Differential Effects of Dry vs. Wet Heating of β-Lactoglobulin on Formation of sRAGE Binding Ligands and sIgE Epitope Recognition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. BLG Isolation and Purification
2.3. Heat Treatment of BLG
2.4. Quantification of CML Using uHPLC-MS/MS
2.5. SDS-PAGE Gelelectorphoresis
2.6. Thioflavin-T Assay
2.7. Inhibition sRAGE ELISA
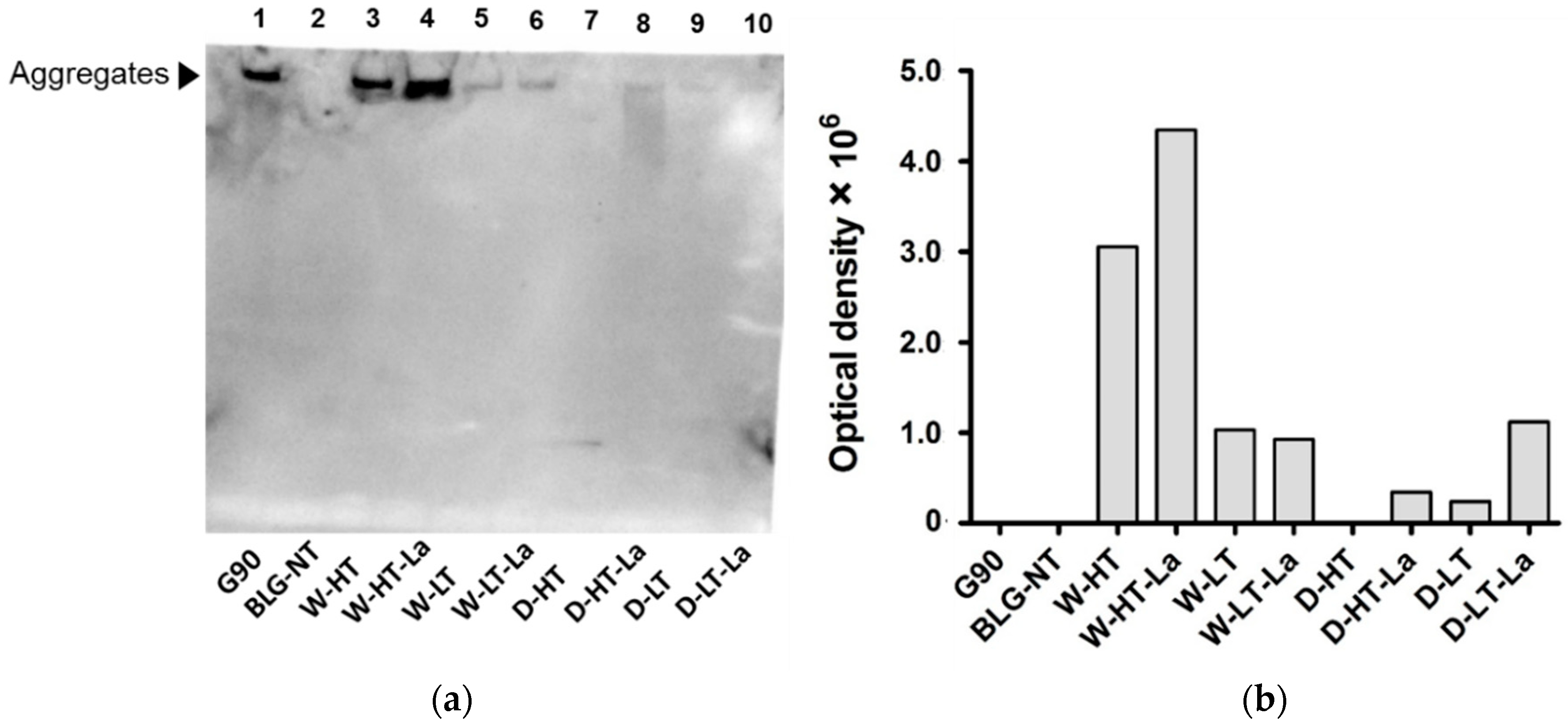
2.8. sRAGE Western Blot
2.9. CML Western Blot
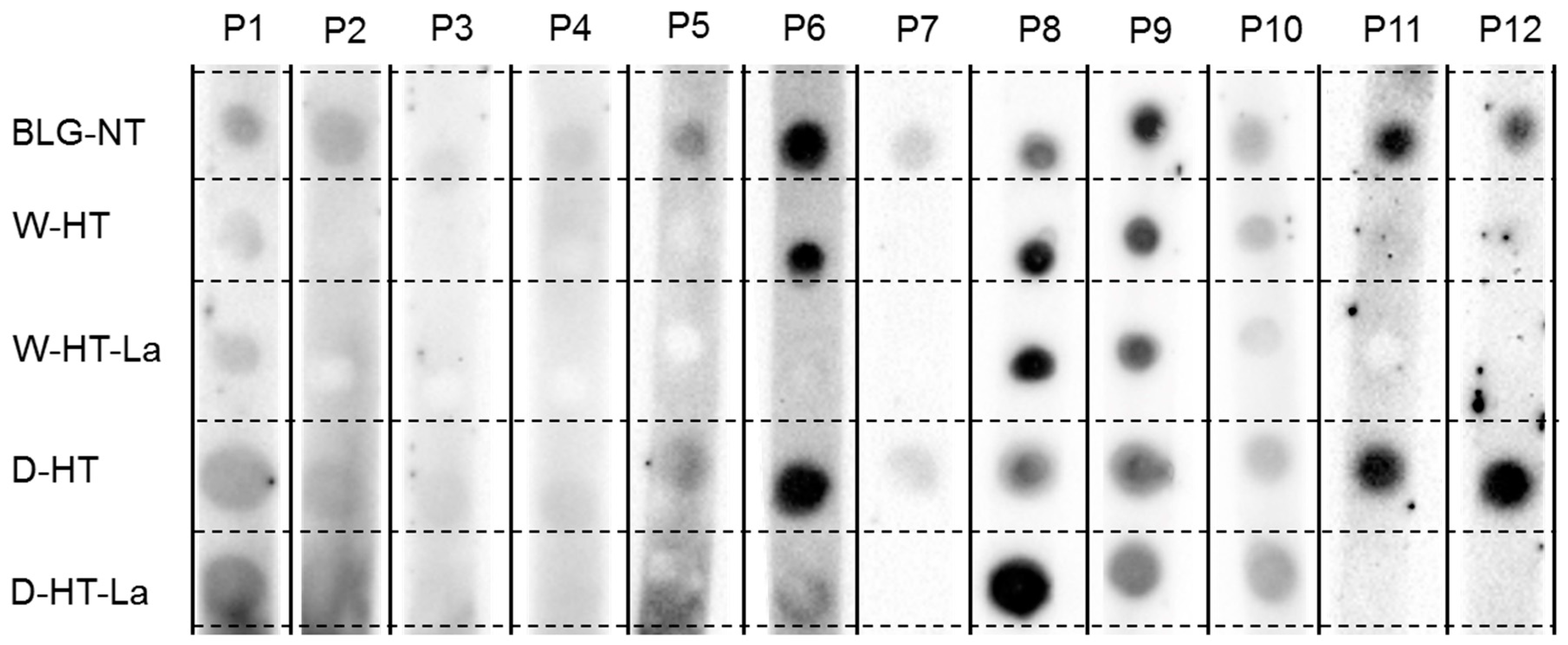
2.10. sIgE Binding Dot Blot
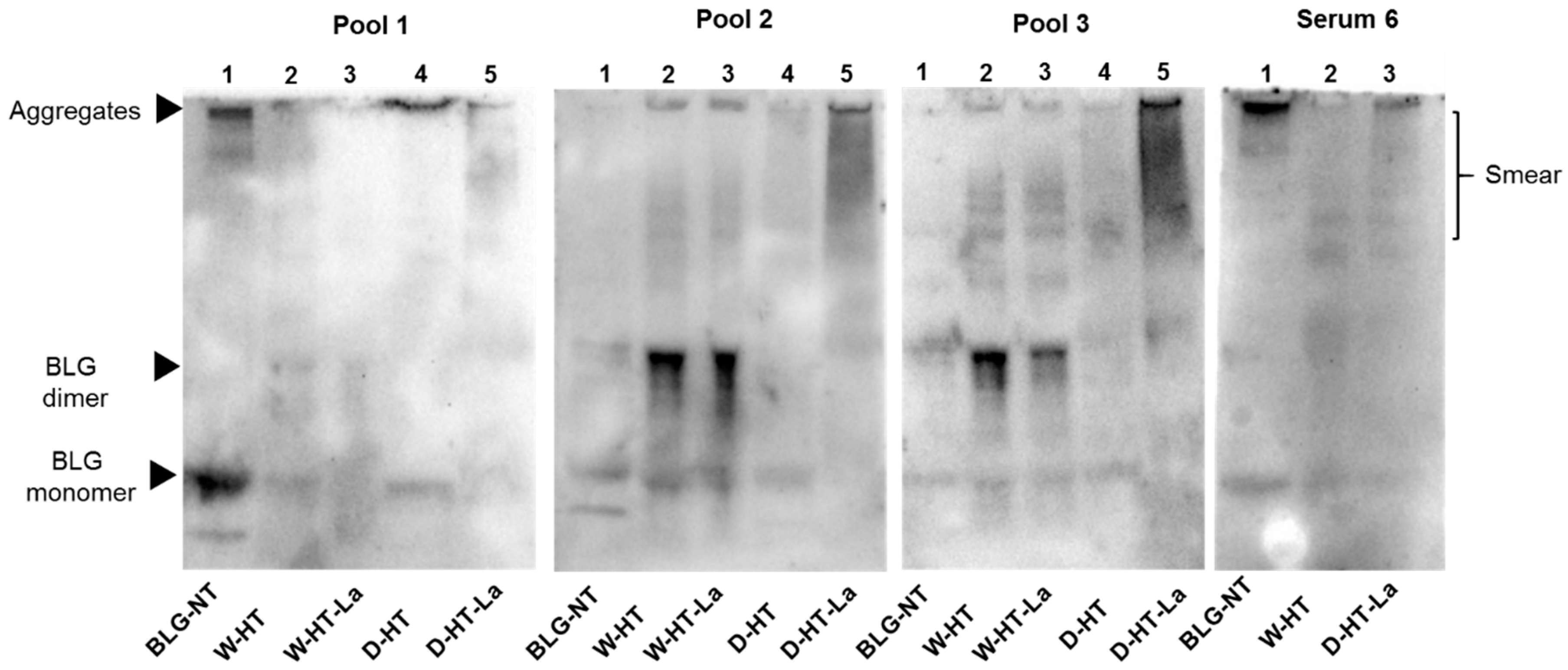
2.11. sIgE Binding Western Blot
2.12. Statistical Analysis
3. Results
3.1. Solubility of BLG after Heat Treatments
3.2. Quantification of Nε-carboxymethyl-L-lysine
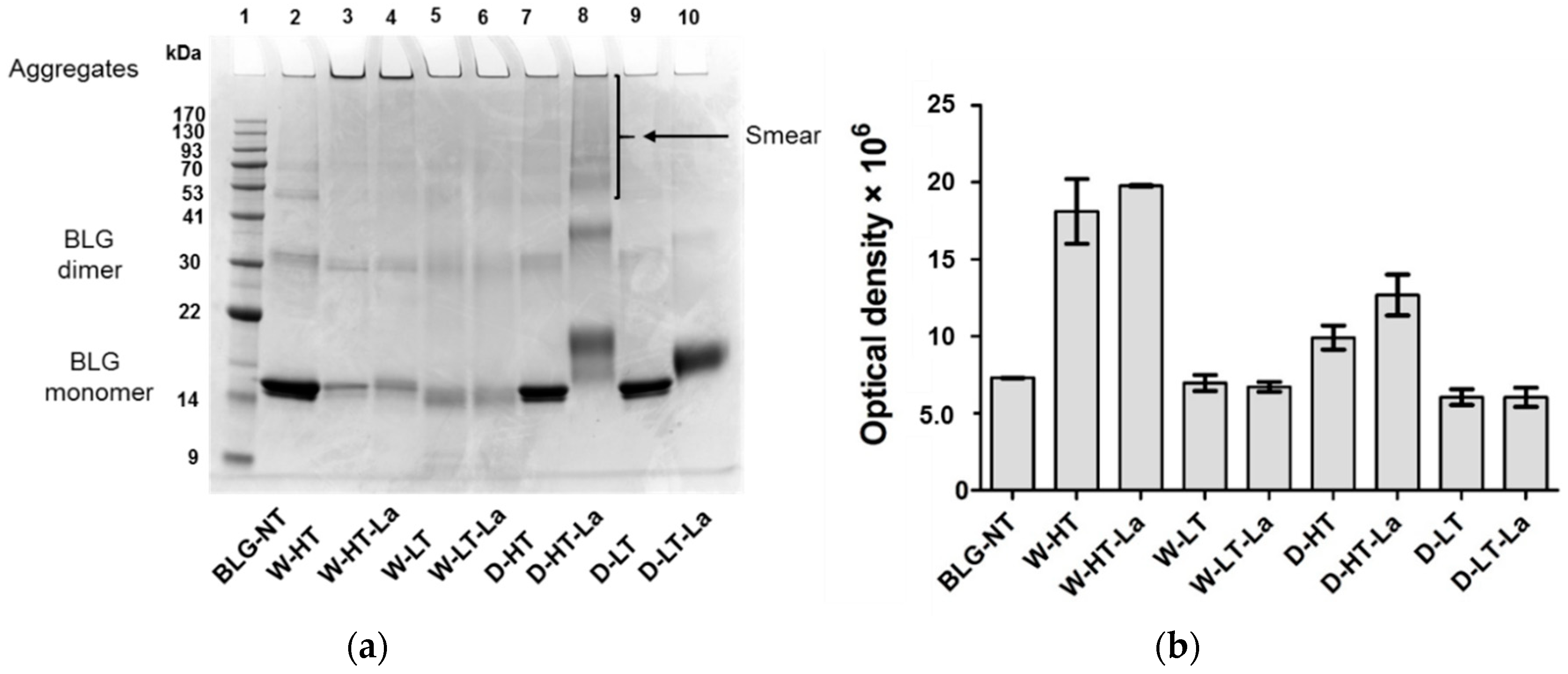
3.3. SDS-PAGE
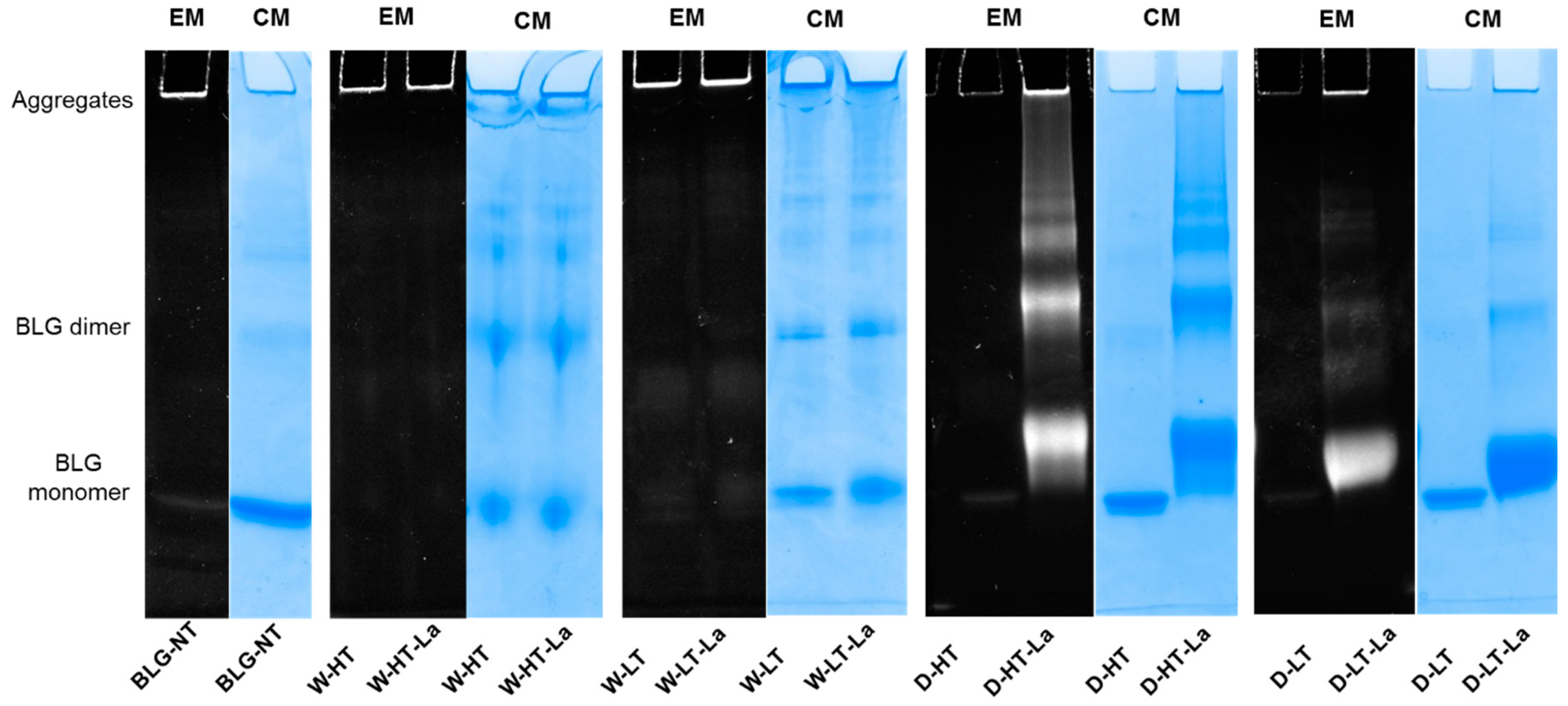
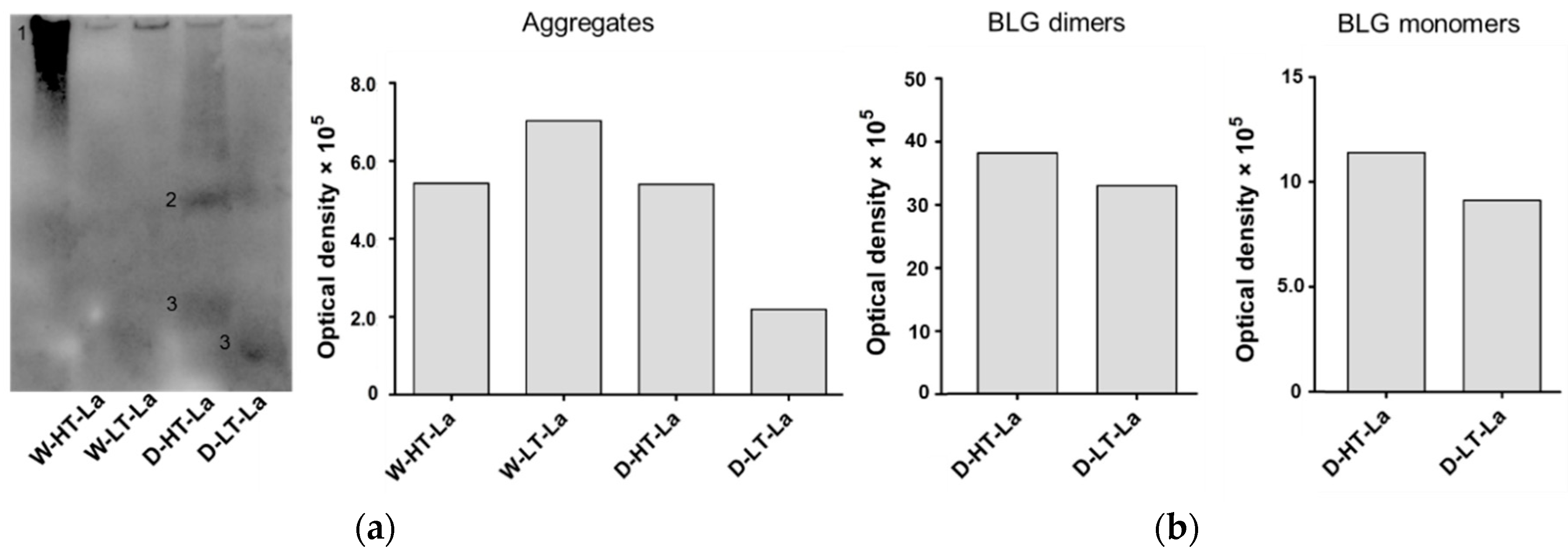
3.4. Detection of Glycation Strucutres on SDS-PAGE Visible Proteins
3.5. Formation of Fibril Structures
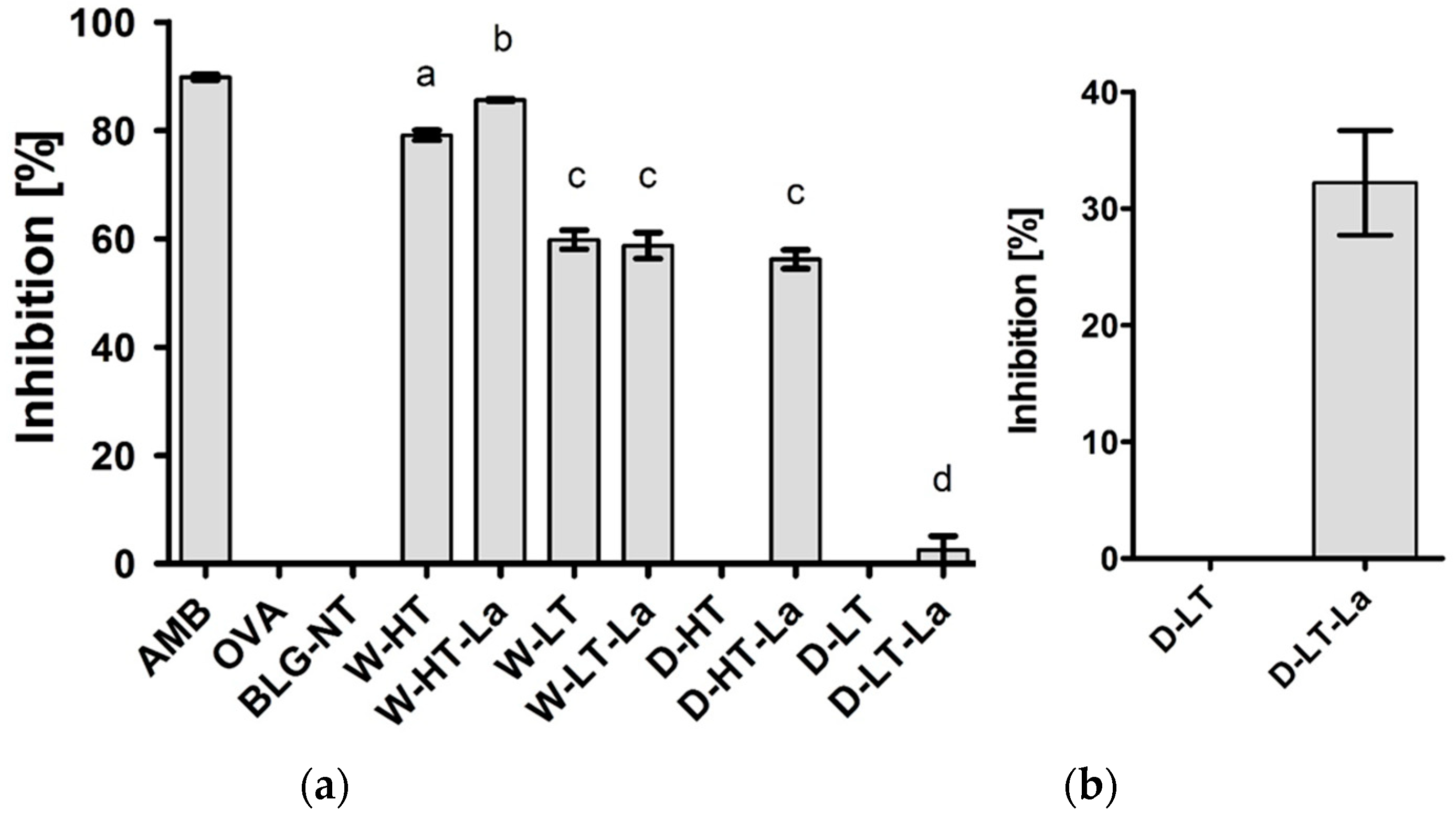
3.6. Binding of sRAGE to Heated and Glycated BLG
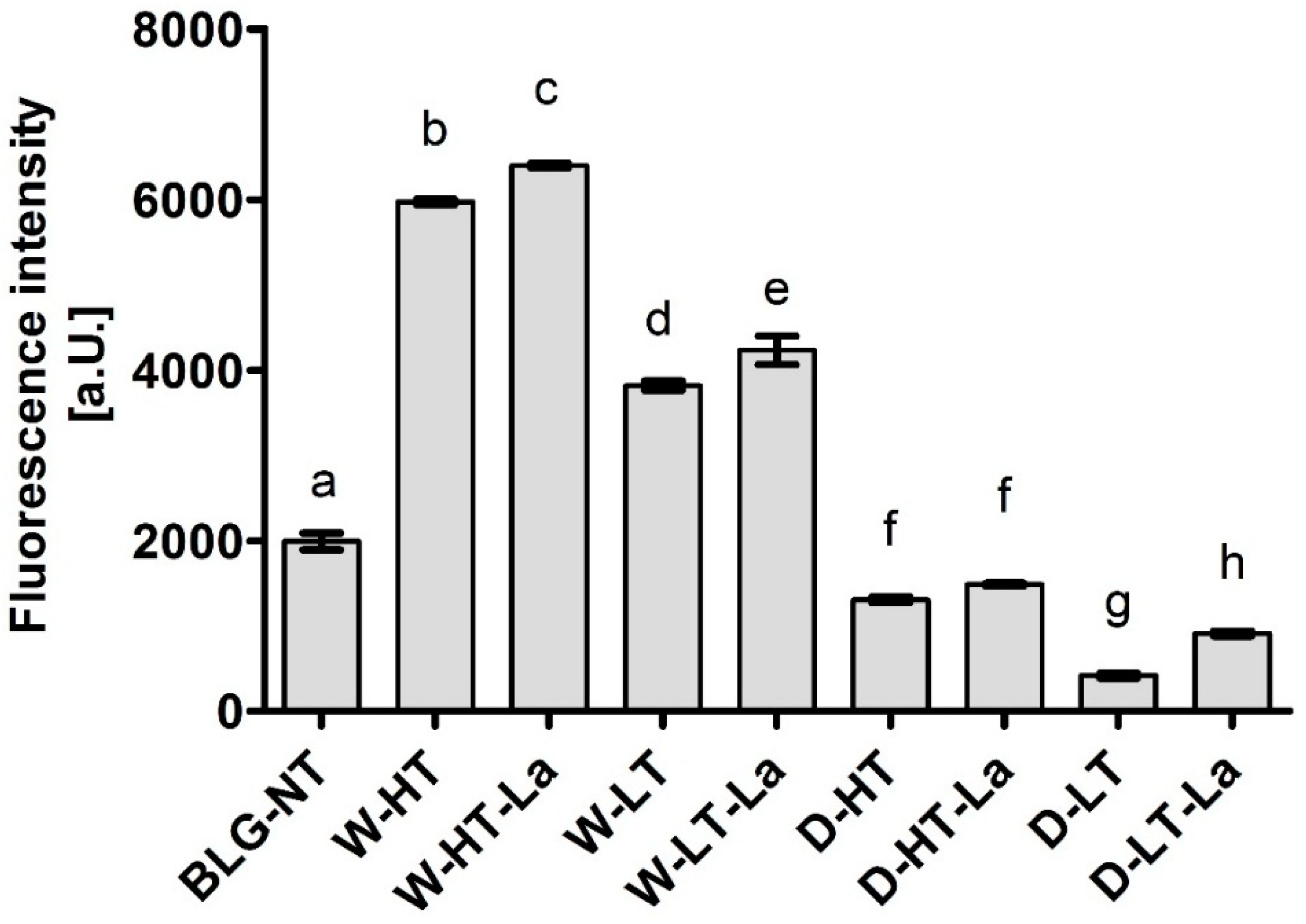
3.7. sIgE Binding to Heated and Glycated BLG
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deng, Y.; Govers, C.; Bastiaan-Net, S.; van der Hulst, N.; Hettinga, K.; Wichers, H.J. Hydrophobicity and aggregation, but not glycation, are key determinants for uptake of thermally processed β-lactoglobulin by thp-1 macrophages. Food Res. Int. 2019, 120, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Ames, J.M. Control of the maillard reaction in food systems. Trends Food Sci. Technol. 