A New Light on Vitamin D in Obesity: A Novel Association with Trimethylamine-N-Oxide (TMAO)
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
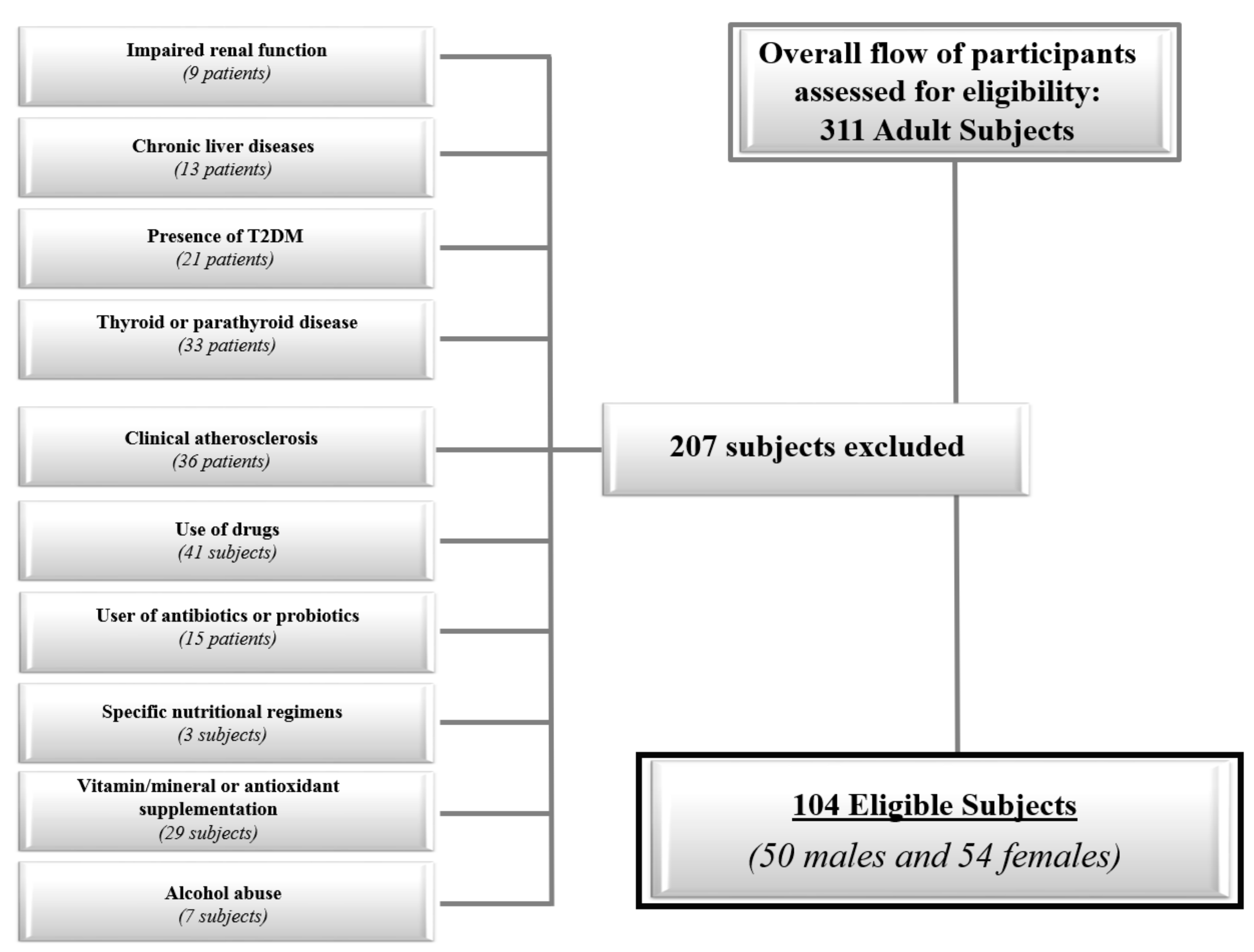
2.2. Population Study
- Impaired renal function (normal values: Estimated glomerular filtration rate ≥90 mL/min/1.73 m2 calculated by a chronic kidney disease epidemiology collaboration equation; Chronic Kidney Disease Epidemiology Collaboration (CKD EPI) (9 subjects);
- Chronic liver diseases, viral hepatitis patients, hemochromatosis, hepatic malignancy (13 subjects);
- Presence of type 2 diabetes (T2DM) (defined by the criteria of the American Diabetes Association as follows: Basal plasma glucose level ≥ 126 mg/dL on two occasions, or glycated haemoglobin (HbA1c) ≥ 6.5% (≥48 mmoL/moL) on two occasions, or both at the same time. Participants on antidiabetic medication were considered to have T2DM [17] (21 subjects);
- Uncontrolled thyroid or parathyroid disease (33 subjects);
- Clinical atherosclerosis (coronary artery disease, peripheral vascular disease) (36 subjects);
- Current therapy with calcium, vitamin D supplementation, or osteoporosis therapies, anti-inflammatory drugs, statin and other hypolipidemic agents (41 subjects);
- User of antibiotics or probiotics within two months of recruitment (15 subjects);
- Specific nutritional regimens, including vegan or vegetarian diets (three subjects);
- Vitamin/mineral or antioxidant supplementation (29 subjects);
- Alcohol abuse according to the Diagnostic and Statistical Manual of Mental Disorders (DSM)-V diagnostic criteria (7 subjects).
