Vitamin D and Obesity: Two Interacting Players in the Field of Infertility
Abstract
1. Introduction
2. Vitamin D and Infertility
2.1. Vitamin D and Male Infertility (Observational Studies)
2.2. Vitamin D and Male Infertility (Interventional Studies)
2.3. Vitamin D and Female Infertility (Observational Studies)
2.4. Vitamin D and Female Infertility (Interventional Studies)
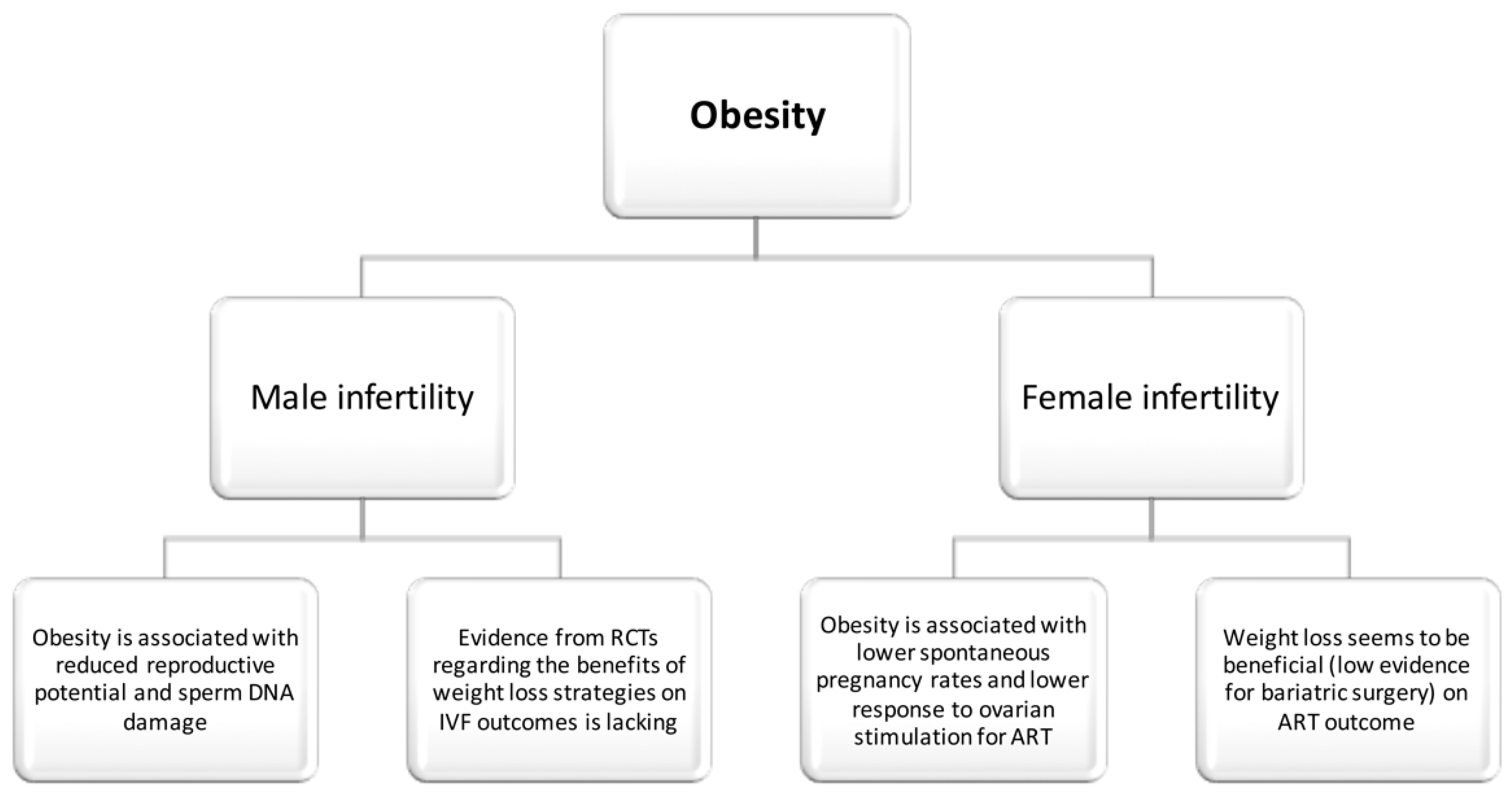
3. Obesity and Human Infertility
3.1. Obesity and Male Infertility (Observational Studies)
3.2. Obesity and Male Infertility (Interventional Studies)
3.3. Obesity and Female Infertility (Observational Studies)
3.4. Obesity and Female Infertility (Interventional Studies)
4. Potential Interactions between Vitamin D and Obesity
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Lieben, L.; Carmeliet, G.; Masuyama, R. Calcemic actions of vitamin D: Effects on the intestine, kidney and bone. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Extra Renal Synthesis of 1,25-dihydroxyvitamin D and its Health Implications. Clin. Rev. Bone Miner. Metab. 2009, 7, 114–125. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and extra-skeletal actions of vitamin D: Current evidence and outstanding questions. Endocr. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Haimi, M.; Kremer, R. Vitamin D deficiency/insufficiency from childhood to adulthood: Insights from a sunny country. World J. Clin. Pediatr. 2017, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van Schoor, N.; Lips, P. Global Overview of Vitamin D Status. Endocrinol. Metab. Clin. North Am. 2017, 46, 845–870. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Anagnostis, P.; Athyros, V.G.; Adamidou, F.; Florentin, M.; Karagiannis, A. Vitamin D and cardiovascular disease: A novel agent for reducing cardiovascular risk? Curr. Vasc. Pharmacol. 2010, 8, 720–730. [Google Scholar] [CrossRef]
- Anagnostis, P.; Karras, S.; Goulis, D.G. Vitamin D in human reproduction: A narrative review. Int. J. Clin. Pract. 2013, 67, 225–235. [Google Scholar] [CrossRef]
- WHO. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Dag, Z.O.; Dilbaz, B. Impact of obesity on infertility in women. J. Turk. Ger. Gynecol. Assoc. 2015, 16, 111–117. [Google Scholar] [PubMed]
- Boulange, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome. Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Kasum, M.; Oreskovic, S.; Cehic, E.; Lila, A.; Ejubovic, E.; Soldo, D. The role of female obesity on in vitro fertilization outcomes. Gynecol. Endocrinol. 2018, 34, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Gautier, A.; Bonnet, F.; Dubois, S.; Massart, C.; Grosheny, C.; Bachelot, A.; Aube, C.; Balkau, B.; Ducluzeau, P.H. Associations between visceral adipose tissue, inflammation and sex steroid concentrations in men. Clin. Endocrinol. (Oxf.) 2013, 78, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Raad, G.; Hazzouri, M.; Bottini, S.; Trabucchi, M.; Azoury, J.; Grandjean, V. Paternal obesity: How bad is it for sperm quality and progeny health? Basic Clin. Androl. 2017, 27, 20. [Google Scholar] [CrossRef]
- Blank, D.M.; Clark, R.V.; Heymsfield, S.B.; Rudman, D.R.; Blank, M.S. Endogenous opioids and hypogonadism in human obesity. Brain Res. Bull 1994, 34, 571–574. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Cavaco, J.E.; Oliveira, P.F. High-energy diets: A threat for male fertility? Obes. Rev. 2014, 15, 996–1007. [Google Scholar] [CrossRef]