1990, 1, 150–154. [Google Scholar] [CrossRef]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Berin, M.C.; Arnaboldi, P.; Escalante, C.R.; Dahan, S.; Rauch, J.; Jensen-Jarolim, E.; Mayer, L. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through peyer’s patches. Eur. J. Allergy Clin. Immunol. 2008, 63, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Nowak-Wgrzyn, A.; Sicherer, S.H.; Noone, S.; Moshier, E.L.; Sampson, H.A. Dietary baked milk accelerates the resolution of cow’s milk allergy in children. J. Allergy Clin. Immunol. 2011, 128, 125–131. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Bloom, K.A.; Sicherer, S.H.; Shreffler, W.G.; Noone, S.; Wanich, N.; Sampson, H.A. Tolerance to extensively heated milk in children with cow’s milk allergy. J. Allergy Clin. Immunol. 2008, 122, 342–347. [Google Scholar] [CrossRef]
- Esmaeilzadeh, H.; Alyasin, S.; Haghighat, M.; Nabavizadeh, H.; Esmaeilzadeh, E.; Mosavat, F. The effect of baked milk on accelerating unheated cow’s milk tolerance: A control randomized clinical trial. Pediatr. Allergy Immunol. 2018, 29, 747–753. [Google Scholar] [CrossRef]
- Taheri-Kafrani, A.; Gaudin, J.C.; Rabesona, H.; Nioi, C.; Agarwal, D.; Drouet, M.; Chobert, J.M.; Bordbar, A.K.; Haertle, T. Effects of heating and glycation of β-lactoglobulin on its recognition by IgE of sera from cow milk allergy patients. J. Agric. Food Chem. 2009, 57, 4974–4982. [Google Scholar] [CrossRef]