2.3. Anthropometric Measurements and Blood Pressure
2.4. Assay Methods
2.5. Statistical Analysis
3. Results
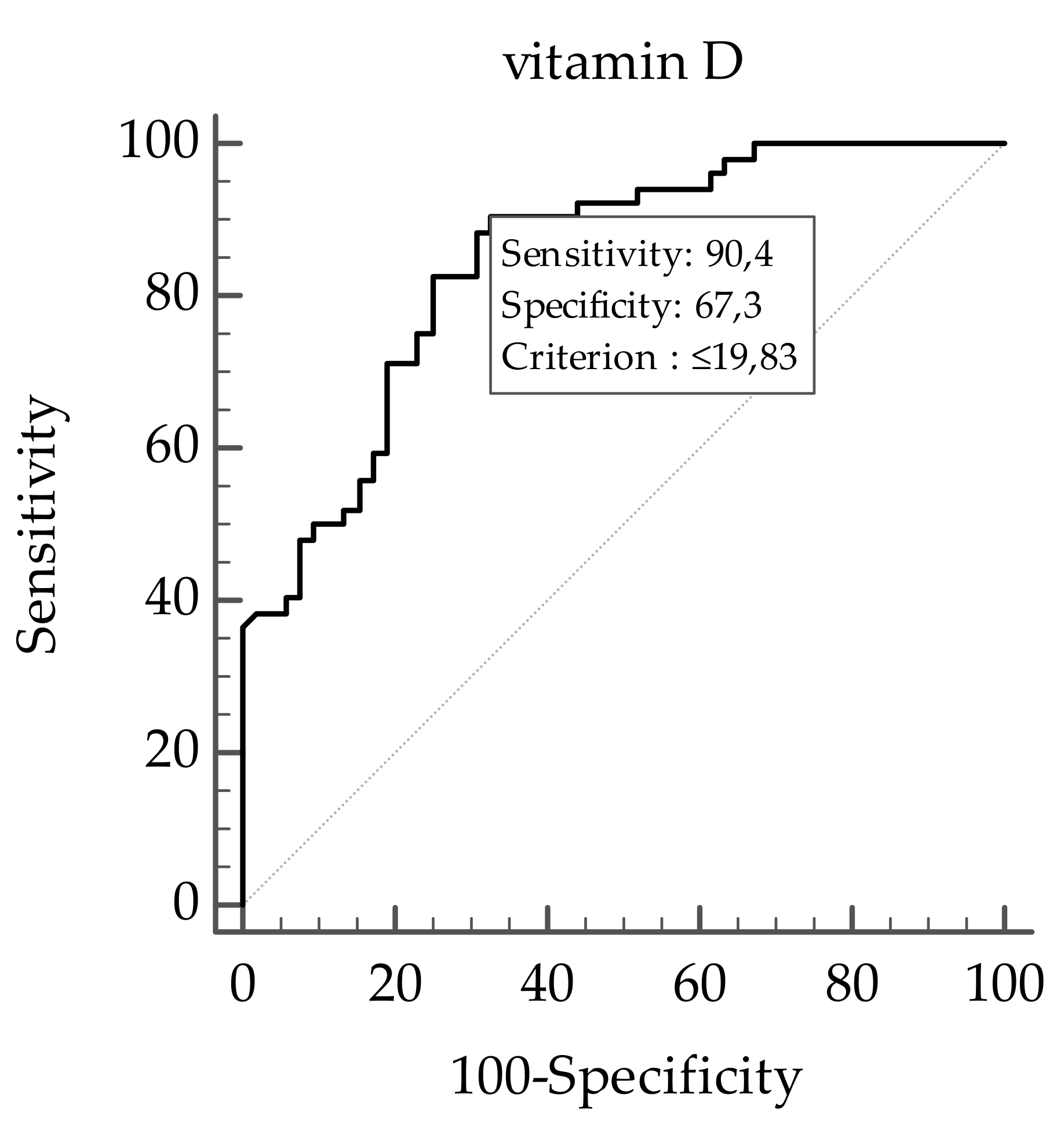
Correlation Analysis
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bassatne, A.; Chakhtoura, M.; Saad, R.; Fuleihan, G.E. Vitamin D supplementation in obesity and during weight loss: A review of randomized controlled trials. Metabolism 2019, 92, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Pramono, A.; Jocken, J.W.E.; Blaak, E.E. Vitamin D deficiency in the aetiology of obesity-related insulin resistance. Diabetes Metab. Res. Rev. 2019, e3146. [Google Scholar] [CrossRef] [PubMed]
- Savastano, S.; Barrea, L.; Savanelli, M.C.; Nappi, F.; Di Somma, C.; Orio, F.; Colao, A. Low vitamin D status and obesity: Role of nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Bakke, D.; Chatterjee, I.; Agrawal, A.; Dai, Y.; Sun, J. Regulation of Microbiota by Vitamin D Receptor: A Nuclear Weapon in Metabolic Diseases. Nucl. Recept. Res. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Canyelles, M.; Tondo, M.; Cedó, L.; Farràs, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Trimethylamine N-Oxide: A Link among Diet, Gut Microbiota, Gene Regulation of Liver and Intestine Cholesterol Homeostasis and HDL Function. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Kanitsoraphan, C.; Rattanawong, P.; Charoensri, S.; Senthong, V. Trimethylamine N-Oxide and Risk of Cardiovascular Disease and Mortality. Curr. Nutr. Rep. 2018, 7, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Awwad, H.M.; Kirsch, S.H.; Waldura, C.; Herrmann, W.; Graeber, S.; Geisel, J. Plasma trimethylamine-N-oxide following supplementation with vitamin D or D plus B vitamins. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with early Atherosclerosis in Humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Q.; Li, H. Gut Microbiota and Nonalcoholic Fatty Liver Disease: Insights on Mechanisms and Therapy. Nutrients 2017, 9, 1124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Liu, Y.; Zhou, R.F.; Chen, X.L.; Wang, C.; Tan, X.Y.; Wang, L.J.; Zheng, R.D.; Zhang, H.W.; Ling, W.H.; et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci. Rep. 2016, 6, 19076. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Hung, H.F.; Lu, C.W.; Chang, H.H.; Lee, L.T.; Huang, K.C. Association of Non-alcoholic Fatty Liver Disease with Metabolic Syndrome Independently of Central Obesity and Insulin Resistance. Sci. Rep. 2016, 6, 27034. [Google Scholar] [CrossRef] [PubMed]
