Impact of Percent Body Fat on All-Cause Mortality among Adequate Dialysis Patients with and without Insulin Resistance: A Multi-Center Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
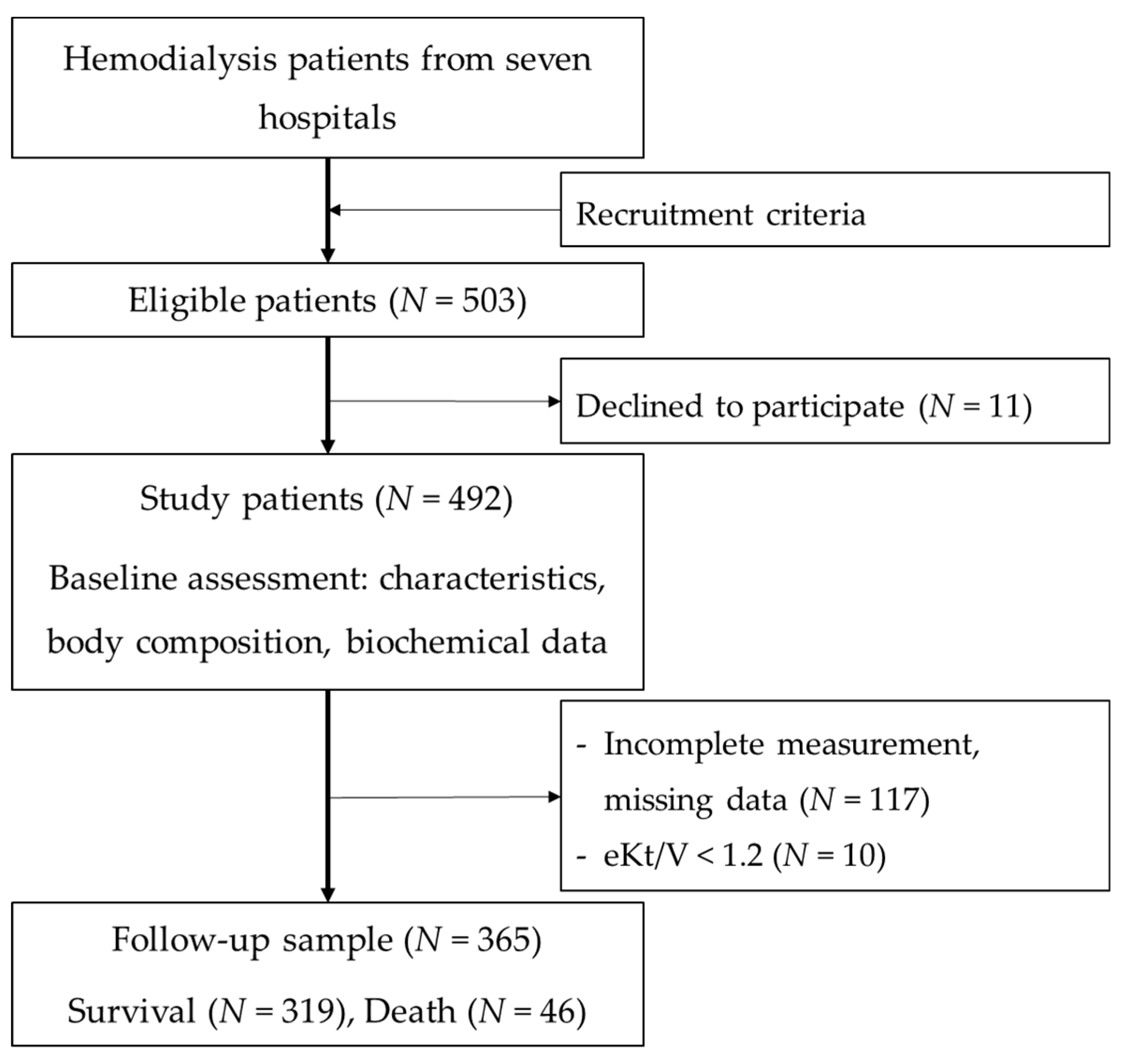
2.2. Patient Recruitments
2.3. Patients’ Characteristics
2.4. Clinical Parameters
2.5. Biochemical Parameters
2.6. Ethical Consideration
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, P.; Ma, F.; Lou, H.; Liu, Y. The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Public Health 2013, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef]
- De Gonzalez, A.B.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J.; Moore, S.C.; Tobias, G.S.; Anton-Culver, H.; Freeman, L.B.; et al. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, G.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J. Obesity paradox in patients on maintenance dialysis. In Obesity and the Kidney; Wolf, G., Ed.; Karger: Basel, Switzerland, 2006; Volume 151, pp. 57–69. [Google Scholar]
- Kalantar-Zadeh, K.; Rhee, C.M.; Chou, J.; Ahmadi, S.F.; Park, J.; Chen, J.L.; Amin, A.N. The obesity paradox in kidney disease: How to reconcile it with obesity management. Kidney Int. Rep. 2017, 2, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ahmadi, S.-F.; Streja, E.; Molnar, M.Z.; Flegal, K.M.; Gillen, D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Obesity paradox in end-stage kidney disease patients. Prog. Cardiovasc. Dis. 2014, 56, 415–425. [Google Scholar] [CrossRef]
- Yajima, T.; Yajima, K.; Takahashi, H.; Yasuda, K. The impact of abdominal fat levels on all-cause mortality risk in patients undergoing hemodialysis. Nutrients 2018, 10, 480. [Google Scholar] [CrossRef]
- Fragoso, A.; Mendes, F.; Silva, A.P.; Neves, P.L. Insulin resistance as a predictor of cardiovascular morbidity and end-stage renal disease. J. Diabetes Complicat. 2015, 29, 1098–1104. [Google Scholar] [CrossRef]
- Shinohara, K.; Shoji, T.; Emoto, M.; Tahara, H.; Koyama, H.; Ishimura, E.; Miki, T.; Tabata, T.; Nishizawa, Y. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J. Am. Soc. Nephrol. 2002, 13, 1894–1900. [Google Scholar] [CrossRef]
- Ricks, J.; Molnar, M.Z.; Kovesdy, C.P.; Shah, A.; Nissenson, A.R.; Williams, M.; Kalantar-Zadeh, K. Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes. Diabetes 2012, 61, 708–715. [Google Scholar] [CrossRef]
- Hung, A.M.; Ikizler, T.A. Factors determining insulin resistance in chronic hemodialysis patients. Contrib. Nephrol. 2011, 171, 127–134. [Google Scholar] [PubMed]
