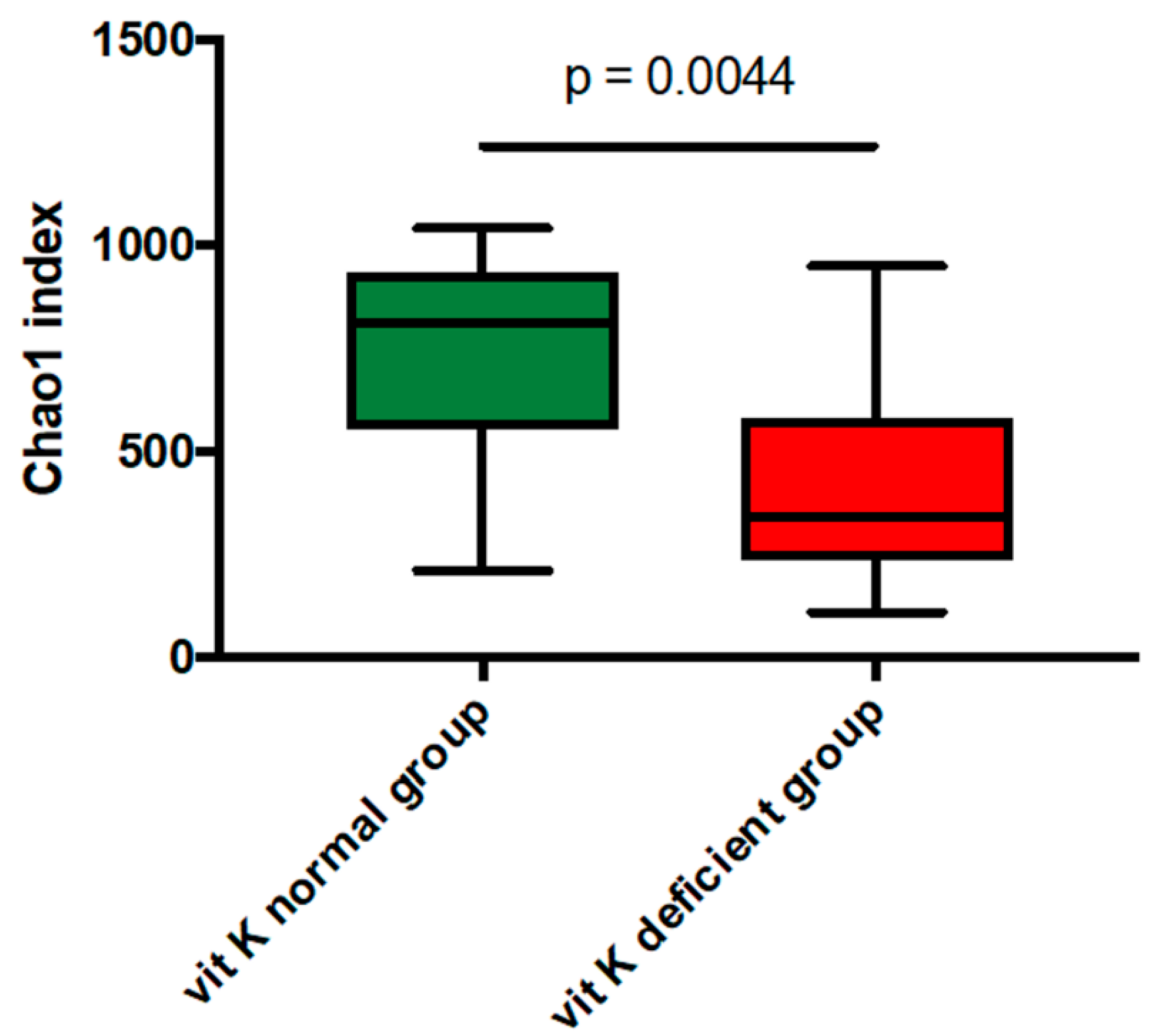
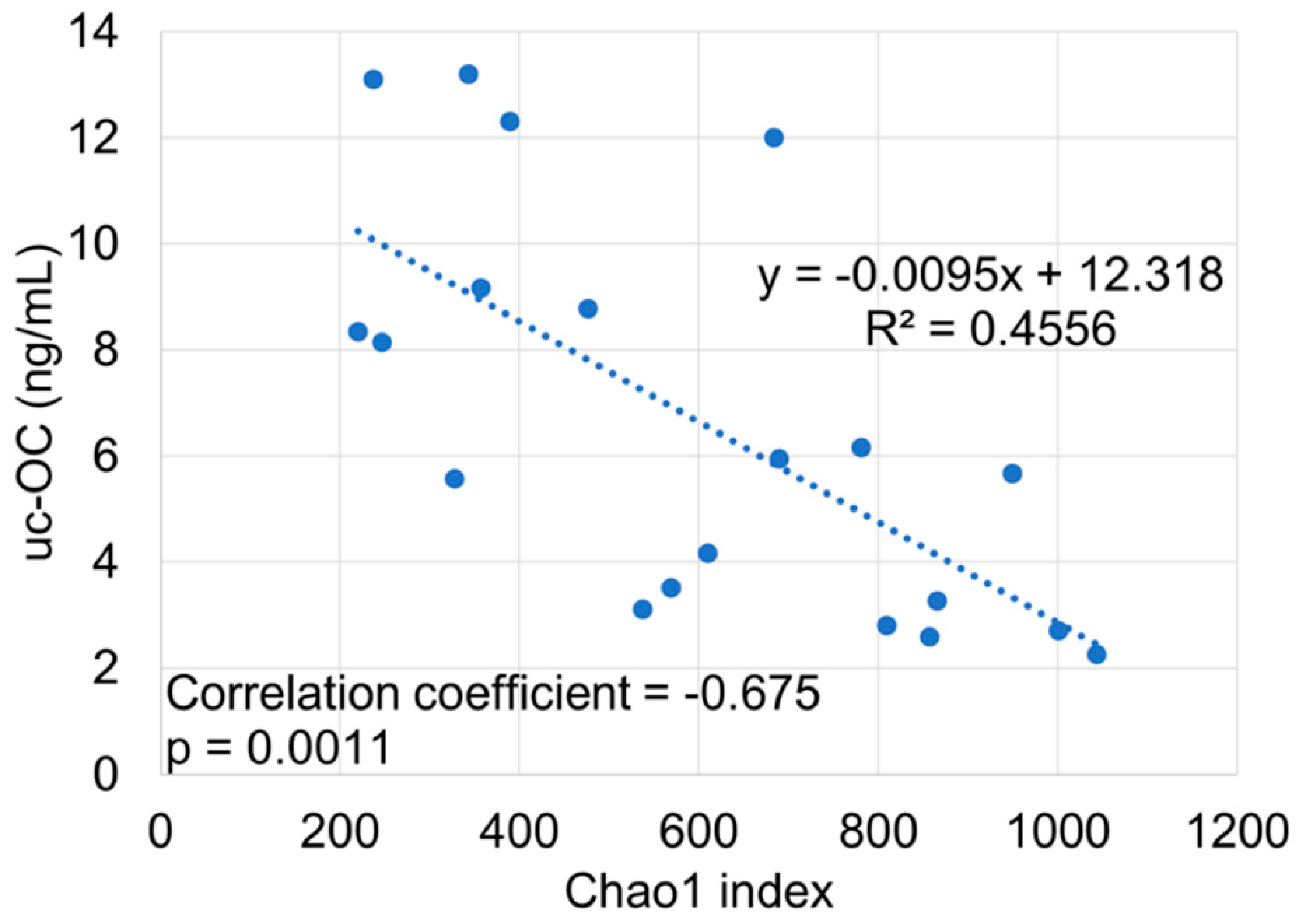
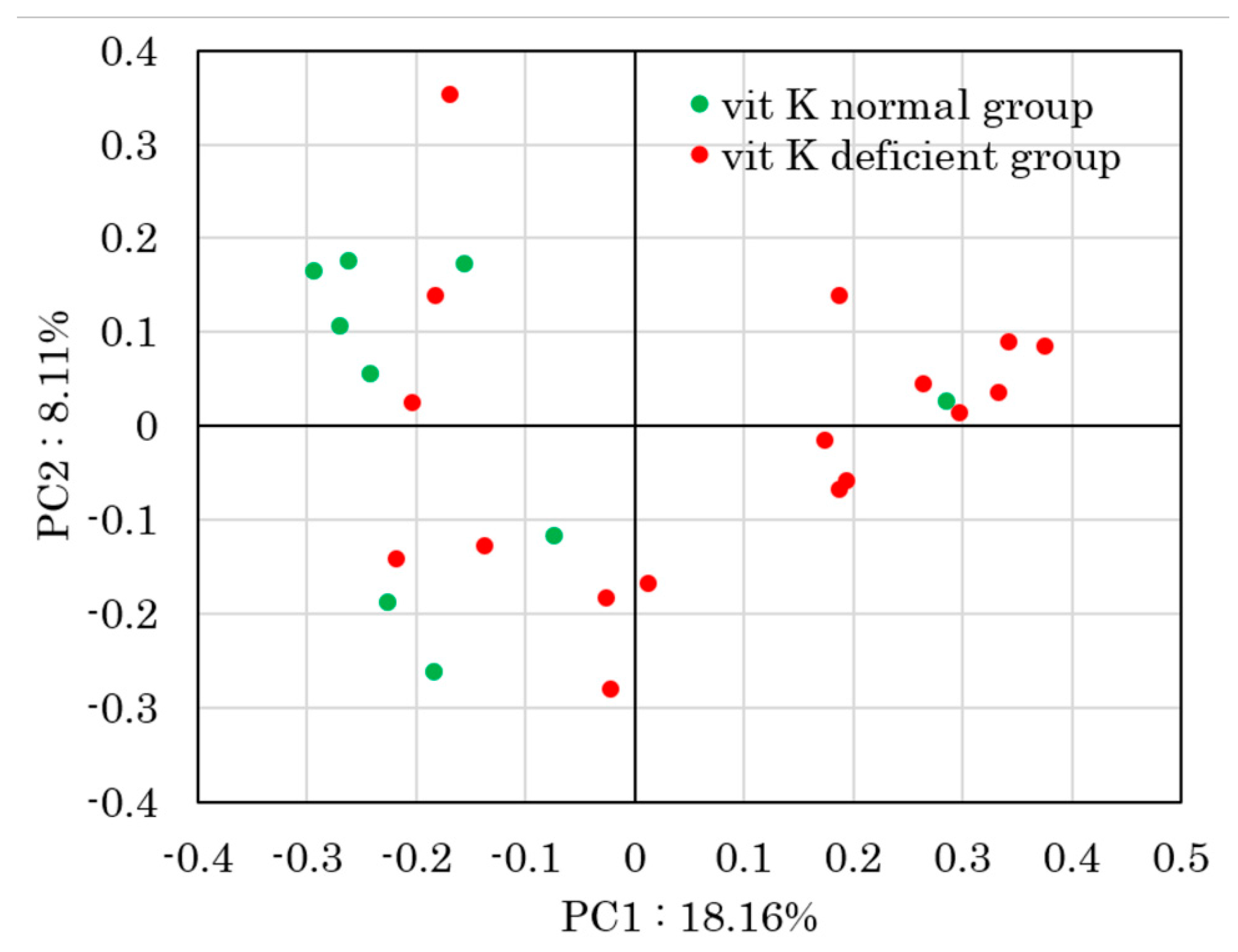
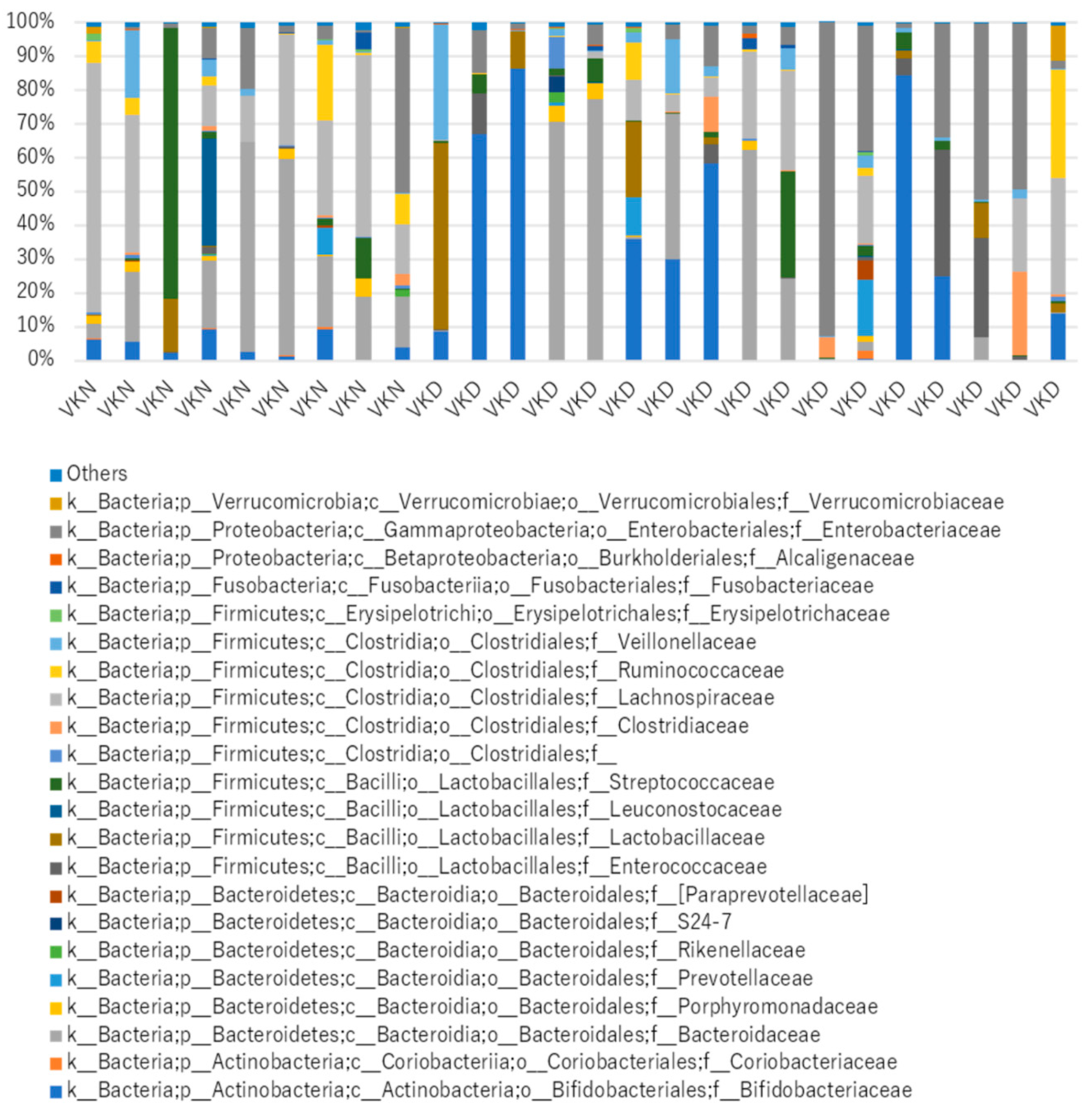
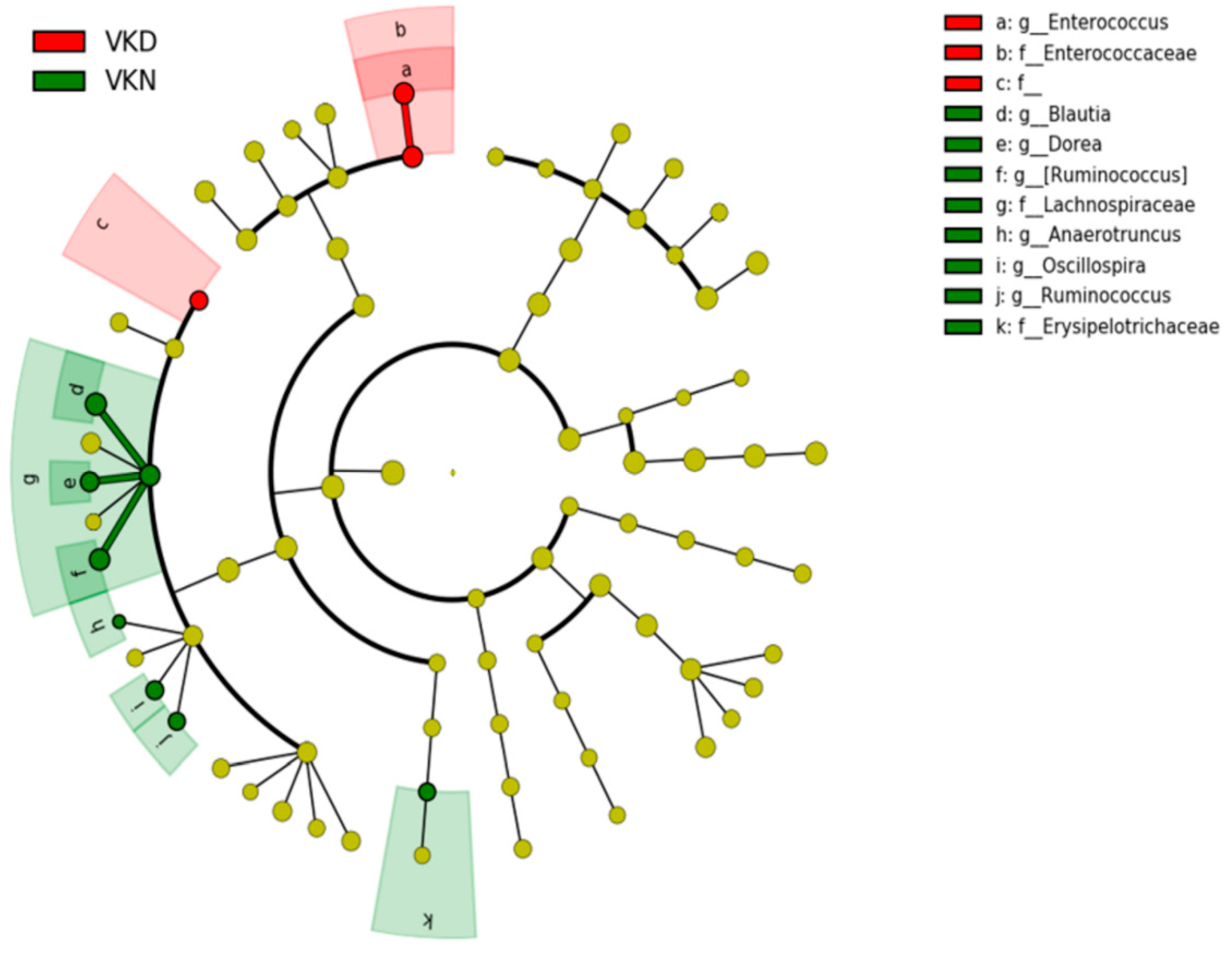
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Furie, B.; Bouchard, B.A.; Furie, B.C. Vitamin K-dependent biosynthesis of gamma-carboxyglutamic acid. Blood 1999, 93, 1798–1808. [Google Scholar] [PubMed]
- Fu, X.; Harshman, S.G.; Shen, X.; Haytowitz, D.B.; Karl, J.P.; Wolfe, B.E.; Booth, S.L. Multiple Vitamin K Forms Exist in Dairy Foods. Curr. Dev. Nutr. 2017, 1, e000638. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Vermeer, C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis 2000, 30, 298–307. [Google Scholar] [PubMed]
- Mathers, J.C.; Fernandez, F.; Hill, M.J.; McCarthy, P.T.; Shearer, M.J.; Oxley, A. Dietary modification of potential vitamin K supply from enteric bacterial menaquinones in rats. Br. J. Nutr. 1990, 63, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Dam, H. The antihæmorrhagic vitamin of the chick: Occurrence and chemical nature. Nature 1935, 135, 652–653. [Google Scholar] [CrossRef]
- Vermeer, C. γ-Carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase. Biochem. J. 1990, 266, 625–636. [Google Scholar] [CrossRef]
- Bouckaert, J.H.; Said, A.H. Fracture Healing by Vitamin K. Nature 1960, 185, 849. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, J.; Sato, Y. Menatetrenone for the treatment of osteoporosis. Expert Opin. Pharmacother. 2013, 14, 449–458. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Vermeer, C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim. Biophys. Acta 2002, 1570, 27–32. [Google Scholar] [CrossRef]