- Forlani, G.; Giorda, C.; Manti, R.; Mazzella, N.; De Cosmo, S.; Rossi, M.C.; Nicolucci, A.; Di Bartolo, P.; Ceriello, A.; Guida, P.; AMD-Annals Study Group. The Burden of NAFLD and Its Characteristics in a Nationwide Population with Type 2 Diabetes. J. Diabetes Res. 2016, 2016, 2931985. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Wu, F.Z.; Lin, K.H.; Chen, Y.H.; Wu, P.C.; Chen, Y.H.; Chen, C.S.; Wang, W.H.; Mar, G.Y.; Yu, H.C. Role of Fatty Liver Index and Metabolic Factors in the Prediction of Nonalcoholic Fatty Liver Disease in a Lean Population Receiving Health Checkup. Clin. Transl. Gastroenterol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes-2017: Summary of Revisions. Diabetes Care 2017, 40, S4–S5. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Waist Circumference and Waist-Hip Ratio. Report of WHO Expert Consultation, Geneva, 8–11 December 2008. 2011. Available online: http://apps.who.int/iris/bitstream/10665/44583/1/9789241501491_eng.pdf (accessed on 21 May 2019).
- National Center for Health Statistics. Anthropometry Procedures Manual—National Health and Nutrition Examination Survey (NHANES). 2013. Available online: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/Anthropometry_Procedures_Manual.pdf (accessed on 21 May 2019).
- Savanelli, M.C.; Barrea, L.; Macchia, P.E.; Savastano, S.; Falco, A.; Renzullo, A.; Scarano, E.; Nettore, I.C.; Colao, A.; Di Somma, C. Preliminary results demonstrating the impact of Mediterranean diet on bone health. J. Transl. Med. 2017, 15, 81. [Google Scholar] [CrossRef]
- Barrea, L.; Macchia, P.E.; Di Somma, C.; Napolitano, M.; Balato, A.; Falco, A.; Savanelli, M.C.; Balato, N.; Colao, A.; Savastano, S. Bioelectrical phase angle and psoriasis: A novel association with psoriasis severity, quality of life and metabolic syndrome. J. Transl. Med. 2016, 14, 130. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Macchia, P.E.; Di Somma, C.; Falco, A.; Savanelli, M.C.; Colao, A.; Savastano, S. Mediterranean Diet and Phase Angle in a Sample of Adult Population: Results of a Pilot Study. Nutrients 2017, 9, 151. [Google Scholar] [CrossRef]
- Barrea, L.; Tarantino, G.; Somma, C.D.; Muscogiuri, G.; Macchia, P.E.; Falco, A.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet and Circulating Levels of Sirtuin 4 in Obese Patients: A Novel Association. Oxid. Med. Cell Longev. 2017, 2017, 6101254. [Google Scholar] [CrossRef] [PubMed]
- Savastano, S.; Barbato, A.; Di Somma, C.; Guida, B.; Pizza, G.; Barrea, L.; Avallone, S.; Schiano di Cola, M.; Strazzullo, P.; Colao, A. Beyond waist circumference in an adult male population of Southern Italy: Is there any role for subscapular skinfold thickness in the relationship between insulin-like growth factor-I system and metabolic parameters? J. Endocrinol. Investig. 2012, 35, 925–929. [Google Scholar] [CrossRef]