- Teta, D. Insulin resistance as a therapeutic target for chronic kidney disease. J. Ren. Nutr. 2015, 25, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Wesołowski, P.; Saracyn, M.; Nowak, Z.; Wańkowicz, Z. Insulin resistance as a novel therapeutic target in patients with chronic kidney disease treated with dialysis. Pol. Arch. Med. Wewn. 2010, 120, 54–57. [Google Scholar] [PubMed]
- Doshi, M.; Streja, E.; Rhee, C.M.; Park, J.; Ravel, V.A.; Soohoo, M.; Moradi, H.; Lau, W.L.; Mehrotra, R.; Kuttykrishnan, S.; et al. Examining the robustness of the obesity paradox in maintenance hemodialysis patients: A marginal structural model analysis. Nephrol. Dial. Transplant. 2016, 31, 1310–1319. [Google Scholar] [CrossRef]
- Huang, J.-C.; Lin, H.Y.-H.; Lim, L.-M.; Chen, S.-C.; Chang, J.-M.; Hwang, S.-J.; Tsai, J.-C.; Hung, C.-C.; Chen, H.-C. Body mass index, mortality, and gender difference in advanced chronic kidney disease. PLoS ONE 2015, 10, e0126668. [Google Scholar] [CrossRef]
- Noori, N.; Kovesdy, C.P.; Dukkipati, R.; Kim, Y.; Duong, U.; Bross, R.; Oreopoulos, A.; Luna, A.; Benner, D.; Kopple, J.D.; et al. Survival predictability of lean and fat mass in men and women undergoing maintenance hemodialysis. Am. J. Clin. Nutr. 2010, 92, 1060–1070. [Google Scholar] [CrossRef]
- Jialin, W.; Yi, Z.; Weijie, Y. Relationship between body mass index and mortality in hemodialysis patients: A meta-analysis. Nephron Clin. Pract. 2012, 121, c102–c111. [Google Scholar] [CrossRef]
- Duong, T.V.; Wong, T.-C.; Su, C.-T.; Chen, H.-H.; Chen, T.-W.; Chen, T.-H.; Hsu, Y.-H.; Peng, S.-J.; Kuo, K.-L.; Liu, H.-C.; et al. Associations of dietary macronutrients and micronutrients with the traditional and nontraditional risk factors for cardiovascular disease among hemodialysis patients: A clinical cross-sectional study. Medicine (Baltim.) 2018, 97, e11306. [Google Scholar] [CrossRef]
- Hemmelgarn, B.R.; Manns, B.J.; Quan, H.; Ghali, W.A. Adapting the Charlson comorbidity index for use in patients with ESRD. Am. J. Kidney Dis. 2003, 42, 125–132. [Google Scholar] [CrossRef]
- Liou, Y.M.; Jwo, C.J.C.; Yao, K.G.; Chiang, L.-C.; Huang, L.-H. Selection of appropriate Chinese terms to represent intensity and types of physical activity terms for use in the Taiwan version of IPAQ. J. Nurs. Res. 2008, 16, 252–263. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.J.; Lee, J.E.; Kim, J.K.; Yoon, S.Y.; Kang, S.W.; Choi, K.H.; Ha, S.K.; Park, H.C. The relationship between hemodialysis modality and insulin resistance in non-diabetic hemodialysis patients. Blood Purif. 2015, 39, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Omae, K.; Kondo, T.; Tanabe, K. High preoperative C-reactive protein values predict poor survival in patients on chronic hemodialysis undergoing nephrectomy for renal cancer. Urol. Oncol. 2015, 33, 67e9–67e13. [Google Scholar] [CrossRef] [PubMed]