- Ehn, B.M.; Ekstrand, B.; Bengtsson, U.; Ahlstedt, S. Modification of IgE binding during heat processing of the cow’s milk allergen β-lactoglobulin. J. Agric. Food Chem. 2004, 52, 1398–1403. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Santos, A.N.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [Green Version]
- Fritz, G. Rage: A single receptor fits multiple ligands. Trends Biochem. Sci. 2011, 36, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, K.; Fritz, G. Rage regulation and signaling in inflammation and beyond. J. Leukoc. Biol. 2013, 94, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Teodorowicz, M.; Wichers, H.J.; Van Boekel, M.A.J.S.; Hettinga, K.A. Generation of soluble advanced glycation end products receptor (srage)-binding ligands during extensive heat treatment of whey protein/lactose mixtures is dependent on glycation and aggregation. J. Agric. Food Chem. 2016, 64, 6477–6486. [Google Scholar] [CrossRef] [PubMed]
- Perkins, T.N.; Oczypok, E.A.; Dutz, R.E.; Donnell, M.L.; Myerburg, M.M.; Oury, T.D. The receptor for advanced glycation endproducts is a critical mediator of type 2 cytokine signaling in the lungs. J. Allergy Clin. Immunol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, L.P.; Gold, D.R.; Tzianabos, A.O.; Weiss, S.T.; Celedón, J.C. Cytokines, allergy, and asthma. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, H.B.; Wierenga, P.A.; Gruppen, H.; Schols, H.A. Maillard induced aggregation of individual milk proteins and interactions involved. Food Chem. 2019, 276, 652–661. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, H.H.J.; Gröneveld, T.; De Groot, J. Mild isolation procedure discloses new protein structural properties of β-lactoglobulin. J. Dairy Sci. 2001, 84, 562–571. [Google Scholar] [CrossRef]