- Savastano, S.; Di Somma, C.; Colao, A.; Barrea, L.; Orio, F.; Finelli, C.; Pasanisi, F.; Contaldo, F.; Tarantino, G. Preliminary data on the relationship between circulating levels of Sirtuin 4, anthropometric and metabolic parameters in obese subjects according to growth hormone/insulin-like growth factor-1 status. Growth Horm. IGF Res. 2015, 25, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Savanelli, M.C.; Scarano, E.; Muscogiuri, G.; Barrea, L.; Vuolo, L.; Rubino, M.; Savastano, S.; Colao, A.; Di Somma, C. Cardiovascular risk in adult hypopituitaric patients with growth hormone deficiency: Is there a role for vitamin D? Endocrine 2016, 52, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Barrea, L.; Capone, D.; Citro, V.; Mosca, T.; Savastano, S. Hematocrit Values Predict Carotid Intimal-Media Thickness in Obese Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study. Front. Endocrinol. (Lausanne) 2018, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Levison, B.S.; Hazen, J.E.; Donahue, L.; Li, X.M.; Hazen, S.L. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal. Biochem. 2014, 455, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Beale, R.; Airs, R. Quantification of glycine betaine, choline and trimethylamine N-oxide in seawater particulates: Minimisation of seawater associated ion suppression. Anal. Chim. Acta 2016, 938, 114–122. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Di Somma, C.; Maisto, M.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: Also a matter of sex? Nutrition 2019, 62, 7–17. [Google Scholar] [CrossRef]
- Yu, W.; Xu, C.; Li, G.; Hong, W.; Zhou, Z.; Xiao, C.; Zhao, Y.; Cai, Y.; Huang, M.; Jin, J. Simultaneous determination of trimethylamine N-oxide, choline, betaine by UPLC-MS/MS in human plasma: An application in acute stroke patients. J. Pharm. Biomed. Anal. 2018, 152, 179–187. [Google Scholar] [CrossRef]
- Lee, J.H.; O’Keefe, J.H.; Bell, D.; Hensrud, D.D.; Holick, M.F. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J. Am. Coll. Cardiol. 2008, 52, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, W.H.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 2014, 35, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A. The Biphasic Effect of Vitamin D on the Musculoskeletal and Cardiovascular System. Int. J. Endocrinol. 2017, 2017, 3206240. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Pichiah, P.B.T.; Cha, Y.S. Vitamin D and Metabolic Diseases: Growing Roles of Vitamin D. J. Obes. Metab. Syndr. 2018, 27, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, N.; Rainsbury, J.; Kimball, S.M. Vitamin D Supplementation, Serum 25(OH)D Concentrations and Cardiovascular Disease Risk Factors: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, T.; Lim, K.; Thadhani, R.; Manson, J.E. Vitamin D and Atherosclerotic Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2019. [Google Scholar] [CrossRef]