- KDOQI; National Kidney Foundation. Clinical practice guidelines and clinical practice recommendations for Anemia in chronic kidney disease in adults. Am. J. Kidney Dis. 2006, 47, S16–S85. [Google Scholar] [CrossRef]
- Ascaso, J.F.; Pardo, S.; Real, J.T.; Lorente, R.I.; Priego, A.; Carmena, R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care 2003, 26, 3320–3325. [Google Scholar] [CrossRef]
- Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- European Association for Cardiovascular Prevention & Rehabilitation; Reiner, Z.; Catapano, A.L.; De Backer, G.; Graham, I.; Taskinen, M.-R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; et al. ESC/EAS guidelines for the management of dyslipidaemias: The task force for the management of dyslipidaemias of the European society of cardiology (ESC) and the European atherosclerosis society (EAS). Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar]
- Kidney Disease Outcomes Quality Initiative (K/DOQI) Group. K/DOQI clinical practice guidelines for management of dyslipidemias in patients with kidney disease. Am. J. Kidney Dis. 2003, 41, S1–S91. [Google Scholar]
- Hager, M.R.; Narla, A.D.; Tannock, L.R. Dyslipidemia in patients with chronic kidney disease. Rev. Endocr. Metab. Disord. 2017, 18, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD). Kidney Int. 2009, 76, S1–S130. [Google Scholar]
- Yerram, P.; Karuparthi, P.R.; Hesemann, L.; Horst, J.; Whaley-Connell, A. Chronic kidney disease and cardiovascular risk. J. Am. Soc. Hypertens. 2007, 1, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Regidor, D.L.; Mehrotra, R.; Jing, J.; McAllister, C.J.; Greenland, S.; Kopple, J.D.; Kalantar-Zadeh, K. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2007, 2, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Razali, N.M.; Wah, Y.B. Power comparisons of shapiro-wilk, kolmogorov-smirnov, lilliefors and anderson-darling tests. J. Stat. Model. Anal. 2011, 2, 21–23. [Google Scholar]
- Stosovic, M.; Stanojevic, M.; Radovic, M.; Simic-Ogrizovic, S.; Lezaic, V.; Naumovic, R.; Jovanovic, D.; Ristic, G.; Djukanovic, L.; Marinkovic, J. Hemodialysis modality, percentage of body fat, and patient survival. Int. J. Artif. Organs 2009, 32, 20–30. [Google Scholar] [CrossRef]
- Caetano, C.; Valente, A.; Oliveira, T.; Garagarza, C. Body composition and mortality predictors in hemodialysis patients. J. Ren. Nutr. 2016, 26, 81–86. [Google Scholar] [CrossRef]