- Troise, A.D.; Fiore, A.; Roviello, G.; Monti, S.M.; Fogliano, V. Simultaneous quantification of amino acids and amadori products in foods through ion-pairing liquid chromatography-high-resolution mass spectrometry. Amino Acids 2015, 47, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.S.P.; Naranjo, G.B.; Leiva, G.E.; Malec, L.S. Maillard reaction kinetics in milk powder: Effect of water activity at mild temperatures. Int. Dairy J. 2010, 20, 40–45. [Google Scholar] [CrossRef]
- Hoffmann, M.A.M.; Van Mil, P.J.J.M. Heat-induced aggregation of β-lactoglobulin: Role of the free thiol group and disulfide bonds. J. Agric. Food Chem. 1997, 45, 2942–2948. [Google Scholar] [CrossRef]
- Roefs, S.P.F.M.; De Kruif, K.G. A model for the denaturation and aggregation of β-lactoglobulin. Eur. J. Biochem. 1994, 226, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Fenaille, F.; Morgan, F.; Parisod, V.; Tabet, J.C.; Guy, P.A. Solid-state glycation of β-lactoglobulin by lactose and galactose: Localization of the modified amino acids using mass spectrometric techniques. J. Mass Spectrom. 2004, 39, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Akıllıoglu, H.G.; Çelikbıçak, O.; Salih, B.; Gokmen, V. Monitoring protein glycation by electrospray ionization (ESI) quadrupole time-of-flight (Q-TOF) mass spectrometer. Food Chem. 2017, 217, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, C.; Irace, G.; Sirangelo, I. Differential effects of glycation on protein aggregation and amyloid formation. Front. Mol. Biosci. 