- Earthman, C.P.; Beckman, L.M.; Masodkar, K.; Sibley, S.D. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: Considerations and implications. Int. J. Obes. (Lond.) 2012, 36, 387–396. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Altieri, B.; Di Somma, C.; Bhattoa, H.; Laudisio, D.; Duval, G.T.; Pugliese, G.; Annweiler, C.; Orio, F.; et al. Calcium and vitamin D supplementation. Myths and realities with regard to cardiovascular risk. Curr. Vasc. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Zittermann, A.; Schleithoff, S.S.; Koerfer, R. Vitamin D and vascular calcification. Curr. Opin. Lipidol. 2007, 18, 41–46. [Google Scholar] [CrossRef]
- Zittermann, A.; Gummert, J.F. Sun, vitamin D, and cardiovascular disease. J. Photochem. Photobiol. B 2010, 101, 124–129. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Wishnok, J.S.; Blusztajn, J.K. Formation of methylamines from ingested choline and lecithin. J. Pharmacol. Exp. Ther. 1983, 225, 320–324. [Google Scholar] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; You, T.; Li, J.; Pan, T.; Xiang, L.; Han, Y.; Zhu, L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: A systematic review and meta-analysis of 11 prospective cohort studies. J. Cell Mol. Med. 2018, 22, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017, 19, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Sherriff, J.L.; O’Sullivan, T.A.; Properzi, C.; Oddo, J.L.; Adams, L.A. Choline, Its Potential Role in Nonalcoholic Fatty Liver Disease, and the Case for Human and Bacterial Genes. Adv. Nutr. 2016, 7, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Snyder, L.; Arora, J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019, 1–9. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Z.; Musch, M.W.; Ning, G.; Sun, J.; Hart, J.; Bissonnette, M.; Li, Y.C. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G208–G216. [Google Scholar] [CrossRef]
- Borges, C.C.; Salles, A.F.; Bringhenti, I.; Mandarim-DE-Lacerda, C.A.; Aguila, M.B. Vitamin D Deficiency Increases Lipogenesis and Reduces Beta-Oxidation in the Liver of Diet-Induced Obese Mice. J. Nutr. Sci. Vitaminol. (Tokyo) 2018, 64, 106–115. [Google Scholar] [CrossRef]