- Huang, C.X.; Tighiouart, H.; Beddhu, S.; Cheung, A.K.; Dwyer, J.T.; Eknoyan, G.; Beck, G.J.; Levey, A.S.; Sarnak, M.J. Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney Int. 2010, 77, 624–629. [Google Scholar] [CrossRef]
- Yajima, T.; Arao, M.; Yajima, K.; Takahashi, H.; Yasuda, K. The associations of fat tissue and muscle mass indices with all-cause mortality in patients undergoing hemodialysis. PLoS ONE 2019, 14, e0211988. [Google Scholar] [CrossRef]
- Duong, T.V.; Wu, P.-Y.; Wong, T.-C.; Chen, H.-H.; Chen, T.-H.; Hsu, Y.-H.; Peng, S.-J.; Kuo, K.-L.; Liu, H.-C.; Lin, E.-T.; et al. Mid-arm circumference, body fat, nutritional and inflammatory biomarkers, blood glucose, dialysis adequacy influence all-cause mortality in hemodialysis patients: A prospective cohort study. Medicine (Baltim.) 2019, 98, e14930. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kuwae, N.; Wu, D.Y.; Shantouf, R.S.; Fouque, D.; Anker, S.D.; Block, G.; Kopple, J.D. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am. J. Clin. Nutr. 2006, 83, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ito, Y.; Takahashi, H.; Ishii, H.; Kasuga, H.; Mizuno, M.; Suzuki, Y.; Yuzawa, Y.; Maruyama, S.; Murohara, T.; et al. Combined values of serum albumin, C-reactive protein and body mass index at dialysis initiation accurately predicts long-term mortality. Am. J. Nephrol. 2012, 36, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kimata, N.; Albert, J.M.; Akiba, T.; Yamazaki, S.; Kawaguchi, Y.; Fukuhara, S.; Akizawa, T.; Saito, A.; Asano, Y.; Kurokawa, K.; et al. Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: The Japan dialysis outcomes and practice patterns study. Hemodial. Int. 2007, 11, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R.; Rodriguez-Osorio, L.; Mas, S.; Abadi, Y.; Rubert, M.; de la Piedra, C.; Gracia-Iguacel, C.; Mahillo, I.; Ortiz, A.; Egido, J.; et al. A decrease in intact parathyroid hormone (iPTH) levels is associated with higher mortality in prevalent hemodialysis patients. PLoS ONE 2017, 12, e0173831. [Google Scholar] [CrossRef] [PubMed]
- Kleber, M.E.; Delgado, G.; Grammer, T.B.; Silbernagel, G.; Huang, J.; Krämer, B.K.; Ritz, E.; März, W. Uric acid and cardiovascular events: A mendelian randomization study. J. Am. Soc. Nephrol. 2015, 26, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Beberashvili, I.; Sinuani, I.; Azar, A.; Shapiro, G.; Feldman, L.; Stav, K.; Sandbank, J.; Averbukh, Z. Serum uric acid as a clinically useful nutritional marker and predictor of outcome in maintenance hemodialysis patients. Nutrition 2015, 31, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Huang, L.; Song, M.; Song, Y. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: A meta-analysis of prospective studies. Atherosclerosis 2013, 231, 61–68. [Google Scholar] [CrossRef]