- Jungheim, E.S.; Moley, K.H. Current knowledge of obesity’s effects in the pre- and periconceptional periods and avenues for future research. Am. J. Obstet. Gynecol. 2010, 203, 525–530. [Google Scholar] [CrossRef]
- Mahutte, N.; Kamga-Ngande, C.; Sharma, A.; Sylvestre, C. Obesity and Reproduction. J. Obstet. Gynaecol. Can. 2018, 40, 950–966. [Google Scholar] [CrossRef]
- Brewer, C.J.; Balen, A.H. The adverse effects of obesity on conception and implantation. Reproduction (Cambridge, England) 2010, 140, 347–364. [Google Scholar] [CrossRef]
- Pasquali, R.; Pelusi, C.; Genghini, S.; Cacciari, M.; Gambineri, A. Obesity and reproductive disorders in women. Hum. Reprod. Update 2003, 9, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Abbasihormozi, S.; Kouhkan, A.; Alizadeh, A.R.; Shahverdi, A.H.; Nasr-Esfahani, M.H.; Sadighi Gilani, M.A.; Salman Yazdi, R.; Matinibehzad, A.; Zolfaghari, Z. Association of vitamin D status with semen quality and reproductive hormones in Iranian subfertile men. Andrology 2017, 5, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Tirabassi, G.; Cutini, M.; Muscogiuri, G.; Delli Muti, N.; Corona, G.; Galdiero, M.; Pivonello, R.; Colao, A.; Balercia, G. Association between vitamin D and sperm parameters: Clinical evidence. Endocrine 2017, 58, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.L.; Xu, Q.F.; Li, S.X.; Wei, Y.C.; Zhu, G.C.; Yang, C.; Shi, Y.C. Investigation of serum vitamin D levels in Chinese infertile men. Andrologia 2016, 48, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Blomberg Jensen, M.; Bjerrum, P.J.; Jessen, T.E.; Nielsen, J.E.; Joensen, U.N.; Olesen, I.A.; Petersen, J.H.; Juul, A.; Dissing, S.; Jorgensen, N. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Human Reprod. (Oxford, England) 2011, 26, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Blomberg Jensen, M.; Gerner Lawaetz, J.; Andersson, A.M.; Petersen, J.H.; Nordkap, L.; Bang, A.K.; Ekbom, P.; Joensen, U.N.; Praetorius, L.; Lundstrom, P.; et al. Vitamin D deficiency and low ionized calcium are linked with semen quality and sex steroid levels in infertile men. Human Reprod. (Oxford, England) 2016, 31, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, A.O.; Meikle, A.W.; Peterson, C.M.; Stanford, J.; Gibson, M.; Carrell, D.T. Association of 25-hydroxy-vitamin D levels with semen and hormonal parameters. Asian J. Androl. 2012, 14, 855–859. [Google Scholar] [CrossRef]
- Rehman, R.; Lalani, S.; Baig, M.; Nizami, I.; Rana, Z.; Gazzaz, Z.J. Association Between Vitamin D, Reproductive Hormones and Sperm Parameters in Infertile Male Subjects. Front. Endocrinol. (Lausanne) 2018, 9, 607. [Google Scholar] [CrossRef]
- Wehr, E.; Pilz, S.; Boehm, B.O.; Marz, W.; Obermayer-Pietsch, B. Association of vitamin D status with serum androgen levels in men. Clin. Endocrinol. (Oxf.) 2010, 73, 243–248. [Google Scholar] [CrossRef]
- Lee, D.M.; Tajar, A.; Pye, S.R.; Boonen, S.; Vanderschueren, D.; Bouillon, R.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Finn, J.D.; et al. Association of hypogonadism with vitamin D status: The European Male Ageing Study. Eur. J. Endocrinol. 2012, 166, 77–85. [Google Scholar] [CrossRef]