- Kuwabara, A.; Tanaka, K.; Tsugawa, N.; Nakase, H.; Tsuji, H.; Shide, K.; Kamao, M.; Chiba, T.; Inagaki, N.; Okano, T.; et al. High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporos. Int. 2009, 20, 935–942. [Google Scholar] [CrossRef]
- O’Connor, E.M.; Grealy, G.; McCarthy, J.; Desmond, A.; Craig, O.; Shanahan, F.; Cashman, K.D. Effect of phylloquinone (vitamin K1) supplementation for 12 months on the indices of vitamin K status and bone health in adult patients with Crohn’s disease. Br. J. Nutr. 2014, 112, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Zhang, B.; Gu, M.; Chen, C.; Zhang, Q.; Zhang, G.; Cao, X. Vitamin K intake and the risk of fractures: A meta-analysis. Medicine 2017, 96, e6725. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.A.; Haney, B.B.; Stone, H.H. Gastrointestinal bleeding due to vitamin K deficiency in patients on parenteral cefamandole. Lancet 1980, 1, 39–40. [Google Scholar] [CrossRef]
- Shearer, M.J.; Bechtold, H.; Andrassy, K.; Koderisch, J.; McCarthy, P.T.; Trenk, D.; Jähnchen, E.; Ritz, E. Mechanism of cephalosporin-induced hypoprothrombinemia: Relation to cephalosporin side chain, vitamin K metabolism, and vitamin K status. J. Clin. Pharmacol. 1988, 28, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.V.; Deshmukh, C.T. A study of Vitamin K status in children on prolonged antibiotic therapy. Indian Pediatr. 2003, 40, 36–40. [Google Scholar] [PubMed]
- Komai, M.; Shirakawa, H.; Kimura, S. Newly developed model for vitamin K deficiency in germfree mice. Int. J. Vitam. Nutr. Res. 1988, 58, 55–59. [Google Scholar]
- Allen, A.M.; Hansen, C.T.; Moore, T.D.; Knapka, J.; Ediger, R.D.; Long, P.H. Hemorrhagic cardiomyopathy and hemothorax in vitamin K deficient mice. Toxicol. Pathol. 1991, 19, 589–596. [Google Scholar] [CrossRef]
- Sjögren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Bäckhed, F.; Ohlsson, C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012, 27, 1357–1367. [Google Scholar] [CrossRef]