- Bae, E.; Cho, H.-J.; Shin, N.; Kim, S.M.; Yang, S.H.; Kim, D.K.; Kim, Y.-L.; Kang, S.-W.; Yang, C.W.; Kim, N.H.; et al. Lower serum uric acid level predicts mortality in dialysis patients. Medicine (Baltim.) 2016, 95, e3701. [Google Scholar] [CrossRef]
- Park, C.; Obi, Y.; Streja, E.; Rhee, C.M.; Catabay, C.J.; Vaziri, N.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum uric acid, protein intake and mortality in hemodialysis patients. Nephrol. Dial. Transplant. 2017, 32, 1750–1757. [Google Scholar] [CrossRef]
- Jeon, J.S.; Chung, S.H.; Han, D.C.; Noh, H.; Kwon, S.H.; Lindholm, B.; Lee, H.B. Mortality predictive role of serum uric acid in diabetic hemodialysis patients. J. Ren. Nutr. 2014, 24, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Kleine, C.-E.; Moradi, H.; Streja, E.; Kalantar-Zadeh, K. Racial and ethnic disparities in the obesity paradox. Am. J. Kidney Dis. 2018, 72, S26–S32. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (N = 365) | Total (N = 365) | HOMA-IR < 5.18 (N = 183) | HOMA-IR ≥ 5.18 (N = 182) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survival (N = 319) | Death (N = 46) | p1 | Survival (N = 157) | Death (N = 26) | p1 | Survival (N = 162) | Death (N = 20) | p1 | ||
| Age ≥ 65 years | 129 (35.3) | 108 (33.9) | 21 (45.7) | 0.118 | 53 (33.8) | 11 (42.3) | 0.397 | 55 (34.0) | 10 (50.0) | 0.158 |
| Gender, male | 205 (56.2) | 175 (54.9) | 30 (65.2) | 0.186 | 88 (56.1) | 14 (53.8) | 0.834 | 87 (53.7) | 16 (80.0) | 0.025 |
| Hemodialysis vintage, year | 5.8 ± 5.0 | 5.9 ± 5.1 | 4.7 ± 4.1 | 0.137 | 6.7 ± 5.4 | 4.1 ± 2.1 | 0.018 | 5.1 ± 4.6 | 5.6 ± 5.5 | 0.717 |
| CCI | 4.7 ± 1.6 | 4.6 ± 1.5 | 5.5 ± 1.6 | <0.001 | 4.4 ± 1.5 | 5.3 ± 1.8 | 0.008 | 4.7 ± 1.5 | 5.7 ± 1.3 | 0.004 |
| PA, MET-min/week | 4886.1 ± 1887.1 | 4999.4 ± 1895.2 | 4100.5 ± 1643.6 | 0.002 | 4865.4 ± 2033.4 | 3871.1 ± 1701.3 | 0.019 | 5129.2 ± 1747.5 | 4398.8 ± 1556.7 | 0.076 |
| Clinical parameters | ||||||||||
| SBP ≥ 130 mmHg | 297 (81.4) | 257 (80.6) | 40 (87.0) | 0.298 | 131 (83.4) | 21 (80.8) | 0.737 | 126 (77.8) | 19 (95.0) | 0.071 |
| DBP ≥ 85 mmHg | 90 (24.7) | 80 (25.1) | 10 (21.7) | 0.623 | 40 (25.5) | 5 (19.2) | 0.493 | 40 (24.7) | 5 (25.0) | 0.976 |
| BMI ≥ 24.0 kg/m2 | 146 (40.0) | 127 (39.8) | 19 (41.3) | 0.847 | 48 (30.6) | 9 (34.6) | 0.680 | 79 (48.8) | 10 (50.0) | 0.917 |
| CTR ≥ 50% | 144 (39.5) | 118 (37.8) | 26 (57.8) | 0.011 | 55 (35.9) | 13 (52.0) | 0.126 | 63 (39.6) | 13 (65.0) | 0.030 |
| TMM, kg | 41.3 ± 9.3 | 41.1 ± 9.3 | 42.7 ± 9.9 | 0.272 | 41.0 ± 8.6 | 40.9 ± 11.8 | 0.988 | 41.1 ± 9.9 | 44.9 ± 6.2 | 0.097 |