- Blomberg Jensen, M.; Lawaetz, J.G.; Petersen, J.H.; Juul, A.; Jorgensen, N. Effects of Vitamin D Supplementation on Semen Quality, Reproductive Hormones, and Live Birth Rate: A Randomized Clinical Trial. J. Clin. Endocrinol. Metab. 2018, 103, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.L.; Li, Y.M.; Yang, X.Y.; Huang, J.R.; Guo, S.L.; Song, L.M. Efficacy and safety of vitamin D in the treatment of idiopathic oligoasthenozoospermia. Zhonghua Nan Ke Xue 2014, 20, 1082–1085. [Google Scholar] [PubMed]
- Pilz, S.; Frisch, S.; Koertke, H.; Kuhn, J.; Dreier, J.; Obermayer-Pietsch, B.; Wehr, E.; Zittermann, A. Effect of vitamin D supplementation on testosterone levels in men. Horm. Metab. Res. 2011, 43, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Canguven, O.; Talib, R.A.; El Ansari, W.; Yassin, D.J.; Al Naimi, A. Vitamin D treatment improves levels of sexual hormones, metabolic parameters and erectile function in middle-aged vitamin D deficient men. Aging Male 2017, 20, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.; Anagnostis, P.; Kotsa, K.; Goulis, D.G. Vitamin D and gonadal function in men: A potential inverse U-shaped association? Andrology 2016, 4, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Gallos, I.; Tobias, A.; Tan, B.; Eapen, A.; Coomarasamy, A. Vitamin D and assisted reproductive treatment outcome: A systematic review and meta-analysis. Human Reprod. (Oxford, England) 2018, 33, 65–80. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, X.; Xu, B.; Yan, Y.; Zhang, Q.; Li, Y. Whether vitamin D was associated with clinical outcome after IVF/ICSI: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2018, 16, 13. [Google Scholar] [CrossRef]
- Ciavattini, A.; Serri, M.; Delli Carpini, G.; Morini, S.; Clemente, N. Ovarian endometriosis and vitamin D serum levels. Gynecol. Endocrinol. 2017, 33, 164–167. [Google Scholar] [CrossRef]
- Anastasi, E.; Fuggetta, E.; De Vito, C.; Migliara, G.; Viggiani, V.; Manganaro, L.; Granato, T.; Benedetti Panici, P.; Angeloni, A.; Porpora, M.G. Low levels of 25-OH vitamin D in women with endometriosis and associated pelvic pain. Clin. Chem. Lab. Med. 2017, 55, e282–e284. [Google Scholar] [CrossRef]
- Pal, L.; Zhang, H.; Williams, J.; Santoro, N.F.; Diamond, M.P.; Schlaff, W.D.; Coutifaris, C.; Carson, S.A.; Steinkampf, M.P.; Carr, B.R.; et al. Vitamin D Status Relates to Reproductive Outcome in Women With Polycystic Ovary Syndrome: Secondary Analysis of a Multicenter Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2016, 101, 3027–3035. [Google Scholar] [CrossRef]
- Abedi, S.; Taebi, M.; Nasr Esfahani, M.H. Effect of Vitamin D Supplementation on Intracytoplasmic Sperm Injection Outcomes: A Randomized Double-Blind Placebo-Controlled Trial. Int. J. Fertil. Steril. 2019, 13, 18–23. [Google Scholar] [PubMed]
- Aflatoonian, A.; Arabjahvani, F.; Eftekhar, M.; Sayadi, M. Effect of vitamin D insufficiency treatment on fertility outcomes in frozen-thawed embryo transfer cycles: A randomized clinical trial. Iran J. Reprod. Med. 2014, 12, 595–600. [Google Scholar] [PubMed]