- Karl, J.P.; Meydani, M.; Barnett, J.B.; Vanegas, S.M.; Barger, K.; Fu, X.; Goldin, B.; Kane, A.; Rasmussen, H.; Vangay, P.; et al. Fecal concentrations of bacterially derived vitamin K forms are associated with gut microbiota composition but not plasma or fecal cytokine concentrations in healthy adults. Am. J. Clin. Nutr. 2017, 106, 1052–1061. [Google Scholar] [CrossRef]
- Beulens, J.W.; Booth, S.L.; van den Heuvel, E.G.; Stoecklin, E.; Baka, A.; Vermeer, C. The role of menaquinones (vitamin K₂) in human health. Br. J. Nutr. 2013, 110, 1357–1368. [Google Scholar] [CrossRef]
- Sato, T.; Schurgers, L.J.; Uenishi, K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutr. J. 2012, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Suttie, J.W.; Mummah-Schendel, L.L.; Shah, D.V.; Lyle, B.J.; Greger, J.L. Vitamin K deficiency from dietary vitamin K restriction in humans. Am. J. Clin. Nutr. 1988, 47, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Schoon, E.J.; Müller, M.C.; Vermeer, C.; Schurgers, L.J.; Brummer, R.J.; Stockbrügger, R.W. Low serum and bone vitamin K status in patients with longstanding Crohn’s disease: Another pathogenetic factor of osteoporosis in Crohn’s disease? Gut 2001, 48, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Iijima, H.; Egawa, S.; Shinzaki, S.; Kondo, J.; Inoue, T.; Hayashi, Y.; Ying, J.; Mukai, A.; Akasaka, T.; et al. Association of vitamin K deficiency with bone metabolism and clinical disease activity in inflammatory bowel disease. Nutrition 2011, 27, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Krasinski, S.D.; Russell, R.M.; Furie, B.C.; Kruger, S.F.; Jacques, P.F.; Furie, B. The prevalence of vitamin K deficiency in chronic gastrointestinal disorders. Am. J. Clin. Nutr. 1985, 41, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; Judd, D.; Crawley, E.O.; Evans, W.D.; Evans, C.; Church, H.A.; Reid, E.M.; Rhodes, J. Osteoporosis in patients with inflammatory bowel disease. Gut 1987, 28, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Bartram, S.A.; Peaston, R.T.; Rawlings, D.J.; Walshaw, D.; Francis, R.M.; Thompson, N.P. Mutifactorial analysis of risk factors for reduced bone mineral density in patients with Crohn’s disease. World J. Gastroenterol. 2006, 12, 5680–5686. [Google Scholar] [CrossRef]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Gao, W.; Wang, B.; Zhao, H.; Zeng, Y.; Ji, Y.; Hao, D. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ 2017, 5, e3450. [Google Scholar] [CrossRef]
- Booth, S.L.; Martini, L.; Peterson, J.W.; Saltzman, E.; Dallal, G.E.; Wood, R.J. Dietary phylloquinone depletion and repletion in older women. J. Nutr. 2003, 133, 2565–2569. [Google Scholar] [CrossRef]
- Iwamoto, J.; Sato, Y.; Takeda, T.; Matsumoto, H. High-dose vitamin K supplementation reduces fracture incidence in postmenopausal women: A review of the literature. Nutr. Res. 2009, 29, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Yamaguchi, T.; Nawata, K.; Takaoka, S.; Sugimoto, T. Relationships between undercarboxylated osteocalcin and vitamin K intakes, bone turnover, and bone mineral density in healthy women. Clin. Nutr. 2010, 29, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Urano, A.; Hotta, M.; Ohwada, R.; Araki, M. Vitamin K deficiency evaluated by serum levels of undercarboxylated osteocalcin in patients with anorexia nervosa with bone loss. Clin. Nutr. 2015, 34, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Vergnaud, P.; Garnero, P.; Meunier, P.J.; Bréart, G.; Kamihagi, K.; Delmas, P.D. Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: The EPIDOS Study. J. Clin. Endocrinol. Metab. 