| BFM, kg | 17.8 ± 8.0 | 18.0 ± 8.1 | 16.5 ± 7.2 | 0.233 | 15.6 ± 7.2 | 16.3 ± 7.8 | 0.643 | 20.3 ± 8.3 | 16.7 ± 6.4 | 0.063 |
| PBF, % | 28.4 ± 9.7 | 28.7 ± 9.7 | 26.1 ± 9.3 | 0.092 | 26.0 ± 9.7 | 26.7 ± 10.3 | 0.745 | 31.3 ± 9.0 | 25.4 ± 8.2 | 0.005 |
| Biochemical parameters | ||||||||||
| hs-CRP > 0.5 mg/dL | 105 (28.8) | 81 (25.4) | 24 (52.2) | <0.001 | 30 (19.1) | 16 (61.5) | <0.001 | 51 (31.5) | 8 (40.0) | 0.443 |
| Anemia (Hgb < 11 g/dL) | 209 (57.3) | 178 (55.8) | 31 (67.4) | 0.137 | 80 (51.0) | 18 (69.2) | 0.084 | 98 (60.5) | 13 (65.0) | 0.697 |
| FPG (mg/dL) | 131.5 ± 58.2 | 131.1 ± 58.8 | 134.3 ± 54.8 | 0.725 | 104.4 ± 35.8 | 121.0 ± 49.5 | 0.041 | 156.9 ± 64.9 | 151.7 ± 57.6 | 0.732 |
| IFG 2 | 253 (69.3) | 215 (67.4) | 38 (82.6) | 0.037 | 72 (45.9) | 18 (69.2) | 0.027 | 143 (88.3) | 20 (100.0) | |
| Insulin, µU/mL | 16.7 (8.8–31.8) | 16.9 (8.8–32.2) | 14.5 (7.3–28.7) | 0.386 | 8.8 (5.9–12.7) | 7.6 (6.0–14.0) | 0.886 | 32.0 (24.2–49.3) | 30.4 (19.9–38.9) | 0.412 |
| Insulin ≥ 12.0 µU/mL) | 234 (64.1) | 205 (64.3) | 29 (63.0) | 0.872 | 46 (29.3) | 9 (34.6) | 0.584 | 159 (98.1) | 20 (100.0) | |
| HOMA-IR ≥ 5.18 | 182 (49.9) | 160 (50.8) | 20 (43.5) | 0.354 | ||||||
| TG ≥ 150 mg/dL) | 143 (39.2) | 127 (39.8) | 16 (34.8) | 0.514 | 34 (21.7) | 10 (38.5) | 0.063 | 93 (57.4) | 6 (36.0) | 0.020 |
| Low HDL-C (<40 mg/dL for men, <50 mg/dL for women) | 203 (55.6) | 180 (63.2) | 23 (52.3) | 0.167 | 78 (53.1) | 14 (56.0) | 0.785 | 102 (73.9) | 9 (47.4) | 0.017 |
| LDL-C ≥ 100 mg/dL) | 179 (49.0) | 158 (49.5) | 21 (45.7) | 0.623 | 82 (52.2) | 15 (57.7) | 0.605 | 76 (46.9) | 6 (30.0) | 0.151 |
| TC ≥ 200 mg/dL | 62 (17.0) | 57 (17.9) | 5 (10.9) | 0.237 | 30 (19.1) | 3 (11.5) | 0.352 | 27 (16.7) | 2 (10.0) | 0.442 |
| Dyslipidemia 3 | 299 (81.9) | 365 (83.1) | 34 (73.9) | 0.131 | 121 (77.1) | 20 (76.9) | 0.987 | 144 (88.9) | 14 (70.0) | 0.018 |
| Serum Ca > 9.5 mg/dL | 132 (36.2) | 119 (37.3) | 13 (28.3) | 0.233 | 58 (36.9) | 9 (34.6) | 0.820 | 61 (37.7) | 4 (20.0) | 0.120 |
| Serum PO4 > 5.5 mg/dL | 126 (34.5) | 113 (35.4) | 13 (28.3) | 0.339 | 57 (36.3) | 8 (30.8) | 0.585 | 56 (34.6) | 5 (25.0) | 0.392 |
| Ca x PO4 ≥ 55 mg2/dL2 | 92 (25.2) | 80 (25.1) | 12 (26.1) | 0.883 | 37 (23.6) | 8 (30.8) | 0.430 | 43 (26.5) | 4 (20.0) | 0.528 |
| iPTH ≥ 300 pg/mL | 157 (43.0) | 142 (44.5) | 15 (32.6) | 0.127 | 71 (45.2) | 11 (42.3) | 0.782 | 71 (43.8) | 4 (20.0) | 0.041 |
| Hcy > 14 µmol/L | 314 (86.0) | 276 (86.5) | 38 (82.6) | 0.474 | 133 (84.7) | 21 (80.8) | 0.610 | 143 (88.3) | 17 (85.0) | 0.672 |
| Albumin, g/dL | 4.0 ± 0.4 | 4.0 ± 0.4 | 3.9 ± 0.4 | 0.138 | 4.1 ± 0.3 | 3.9 ± 0.4 | 0.032 | 3.9 ± 0.4 | 3.9 ± 0.4 | 0.746 |