- Fang, F.; Ni, K.; Cai, Y.; Shang, J.; Zhang, X.; Xiong, C. Effect of vitamin D supplementation on polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Complement Ther. Clin. Pract. 2017, 26, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, F.; Mohammadzadeh, A.; Sadeghi, M.R.; Akhondi, M.M.; Mohammadmoradi, S.; Kamali, K.; Lackpour, N.; Jouhari, S.; Zafadoust, S.; Mokhtar, S.; et al. Role of vitamin E and D3 supplementation in Intra-Cytoplasmic Sperm Injection outcomes of women with polycystic ovarian syndrome: A double blinded randomized placebo-controlled trial. Clin. Nutr. ESPEN 2017, 18, 23–30. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.A.; Herbison, G.P.; Showell, M.; Farquhar, C.M. The impact of body mass index on semen parameters and reproductive hormones in human males: A systematic review with meta-analysis. Human Reprod. Update 2010, 16, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Sermondade, N.; Faure, C.; Fezeu, L.; Shayeb, A.G.; Bonde, J.P.; Jensen, T.K.; Van Wely, M.; Cao, J.; Martini, A.C.; Eskandar, M.; et al. BMI in relation to sperm count: An updated systematic review and collaborative meta-analysis. Human Reprod. Update 2013, 19, 221–231. [Google Scholar] [CrossRef]
- Campbell, J.M.; Lane, M.; Owens, J.A.; Bakos, H.W. Paternal obesity negatively affects male fertility and assisted reproduction outcomes: A systematic review and meta-analysis. Reprod. Biomed. Online 2015, 31, 593–604. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, F.; Hu, L.; Bai, R.; Zhang, N.; Yao, G.; Sun, Y. Effect of paternal overweight or obesity on IVF treatment outcomes and the possible mechanisms involved. Sci. Rep. 2016, 6, 29787. [Google Scholar] [CrossRef]
- Best, D.; Avenell, A.; Bhattacharya, S. How effective are weight-loss interventions for improving fertility in women and men who are overweight or obese? A systematic review and meta-analysis of the evidence. Human Reprod. Update 2017, 23, 681–705. [Google Scholar] [CrossRef]
- Hakonsen, L.B.; Thulstrup, A.M.; Aggerholm, A.S.; Olsen, J.; Bonde, J.P.; Andersen, C.Y.; Bungum, M.; Ernst, E.H.; Hansen, M.L.; Ernst, E.H.; et al. Does weight loss improve semen quality and reproductive hormones? Results from a cohort of severely obese men. Reprod. Health 2011, 8, 24. [Google Scholar] [CrossRef]
- Samavat, J.; Cantini, G.; Lotti, F.; Di Franco, A.; Tamburrino, L.; Degl’Innocenti, S.; Maseroli, E.; Filimberti, E.; Facchiano, E.; Lucchese, M.; et al. Massive Weight Loss Obtained by Bariatric Surgery Affects Semen Quality in Morbid Male Obesity: A Preliminary Prospective Double-Armed Study. Obes. Surg. 2018, 28, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Pelusi, C.; Pasquali, R. Polycystic ovary syndrome in adolescents: Pathophysiology and treatment implications. Treat. Endocrinol. 2003, 2, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.K.; Knutsen, S.F.; Oda, K.; Fraser, G.E. Obesity at age 20 and the risk of miscarriages, irregular periods and reported problems of becoming pregnant: The Adventist Health Study-2. Eur. J. Epidemiol. 2012, 27, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Van der Steeg, J.W.; Steures, P.; Eijkemans, M.J.; Habbema, J.D.; Hompes, P.G.; Burggraaff, J.M.; Oosterhuis, G.J.; Bossuyt, P.M.; van der Veen, F.; Mol, B.W. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Human. Reprod. (Oxford, England) 2008, 23, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Broughton, D.E.; Moley, K.H. Obesity and female infertility: Potential mediators of obesity’s impact. Fertil. Steril. 