1997, 82, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P.; Chapuy, M.C.; Meunier, P.J.; Delmas, P.D. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J. Clin. Investig. 1993, 91, 1769–1774. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Peacock, M. Age-related changes in serum undercarboxylated osteocalcin and its relationships with bone density, bone quality, and hip fracture. Calcif. Tissue Int. 1998, 62, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Duggan, P.; O’Brien, M.; Kiely, M.; McCarthy, J.; Shanahan, F.; Cashman, K.D. Vitamin K status in patients with Crohn’s disease and relationship to bone turnover. Am. J. Gastroenterol. 2004, 99, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.S.; Touyama, M.; Yamada, S.; Yamazaki, T.; Benno, Y. Alteration of a human intestinal microbiota under extreme life environment in the Antarctica. Biol. Pharm. Bull. 2014, 37, 1899–1906. [Google Scholar] [CrossRef][Green Version]
- Hayashi, A.; Mikami, Y.; Miyamoto, K.; Kamada, N.; Sato, T.; Mizuno, S.; Naganuma, M.; Teratani, T.; Aoki, R.; Fukuda, S.; et al. Intestinal Dysbiosis and Biotin Deprivation Induce Alopecia through Overgrowth of Lactobacillus murinus in Mice. Cell Rep. 2017, 20, 1513–1524. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, M.; Aoki, C.; Yamazaki, N.; Ito, Y.; Tsugawa, N.; Suhara, Y.; Okano, T. Clinical assessment of undercarboxylated osteocalcin measurement in serum using an electrochemiluminescence immunoassay: Establishments of cut-off value to determine vitamin K insufficiency in bone and to predict fracture leading to clinical use of vitamin K2. Jpn. J. Med. Pharm. Sci. 2007, 57, 537–546. (In Japanese) [Google Scholar]