2014, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Bouma, B.; Kroon-Batenburg, L.M.J.; Wu, Y.P.; Brünjes, B.; Posthuma, G.; Kranenburg, O.; De Groot, P.G.; Voest, E.E.; Gebbink, M.F.B.G. Glycation induces formation of amyloid cross-β structure in albumin. J. Biol. Chem. 2003, 278, 41810–41819. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.S.; Léonil, J.; Henry, G.; Cauty, C.; Carvalho, A.F.; Bouhallab, S. Heating and glycation of β-lactoglobulin and β-casein: Aggregation and in vitro digestion. Food Res. Int. 2014, 55, 70–76. [Google Scholar] [CrossRef]
- Gulzar, M.; Bouhallab, S.; Jardin, J.; Briard-Bion, V.; Croguennec, T. Structural consequences of dry heating on alpha-lactalbumin and beta-lactoglobulin at pH 6.5. Food Res. Int. 2013, 51, 899–906. [Google Scholar] [CrossRef]
- Liu, F.; Teodorowicz, M.; Van Boekel, M.A.J.S.; Wichers, H.J.; Hettinga, K.A. The decrease in the igg-binding capacity of intensively dry heated whey proteins is associated with intense maillard reaction, structural changes of the proteins and formation of rage-ligands. Food Funct. 2016, 7, 239–249. [Google Scholar] [CrossRef]
- Perusko, M.; van Roest, M.; Stanic-Vucinic, D.; Simons, P.J.; Pieters, R.H.H.; Cirkovic Velickovic, T.; Smit, J.J. Glycation of the major milk allergen β-lactoglobulin changes its allergenicity by alterations in cellular uptake and degradation. Mol. Nutr. Food Res. 2018, 62, 1800341. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Zheng, Z.; Zheng, H. Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food Agric. Immunol. 2009, 20, 195–206. [Google Scholar] [CrossRef]