2017, 107, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Zaadstra, B.M.; Seidell, J.C.; Van Noord, P.A.; te Velde, E.R.; Habbema, J.D.; Vrieswijk, B.; Karbaat, J. Fat and female fecundity: Prospective study of effect of body fat distribution on conception rates. BMJ 1993, 306, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Bellver, J.; Ayllon, Y.; Ferrando, M.; Melo, M.; Goyri, E.; Pellicer, A.; Remohi, J.; Meseguer, M. Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertil. Steril. 2010, 93, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Fedorcsak, P.; Dale, P.O.; Storeng, R.; Ertzeid, G.; Bjercke, S.; Oldereid, N.; Omland, A.K.; Abyholm, T.; Tanbo, T. Impact of overweight and underweight on assisted reproduction treatment. Human Reprod. (Oxford, England) 2004, 1, 2523–2528. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; Stofberg, L.; Bhattacharya, S. Effect of overweight and obesity on assisted reproductive technology--a systematic review. Human Reprod. Update 2007, 13, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Souter, I.; Baltagi, L.M.; Kuleta, D.; Meeker, J.D.; Petrozza, J.C. Women, weight, and fertility: The effect of body mass index on the outcome of superovulation/intrauterine insemination cycles. Fertil. Steril. 2011, 95, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Rittenberg, V.; Seshadri, S.; Sunkara, S.K.; Sobaleva, S.; Oteng-Ntim, E.; El-Toukhy, T. Effect of body mass index on IVF treatment outcome: An updated systematic review and meta-analysis. Reprod. Biomed. Online 2011, 23, 421–439. [Google Scholar] [CrossRef] [PubMed]
- Provost, M.P.; Acharya, K.S.; Acharya, C.R.; Yeh, J.S.; Steward, R.G.; Eaton, J.L.; Goldfarb, J.M.; Muasher, S.J. Pregnancy outcomes decline with increasing body mass index: Analysis of 239,127 fresh autologous in vitro fertilization cycles from the 2008-2010 Society for Assisted Reproductive Technology registry. Fertil. Steril. 2016, 105, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Norman, R.J.; Middleton, P. Preconception lifestyle advice for people with subfertility. Cochrane Database Syst. Rev. 2010, 4, CD008189. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.A.; Partridge, S.R.; Sainsbury, A. Does weight loss in overweight or obese women improve fertility treatment outcomes? A systematic review. Obes. Rev. 2014, 15, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.H.; Farland, L.V.; Muir Thomas, A.; Zera, C.A.; Ginsburg, E.S. The Combined Impact of Maternal Age and Body Mass Index on Cumulative Live Birth Following In Vitro Fertilization. Am. J. Obstet. Gynecol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.; De Placido, G.; Musella, M.; Sosa Fernandez, L.M.; Sosa Fernandez, L.V.; Campana, G.; Di Minno, M.N.; Milone, F. Incidence of Successful Pregnancy After Weight Loss Interventions in Infertile Women: A Systematic Review and Meta-Analysis of the Literature. Obes. Surg. 2016, 26, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.; Sosa Fernandez, L.M.; Sosa Fernandez, L.V.; Manigrasso, M.; Elmore, U.; De Palma, G.D.; Musella, M.; Milone, F. Does Bariatric Surgery Improve Assisted Reproductive Technology Outcomes in Obese Infertile Women? Obes. Surg. 2017, 27, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Rafiq, S.; Jeppesen, P.B. Body Mass Index, Vitamin D, and Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Wamberg, L.; Christiansen, T.; Paulsen, S.K.; Fisker, S.; Rask, P.; Rejnmark, L.; Richelsen, B.; Pedersen, S.B. Expression