- Van Horn, D.J.; Wolf, C.R.; Colman, D.R.; Jiang, X.; Kohler, T.J.; McKnight, D.M.; Stanish, L.F.; Yazzie, T.; Takacs-Vesbach, C.D. Patterns of bacterial biodiversity in the glacial meltwater streams of the McMurdo Dry Valleys, Antarctica. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Allegretti, J.; Eysenbach, L.M.; El-Nachef, N.; Fischer, M.; Kelly, C.; Kassam, Z. The Current Landscape and Lessons from Fecal Microbiota Transplantation for Inflammatory Bowel Disease: Past, Present, and Future. Inflamm. Bowel Dis. 2017, 23, 1710–1717. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Schirmer, M.; Franzosa, E.A.; Lloyd-Price, J.; McIver, L.J.; Schwager, R.; Poon, T.W.; Ananthakrishnan, A.N.; Andrews, E.; Barron, G.; Lake, K.; et al. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat. Microbiol. 2018, 3, 337–346. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Kuzuoka, H.; Tsujikawa, T.; Nakamura, S.; Hirai, F.; Suzuki, Y.; Matsui, T.; Fujiyama, Y.; Matsumoto, T. Multicenter analysis of fecal microbiota profiles in Japanese patients with Crohn’s disease. J. Gastroenterol. 2012, 47, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef] [PubMed]
- Mottawea, W.; Chiang, C.K.; Mühlbauer, M.; Starr, A.E.; Butcher, J.; Abujamel, T.; Deeke, S.A.; Brandel, A.; Zhou, H.; Shokralla, S.; et al. Altered intestinal microbiota-host mitochondria crosstalk in new onset Crohn’s disease. Nat. Commun. 2016, 7, 13419. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef] [PubMed]
- Gundberg, C.M.; Nieman, S.D.; Abrams, S.; Rosen, H. Vitamin K status and bone health: An analysis of methods for determination of undercarboxylated osteocalcin. J. Clin. Endocrinol. Metab. 1998, 83, 3258–3266. [Google Scholar] [CrossRef]