- Kleber, N.; Maier, S.; Hinrichs, J. Antigenic response of bovine β-lactoglobulin influenced by ultra-high pressure treatment and temperature. Innov. Food Sci. Emerg. Technol. 2007, 8, 39–45. [Google Scholar] [CrossRef]
- Cellmer, T.; Bratko, D.; Prausnitz, J.M.; Blanch, H.W. Protein aggregation in silico. Trends Biotechnol. 2007, 25, 254–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheurer, S.; Lauer, I.; Foetisch, K.; Moncin, M.S.M.; Retzek, M.; Hartz, C.; Enrique, E.; Lidholm, J.; Cistero-Bahima, A.; Vieths, S. Strong allergenicity of pru av 3, the lipid transfer protein from cherry, is related to high stability against thermal processing and digestion. J. Allergy Clin. Immunol. 2004, 114, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Vissers, Y.M.; Iwan, M.; Adel-Patient, K.; Skov, P.S.; Rigby, N.M.; Johnson, P.E.; Müller, P.M.; Przybylski-Nicaise, L.; Schaap, M.; Ruinemans-Koerts, J.; et al. Effect of roasting on the allergenicity of major peanut allergens ara h 1 and ara h 2/6: The necessity of degranulation assays. Clin. Exp. Allergy 2011, 41, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Gruber, P.; Vieths, S.; Wangorsch, A.; Nerkamp, J.; Hofmann, T. Maillard reaction and enzymatic browning affect the allergenicity of pru av 1, the major allergen from cherry (Prunus avium). J. Agric. Food Chem. 2004, 52, 4002–4007. [Google Scholar] [CrossRef] [PubMed]