- Patel, Y.A.; Henao, R.; Moylan, C.A.; Guy, C.D.; Piercy, D.L.; Diehl, A.M.; Abdelmalek, M.F. Vitamin D is Not Associated with Severity in NAFLD: Results of a Paired Clinical and Gene Expression Profile Analysis. Am. J. Gastroenterol. 2016, 111, 1591–1598. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Long, J.; Chen, S.; Liao, G.; Wu, S.; Li, C.; Wang, L.; Ling, W.; Zhu, H. Trimethylamine N-Oxide Aggravates Liver Steatosis Through Modulation of Bile Acid Metabolism and Inhibition of Farnesoid X Receptor Signaling in Nonalcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2019, e1900257. [Google Scholar] [CrossRef]
- Miao, J.; Ling, A.V.; Manthena, P.V.; Gearing, M.E.; Graham, M.J.; Crooke, R.M.; Croce, K.J.; Esquejo, R.M.; Clish, C.B.; Morbid Obesity Study Group; et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat. Commun. 2015, 6, 6498. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; McCarty, M.; OKeefe, J. Association of moderately elevated trimethylamine N-oxide with cardiovascular risk: Is TMAO serving as a marker for hepatic insulin resistance. Open Heart 2019, 6, e000890. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, X.; Xu, J.; Xue, C.; Xue, Y.; Wang, Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J. Biosci. Bioeng. 2014, 118, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Kühn, T.; Rohrmann, S.; Sookthai, D.; Johnson, T.; Katzke, V.; Kaaks, R.; von Eckardstein, A.; Müller, D. Intra-individual variation of plasma trimethylamine-N-oxide (TMAO), betaine and choline over 1 year. Clin. Chem. Lab. Med. 2017, 55, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Linseisen, J.; Allenspach, M.; von Eckardstein, A.; Müller, D. Plasma Concentrations of Trimethylamine-N-oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J. Nutr. 2016, 146, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Cho, C.E.; Caudill, M.A. Trimethylamine-N-Oxide: Friend, Foe, or Simply Caught in the Cross-Fire? Trends Endocrinol. Metab. 2017, 28, 121–130. [Google Scholar] [CrossRef]
- Krüger, R.; Merz, B.; Rist, M.J.; Ferrario, P.G.; Bub, A.; Kulling, S.E.; Watzl, B. Associations of current diet with plasma and urine TMAO in the KarMeN study: Direct and indirect contributions. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Silaghi, C.A.; Silaghi, H.; Colosi, H.A.; Craciun, A.E.; Farcas, A.; Cosma, D.T.; Hancu, N.; Pais, R.; Georgescu, C.E. Prevalence and predictors of non-alcoholic fatty liver disease as defined by the fatty liver index in a type 2 diabetes population. Clujul Med. 2016, 89, 82–88. [Google Scholar] [CrossRef]
- Fedchuk, L.; Nascimbeni, F.; Pais, R.; Charlotte, F.; Housset, C.; Ratziu, V.; LIDO Study Group. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2014, 40, 1209–1222. [Google Scholar] [CrossRef]

| Parameters | Mean ± SD or Number (%) |
|---|---|
| Age (years) | 35.38 ± 7.49 |
| BMI (kg/m2) | 33.52 ± 9.59 |
| Normal weight | 25, 24.0% |
| Overweight | 23, 22.1% |