- Shea, M.K.; Booth, S.L.; Massaro, J.M.; Jacques, P.F.; D’Agostino, R.B., Sr.; Dawson-Hughes, B.; Ordovas, J.M.; O’Donnell, C.J.; Kathiresan, S.; Keaney, J.F., Jr.; et al. Vitamin K and vitamin D status: Associations with inflammatory markers in the Framingham Offspring Study. Am. J. Epidemiol. 2008, 167, 313–320. [Google Scholar] [CrossRef]
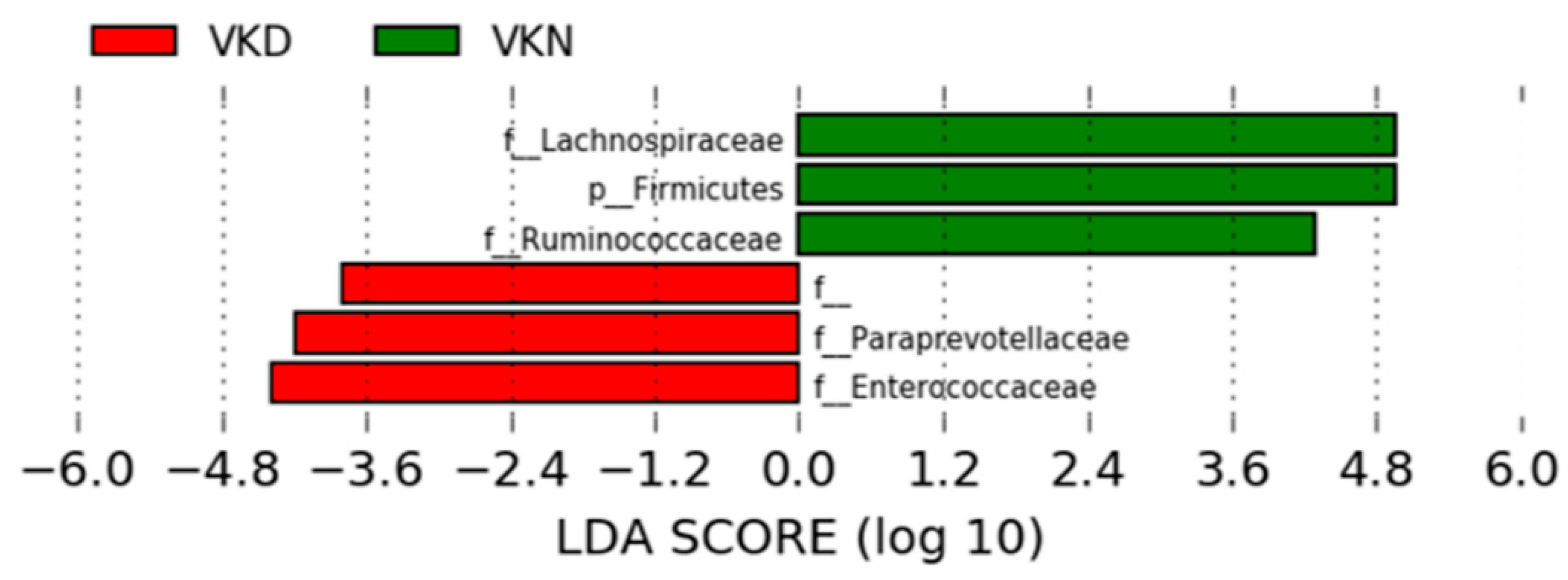
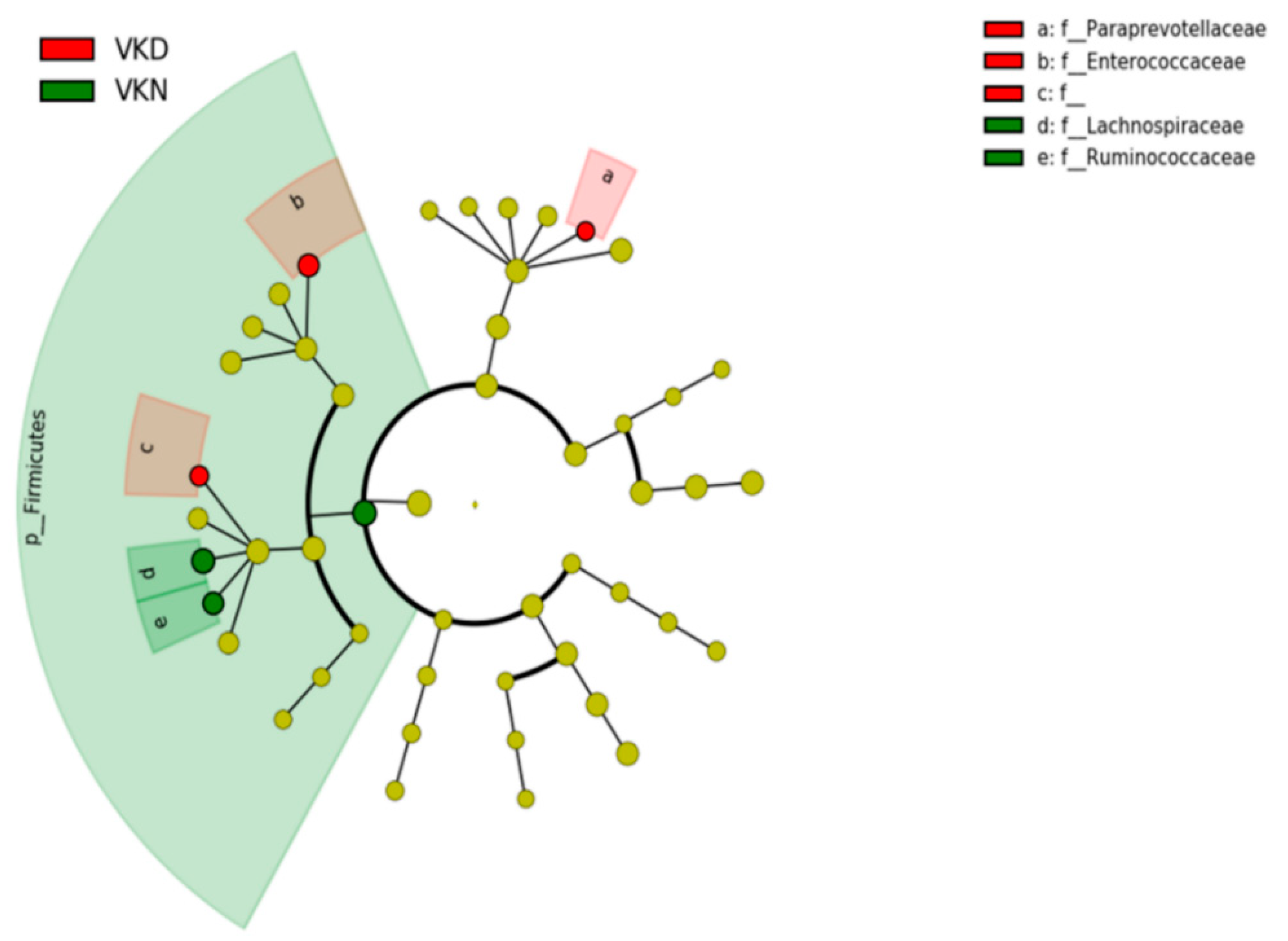
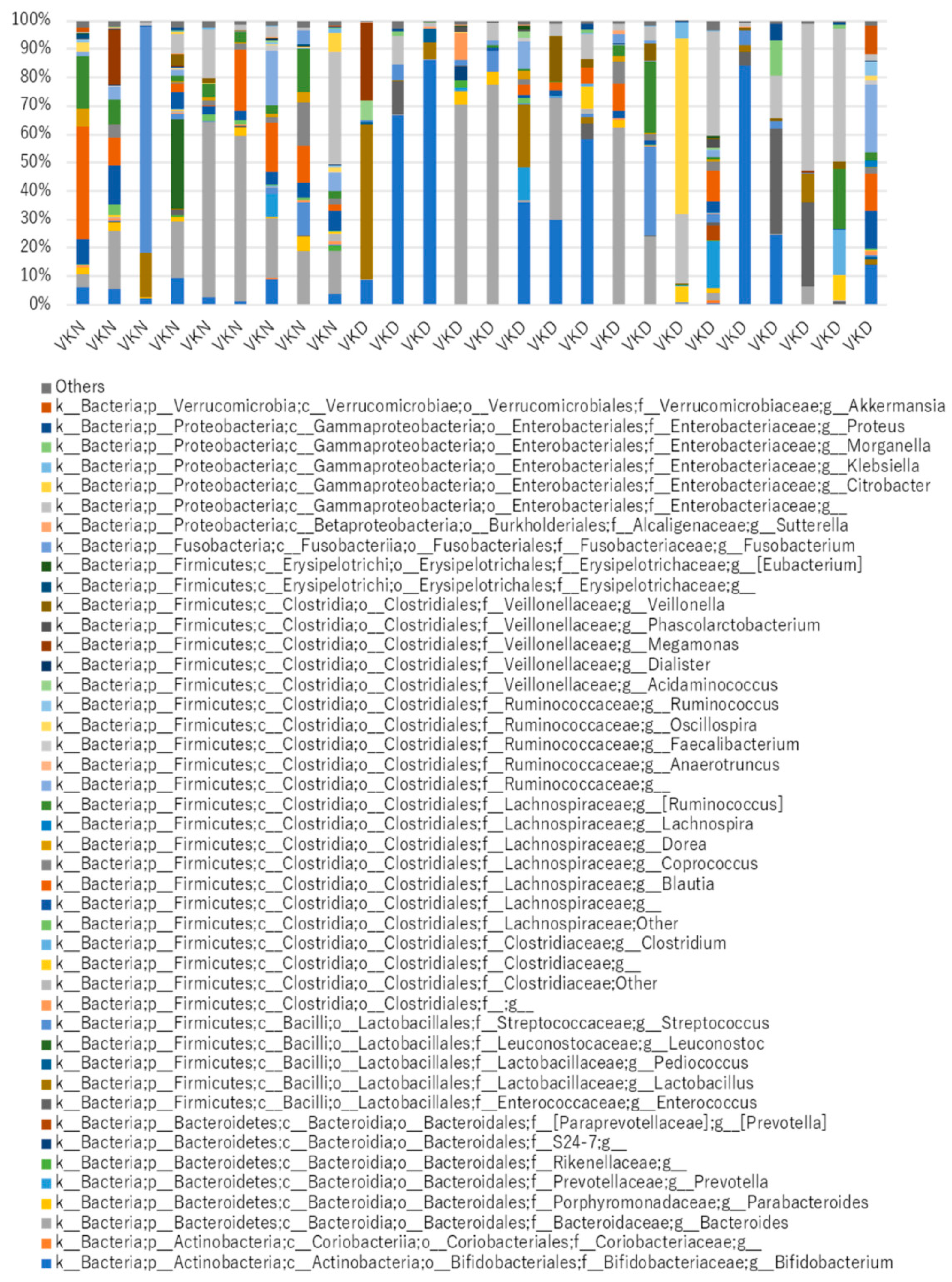
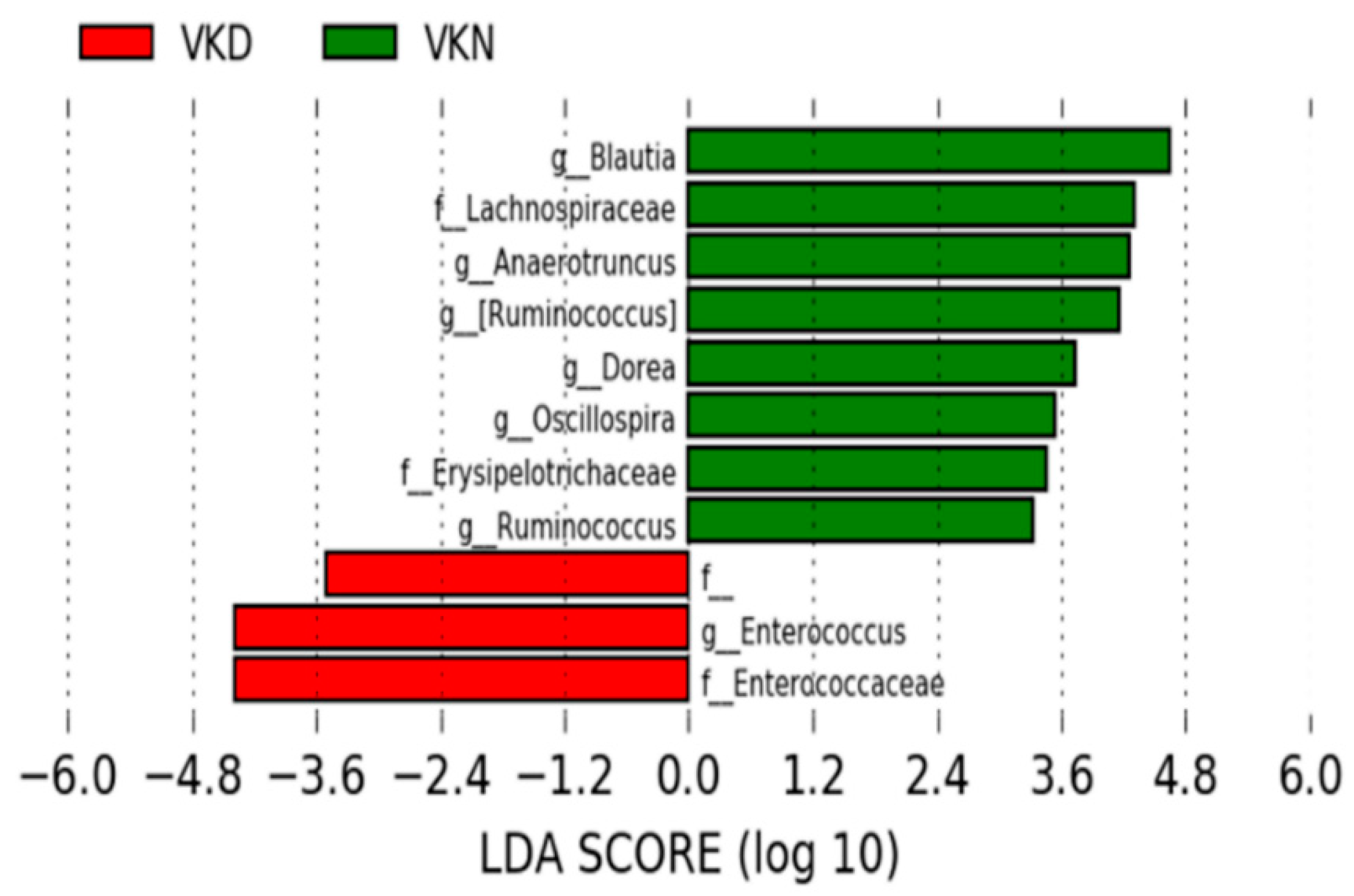
| uc-OC <4.5 ng/mL (Vit K-normal Group) (n = 9) | uc-OC ≥4.5 ng/mL (Vit K-deficient Group) (n = 17) | p-Value | ||
|---|---|---|---|---|
| Age (years; mean, range) | 42.8 (28–59) | 40.5 (21–73) | 0.61 | |
| Duration of disease (years; mean, range) | 23.0 (11–34) | 19.5 (4–53) | 0.41 | |
| Body mass index (kg/m2; mean, range) | 22.4 (15.7–27.8) | 20.8 (17.8–29.3) | 0.32 | |
| Clinical disease type | inflammatory/structuring/penetrating | 1/7/1 | 6/6/5 | 0.19 |
| Disease location range | small/small and large/large intestine | 4/5/0 | 8/6/3 | 0.75 |
| Anal lesion | 3 (33.3%) | 6 (35.3%) | 0.92 | |
| Surgical history | small intestine resection | 3 (33.3%) | 4 (23.5%) | 0.59 |
| ileocecal resection | 3 (33.3%) | 8 (47.1%) | 0.50 | |
| Disease activity (CDAI; mean, range) | 90.7 (−9–208) | 111.0 (34–204) | 0.45 | |
| Blood tests | Albumin (g/dL; mean, range) | 4.0 (3.0–4.6) | 3.9 (3.0–4.1) | 0.69 |
| Total cholesterol (mg/dL; mean, range) | 155 (109–209) | 141 (97–220) | 0.36 | |
| Triglyceride (mg/dL; mean, range) | 136 (49–271) | 97 (35–166) | 0.18 | |
| Cholinesterase (U/L; mean, range) | 315 (236–360) | 293 (172–497) | 0.33 | |
| Calcium (mg/dL; mean, range) | 8.9 (8.3–9.2) | 8.7 (8.4–9.1) | 0.39 | |
| C-reactive protein (mg/dL; mean, range) | 0.3 (0–0.9) | 0.2 (0–2.3) | 0.91 | |
| Intact parathyroid hormone (pg/mL; mean, range) | 39.2 (20.0–61.3) | 46.7 (15.9–98.5) | 0.30 | |
| folic acid | 12.3 (4.4–49.3) | 6.0 (0.9–10.5) | 0.22 | |