- Blanc, F.; Vissers, Y.M.; Adel-Patient, K.; Rigby, N.M.; Mackie, A.R.; Gunning, A.P.; Wellner, N.K.; Skov, P.S.; Przybylski-Nicaise, L.; Ballmer-Weber, B.; et al. Boiling peanut ara h 1 results in the formation of aggregates with reduced allergenicity. Mol. Nutr. Food Res. 2011, 55, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Conformational changes of β-lactoglobulin induced by shear, heat, and pH-effects on antigenicity. J. Dairy Sci. 2015, 98, 4255–4265. [Google Scholar] [CrossRef]
- Iwan, M.; Vissers, Y.M.; Fiedorowicz, E.; Kostyra, H.; Kostyra, E.; Savelkoul, H.F.J.; Wichers, H.J. Impact of maillard reaction on immunoreactivity and allergenicity of the hazelnut allergen cor a 11. J. Agric. Food Chem. 2011, 59, 7163–7171. [Google Scholar] [CrossRef]
- Taylor, S.L.; Lemanske, R.F.; Bush, R.K.; Busse, W.W. Food allergens: Structure and immunologic properties. Ann. Allergy 1987, 59, 93–99. [Google Scholar]
| Patient # | sIgE-Level Cow’s Milk Proteins [kU/L] | Specimen Type | Dilution |
|---|---|---|---|
| 1 | 52.2 | Serum | 1:5 |
| 2 | 0.73 | Serum | 1:3 |
| 3 | 0.96 | Serum | 1:3 |
| 4 | 0.53 | Serum | 1:3 |
| 5 | 0.96 | Serum | 1:3 |
| 6 | 1.69 | Serum | 1:3 |
| 7 | 1.55 | Serum | 1:3 |
| 8 | >100 | Plasma | 1:5 |
| 9 | 91.0 | Plasma | 1:5 |
| 10 | 94.8 | Plasma | 1:5 |
| 11 | 28.4 | Serum | 1:5 |
| 12 | 6.6 | Serum | 1:5 |
| Pool/Serum | Patient Serum/Plasma | Dilution |
|---|---|---|
| Pool 1 | 7, 11, and 12 | 1:5 |
| Serum 6 | 6 | 1:7 |
| Pool 2 | 8 and 9 | 1:7 |
| Pool 3 | 1 and 10 | 1:5 |
| Sample | BLG-NT | W-HT-La | W-HT | W-LT-La | W-LT | D-HT-La | D-HT | D-LT-La | D-LT |