of vitamin D-metabolizing enzymes in human adipose tissue—The effect of obesity and diet-induced weight loss. Int. J. Obes. (Lond.) 2013, 37, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Deriquehem, V.A.; Antunes, R.A.; Reginatto, M.W.; Mancebo, A.C.; Areas, P.; Bloise, E.; Souza Mdo, C.; Ortiga-Carvalho, T.M. Body weight and 25-hidroxyvitamin D follicular levels: A prospective study of women submitted to in vitro fertilization. JBRA Assist. Reprod. 2016, 20, 127–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yildizhan, R.; Kurdoglu, M.; Adali, E.; Kolusari, A.; Yildizhan, B.; Sahin, H.G.; Kamaci, M. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch. Gynecol. Obstet. 2009, 280, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Aghadavod, E.; Mollaei, H.; Nouri, M.; Hamishehkar, H. Evaluation of Relationship between Body Mass Index with Vitamin D Receptor Gene Expression and Vitamin D Levels of Follicular Fluid in Overweight Patients with Polycystic Ovary Syndrome. Int. J. Fertil. Steril. 2017, 11, 105–111. [Google Scholar] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.C.; Chuang, L.M.; Yoon, C. The vitamin D receptor polymorphism in the translation initiation codon is a risk factor for insulin resistance in glucose tolerant Caucasians. BMC Med. Genet. 2001, 2, 2. [Google Scholar] [CrossRef]
| Male infertility Linear or U-shaped correlation between vitamin D concentrations and sperm motility/morphology [23,24,25,26,27,28]. Sufficient vitamin D concentrations associated with high testosterone concentrations [29,30,31]. Supplementation of vitamin D improved semen quality and pregnancy rates [32,33]. Supplementation of vitamin D increased testosterone concentrations [34,35]. |
| Female infertility Contradictory data on whether supplementation of vitamin D is associated with pregnancy rates [42,43]. |
| ART Higher live birth rates in vitamin D-sufficient women [37,38]. |
| Endometriosis Linear correlation between vitamin D concentrations and diameter of ovarian endometriomas [39]. Higher incidence of vitamin D deficiency/insufficiency in women with endometriosis [40]. |
| PCOS Linear correlation between vitamin D levels and reproductive success rates after ovulation induction in women with PCOS [41]. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosdou, J.K.; Konstantinidou, E.; Anagnostis, P.; Kolibianakis, E.M.; Goulis, D.G. Vitamin D and Obesity: Two Interacting Players in the Field of Infertility. Nutrients 2019, 11, 1455. https://doi.org/10.3390/nu11071455
Bosdou JK, Konstantinidou E, Anagnostis P, Kolibianakis EM, Goulis DG. Vitamin D and Obesity: Two Interacting Players in the Field of Infertility. Nutrients. 2019; 11(7):1455. https://doi.org/10.3390/nu11071455
Chicago/Turabian StyleBosdou, Julia K., Eirini Konstantinidou, Panagiotis Anagnostis, Efstratios M. Kolibianakis, and Dimitrios G. Goulis. 2019. "Vitamin D and Obesity: Two Interacting Players in the Field of Infertility" Nutrients 11, no. 7: 1455. https://doi.org/10.3390/nu11071455
APA StyleBosdou, J. K., Konstantinidou, E., Anagnostis, P., Kolibianakis, E. M., & Goulis, D. G. (2019). Vitamin D and Obesity: Two Interacting Players in the Field of Infertility. Nutrients, 11(7), 1455. https://doi.org/10.3390/nu11071455