| vitamin B12 | 412 (151–774) | 440 (172–853) | 0.74 | |
| homocysteine | 15.3 (9.1–30.6) | 20.7 (9.6–76.8) | 0.24 | |
| PIVKA-II (mAU/mL; mean, range) | 22.6 (12–32) | 31.1 (20–48) | 0.01 | |
| uc-OC (ng/mL; mean, range) | 3.02 (2.26–4.17) | 9.48 (5.56–13.1) | <0.001 | |
| Therapy | Antibiotics | 2 (22.2%) | 9 (52.9%) | 0.13 |
| Probiotics | 3 (33.3%) | 8 (47.1%) | 0.50 | |
| Immunosuppressants | 7 (77.8%) | 11 (64.7%) | 0.49 | |
| Biologics | 1 (11.1%) | 9 (52.9%) | 0.08 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagatsuma, K.; Yamada, S.; Ao, M.; Matsuura, M.; Tsuji, H.; Iida, T.; Miyamoto, K.; Oka, K.; Takahashi, M.; Tanaka, K.; et al. Diversity of Gut Microbiota Affecting Serum Level of Undercarboxylated Osteocalcin in Patients with Crohn’s Disease. Nutrients 2019, 11, 1541. https://doi.org/10.3390/nu11071541
Wagatsuma K, Yamada S, Ao M, Matsuura M, Tsuji H, Iida T, Miyamoto K, Oka K, Takahashi M, Tanaka K, et al. Diversity of Gut Microbiota Affecting Serum Level of Undercarboxylated Osteocalcin in Patients with Crohn’s Disease. Nutrients. 2019; 11(7):1541. https://doi.org/10.3390/nu11071541
Chicago/Turabian StyleWagatsuma, Kohei, Satoshi Yamada, Misora Ao, Minoru Matsuura, Hidemi Tsuji, Tomoya Iida, Kentaro Miyamoto, Kentaro Oka, Motomichi Takahashi, Kiyoshi Tanaka, and et al. 2019. "Diversity of Gut Microbiota Affecting Serum Level of Undercarboxylated Osteocalcin in Patients with Crohn’s Disease" Nutrients 11, no. 7: 1541. https://doi.org/10.3390/nu11071541
APA StyleWagatsuma, K., Yamada, S., Ao, M., Matsuura, M., Tsuji, H., Iida, T., Miyamoto, K., Oka, K., Takahashi, M., Tanaka, K., & Nakase, H. (2019). Diversity of Gut Microbiota Affecting Serum Level of Undercarboxylated Osteocalcin in Patients with Crohn’s Disease. Nutrients, 11(7), 1541. https://doi.org/10.3390/nu11071541