|---|---|---|---|---|---|---|---|---|---|
| CML mg/100 g protein | ND | 129 ± 10 a | ND | 77 ± 4 b | ND | 182 ± 7 c | ND | 158 ± 9 d | ND |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenker, H.E.; Ewaz, A.; Deng, Y.; Savelkoul, H.F.J.; van Neerven, R.J.J.; De Jong, N.W.; Wichers, H.J.; Hettinga, K.A.; Teodorowicz, M. Differential Effects of Dry vs. Wet Heating of β-Lactoglobulin on Formation of sRAGE Binding Ligands and sIgE Epitope Recognition. Nutrients 2019, 11, 1432. https://doi.org/10.3390/nu11061432
Zenker HE, Ewaz A, Deng Y, Savelkoul HFJ, van Neerven RJJ, De Jong NW, Wichers HJ, Hettinga KA, Teodorowicz M. Differential Effects of Dry vs. Wet Heating of β-Lactoglobulin on Formation of sRAGE Binding Ligands and sIgE Epitope Recognition. Nutrients. 2019; 11(6):1432. https://doi.org/10.3390/nu11061432
Chicago/Turabian StyleZenker, Hannah E., Arifa Ewaz, Ying Deng, Huub F. J. Savelkoul, R.J. Joost van Neerven, Nicolette W. De Jong, Harry J. Wichers, Kasper A. Hettinga, and Malgorzata Teodorowicz. 2019. "Differential Effects of Dry vs. Wet Heating of β-Lactoglobulin on Formation of sRAGE Binding Ligands and sIgE Epitope Recognition" Nutrients 11, no. 6: 1432. https://doi.org/10.3390/nu11061432
APA StyleZenker, H. E., Ewaz, A., Deng, Y., Savelkoul, H. F. J., van Neerven, R. J. J., De Jong, N. W., Wichers, H. J., Hettinga, K. A., & Teodorowicz, M. (2019). Differential Effects of Dry vs. Wet Heating of β-Lactoglobulin on Formation of sRAGE Binding Ligands and sIgE Epitope Recognition. Nutrients, 11(6), 1432. https://doi.org/10.3390/nu11061432