| Grade I obesity | 15, 14.4% |
| Grade II obesity | 12, 11.5% |
| Grade III obesity | 29, 27.9% |
| WC (cm) | 109.14 ± 24.34 |
| SBP (mmHg) | 124.27 ± 13.06 |
| SDP (mmHg) | 79.86 ± 10.46 |
| Plasma TMAO (µM) | 8.33 ± 3.28 |
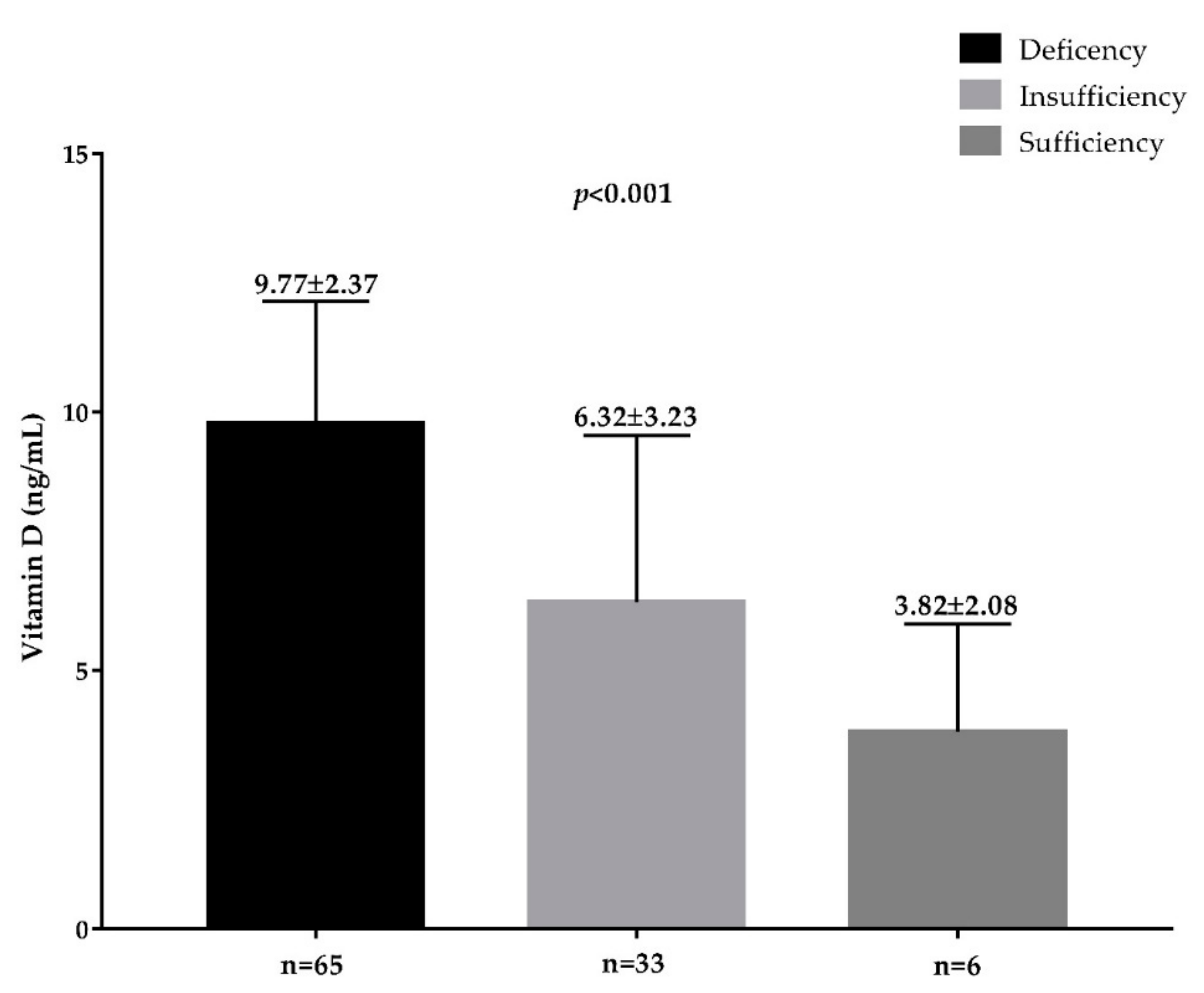
| Vitamin D (ng/mL) | 17.69 ± 6.44 |
| Deficiency | 65, 62.5% |
| Insufficiency | 33, 31.7% |
| Normal | 6, 5.8% |
| Fasting Glucose (mg/dL) | 100.56 ± 17.48 |
| Insulin (µU/mL) | 13.68 ± 12.57 |
| Total cholesterol (mg/dL) | 184.65 ± 40.00 |
| HDL cholesterol (mg/dL) | 46.50 ± 12.10 |
| LDL cholesterol (mg/dL) | 111.51 ± 42.69 |
| Triglycerides (mg/dL) | 139.30 ± 54.74 |
| ALT (U/L) | 31.96 ± 15.25 |
| AST (U/L) | 32.49 ± 15.61 |
| γGT (U/L) | 35.71 ± 20.04 |
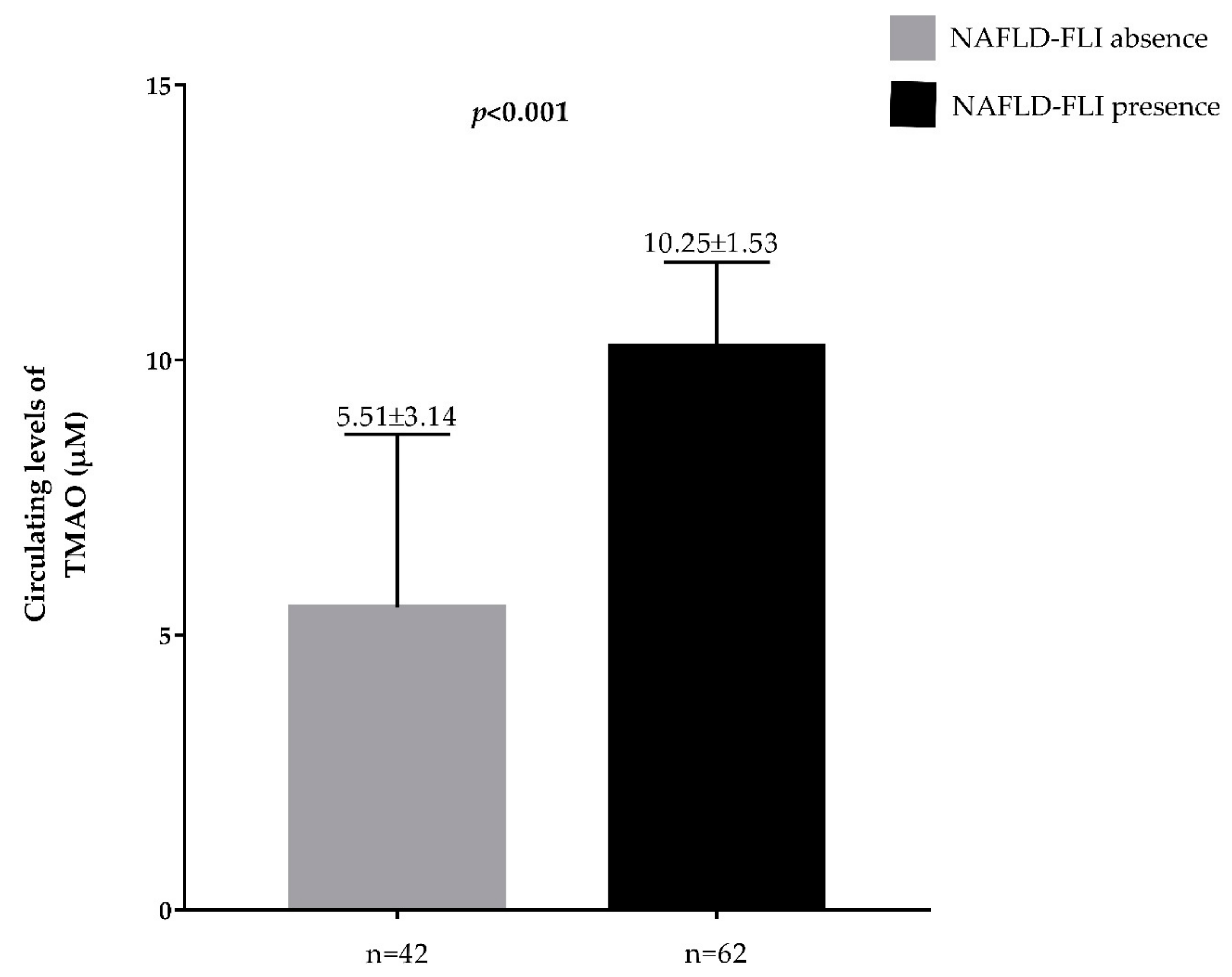
| FLI | 63.78 ± 33.89 |
| FLI NAFLD (presence) | 62, 59.6% |
| Parameters | Normal Weight n = 25; 24.0% | Over Weight n = 23; 22.1% | Grade I Obesity n = 15; 14.4% | Grade II Obesity n = 12; 11.5% | Grade III Obesity n = 29; 27.9% | p-Value |
|---|---|---|---|---|---|---|
| Age (years) | 32.64 ± 7.55 | 38.35 ± 7.40 | 37.60 ± 6.45 | 35.00 ± 8.68 | 34.41 ± 6.81 | 0.063 |
| BMI (kg/m2) | 23.11 ± 1.59 | 27.34 ± 1.24 | 32.37 ± 1.47 | 37.58 ± 1.43 | 46.31 ± 5.31 | <0.001 |
| WC (cm) | 84.17 ± 10.66 | 95.60 ± 12.21 | 109.41 ± 8.08 | 117.91 ± 14.33 | 137.64 ± 16.41 | <0.001 |
| Plasma TMAO (µM) | 3.53 ± 2.49 | 8.32 ± 0.70 | 9.17 ± 1.11 | 9.94 ± 0.90 | 11.39 ± 1.03 | <0.001 |
| Vitamin D (ng/mL) | 23.69 ± 6.35 | 20.29 ± 3.94 | 17.27 ± 4.52 | 14.78 ± 2.45 | 11.89 ± 4.11 | <0.001 |
| Fasting Glucose (mg/dL) | 85.60 ± 10.57 | 95.78 ± 13.15 | 98.33 ± 13.76 | 97.00 ± 12.28 | 119.89 ± 11.15 | <0.001 |
| Insulin (µU/mL) | 2.66 ± 1.15 | 7.16 ± 5.66 | 10.64 ± 5.65 | 14.51 ± 10.77 | 29.57 ± 9.14 | <0.001 |
| Total cholesterol (mg/dL) | 151.36 ± 20.99 | 175.61 ± 27.92 | 168.67 ± 24.38 | 211.58 ± 43.25 | 217.66 ± 35.08 | <0.001 |
| HDL cholesterol (mg/dL) | 57.32 ± 8.45 | 51.43 ± 6.74 | 41.93 ± 13.38 | 41.67 ± 10.59 | 37.62 ± 8.98 | <0.001 |
| LDL cholesterol (mg/dL) | 74.77 ± 23.59 | 99.33 ± 25.22 | 99.51 ± 16.81 | 138.05 ± 46.00 | 147.67 ± 40.54 | <0.001 |
| Triglycerides (mg/dL) | 96.36 ± 26.22 | 124.22 ± 21.03 | 174.47 ± 64.77 | 159.33 ± 35.22 | 161.83 ± 65.63 | <0.001 |
| ALT (U/L) | 23.84 ± 6.53 | 25.08 ± 9.96 | 36.47 ± 11.30 | 37.58 ± 17.71 | 39.76 ± 19.22 | <0.001 |
| AST (U/L) | 21.56 ± 5.95 | 27.73 ± 6.53 | 35.70 ± 15.27 | 39.08 ± 15.44 | 41.28 ± 19.81 | <0.001 |
| γGT (U/L) | 25.20 ± 7.51 | 25.65 ± 10.81 | 38.13 ± 15.93 | 40.00 ± 14.70 | 49.72 ± 27.02 | <0.001 |
| FLI | 19.83 ± 13.66 | 45.02 ± 20.58 | 78.67 ± 11.27 | 89.88 ± 7.33 | 98.04 ± 2.55 | <0.001 |
| FLI-NAFLD (presence) | 0, 0% | 7, 30.4% | 14, 93.3% | 12, 100% | 29, 100% | <0.001 |
| Parameters | Vitamin D (ng/mL) | TMAO (µM) | ||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| Age (years) | −0.055 | 0.580 | 0.174 | 0.078 |
| BMI (kg/m2) | −0.752 | <0.001 | 0.827 | <0.001 |
| WC (cm) | −0.631 | <0.001 | 0.741 | <0.001 |
| Fasting Glucose (mg/dL) | −0.561 | <0.001 | 0.756 | <0.001 |
| Insulin (µU/mL) | −0.654 | <0.001 | 0.656 | <0.001 |
| Total cholesterol (mg/dL) | −0.493 | <0.001 | 0.631 | <0.001 |
| HDL cholesterol (mg/dL) | 0.482 | <0.001 | −0.676 | <0.001 |
| LDL cholesterol (mg/dL) | −0.502 | <0.001 | 0.653 | <0.001 |
| Triglycerides (mg/dL) | −0.491 | <0.001 | 0.495 | <0.001 |
| ALT (U/L) | −0.351 | <0.001 | 0.417 | <0.001 |
| AST (U/L) | −0.301 | <0.001 | 0.502 | <0.001 |
| γGT (U/L) | −0.339 | <0.001 | 0.462 | <0.001 |
| FLI | −0.647 | <0.001 | 0.826 | <0.001 |
| Parameters | Circulating Levels of TMAO (µM) | |||
|---|---|---|---|---|
| OR | p-Value | 95% IC | R2 | |
| BMI | ||||
| Normal weight | 0.08 | 0.004 | 0.016–0.465 | 0.588 |
| Overweight | 0.99 | 0.009 | 0.866–1.150 | 0.232 |
| Grade I obesity | 0.06 | 0.004 | 0.915–1.345 | 0.012 |
| Grade II obesity | 0.02 | 0.002 | 0.972–1.650 | 0.038 |
| Grade III obesity | 7.92 | <0.001 | 3.174–19.77 | 0.525 |
| Vitamin D | ||||
| Deficit | 1.62 | <0.001 | 1.32–1.99 | 0.302 |
| Insufficiency | 0.75 | <0.001 | 0.65–0.87 | 0.161 |
| Sufficiency | 0.68 | 0.004 | 0.53–0.89 | 0.094 |
| FLI-NAFLD | 3.79 | <0.001 | 2.06–6.89 | 0.518 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrea, L.; Muscogiuri, G.; Annunziata, G.; Laudisio, D.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. A New Light on Vitamin D in Obesity: A Novel Association with Trimethylamine-N-Oxide (TMAO). Nutrients 2019, 11, 1310. https://doi.org/10.3390/nu11061310
Barrea L, Muscogiuri G, Annunziata G, Laudisio D, de Alteriis G, Tenore GC, Colao A, Savastano S. A New Light on Vitamin D in Obesity: A Novel Association with Trimethylamine-N-Oxide (TMAO). Nutrients. 2019; 11(6):1310. https://doi.org/10.3390/nu11061310
Chicago/Turabian StyleBarrea, Luigi, Giovanna Muscogiuri, Giuseppe Annunziata, Daniela Laudisio, Giulia de Alteriis, Gian Carlo Tenore, Annamaria Colao, and Silvia Savastano. 2019. "A New Light on Vitamin D in Obesity: A Novel Association with Trimethylamine-N-Oxide (TMAO)" Nutrients 11, no. 6: 1310. https://doi.org/10.3390/nu11061310
APA StyleBarrea, L., Muscogiuri, G., Annunziata, G., Laudisio, D., de Alteriis, G., Tenore, G. C., Colao, A., & Savastano, S. (2019). A New Light on Vitamin D in Obesity: A Novel Association with Trimethylamine-N-Oxide (TMAO). Nutrients, 11(6), 1310. https://doi.org/10.3390/nu11061310