| Pre-BUN, mg/dL | 72.9 ± 19.4 | 72.7 ± 19.8 | 74.4 ± 16.0 | 0.574 | 71.9 ± 21.0 | 74.0 ± 16.7 | 0.626 | 73.4 ± 18.6 | 74.9 ± 15.4 | 0.737 |
| Creatinine, mg/dL | 11.0 ± 2.1 | 11.1 ± 2.1 | 10.4 ± 1.7 | 0.017 | 11.2 ± 1.9 | 10.1 ± 1.6 | 0.004 | 11.1 ± 2.4 | 10.7 ± 1.8 | 0.482 |
| Hyperkalemia (K ≥ 5.0 mEq/L) | 130 (35.6) | 114 (35.7) | 16 (34.8) | 0.899 | 66 (42.0) | 11 (42.3) | 0.979 | 48 (29.6) | 5 (25.0) | 0.667 |
| Uric acid, mg/dL | 7.3 ± 1.2 | 7.3 ± 1.2 | 6.9 ± 1.2 | 0.021 | 7.2 ± 1.2 | 6.9 ± 1.3 | 0.316 | 7.4 ± 1.2 | 6.8 ± 0.9 | 0.025 |
| Variables | Overall (N = 365) | HOMA-IR < 5.18 (N = 183) | HOMA-IR ≥ 5.18 (N = 182) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age ≥ 65 years | 1.58 (0.88–2.82) | 0.125 | 1.32 (0.61–2.88) | 0.481 | 1.95 (0.81–4.69) | 0.138 |
| Gender, male | 1.78 (0.97–3.28) | 0.063 | 1.20 (0.55–2.63) | 0.646 | 2.83 (0.94–8.50) | 0.063 |
| Hemodialysis vintage, year | 0.95 (0.88–1.02) | 0.142 | 0.88 (0.79–0.99) | 0.028 | 1.01 (0.93–1.09) | 0.896 |
| CCI | 1.37 (1.12–1.66) | 0.002 | 1.32 (1.04–1.68) | 0.023 | 1.40 (1.00–1.95) | 0.049 |
| PA, MET-min/week | 0.95 (0.85–1.06) | 0.368 | 0.93 (0.81–1.07) | 0.313 | 0.99 (0.85–1.17) | 0.942 |
| Clinical parameters | ||||||
| SBP ≥ 130 mmHg | 1.78 (0.75–4.20) | 0.190 | 0.95 (0.36–2.53) | 0.923 | 6.71 (0.89–50.42) | 0.064 |
| DBP ≥ 85 mmHg | 1.00 (0.49–2.01) | 0.992 | 0.83 (0.31–2.20) | 0.703 | 1.33 (0.48–3.69) | 0.590 |
| BMI ≥ 24.0 kg/m2 | 1.08 (0.60–1.94) | 0.810 | 1.19 (0.53–2.66) | 0.679 | 0.99 (0.41–2.38) | 0.984 |
| CTR ≥ 50% | 1.85 (1.02–3.34) | 0.043 | 1.47 (0.67–3.24) | 0.338 | 2.66 (1.06–6.67) | 0.037 |
| TMM, kg | 1.02 (0.99–1.05) | 0.249 | 1.01 (0.97–1.05) | 0.739 | 1.02 (0.98–1.06) | 0.350 |
| BFM, kg | 0.98 (0.94–1.02) | 0.227 | 1.01 (0.96–1.06) | 0.796 | 0.94 (0.88–1.00) | 0.062 |
| PBF, % | 0.97 (0.94–1.00) | 0.088 | 1.00 (0.96–1.04) | 0.982 | 0.94 (0.90–0.99) | 0.017 |
| Biochemical parameters | ||||||
| hs-CRP > 0.5 mg/dL | 2.98 (1.67–5.32) | <0.001 | 5.38 (2.44–11.85) | <0.001 | 1.39 (0.57–3.41) | 0.468 |
| Anemia (Hgb < 11 g/dL) | 1.41 (0.76–2.60) | 0.280 | 1.80 (0.78–4.15) | 0.167 | 1.06 (0.42–2.66) | 0.901 |
| IFG 1 | 2.46 (1.15–5.29) | 0.021 | 2.44 (1.06–5.60) | 0.036 | - | |
| Insulin ≥ 12.0 µU/mL) | 1.14 (0.62–2.09) | 0.669 | 1.27 (0.57–2.86) | 0.561 | - | |
| HOMA-IR ≥ 5.18 | 0.94 (0.52–1.69) | 0.839 | - | - | ||
| Dyslipidemia 2 | 0.54 (0.28–1.04) | 0.067 | 0.94 (0.38–2.34) | 0.896 | 0.19 (0.07–0.51) | 0.001 |
| Serum Ca > 9.5 mg/dL | 0.78 (0.41–1.49) | 0.453 | 1.03 (0.46–2.32) | 0.937 | 0.49 (0.16–1.47) | 0.204 |
| Serum PO4 > 5.5 mg/dL | 0.73 (0.38–1.41) | 0.351 | 0.79 (0.34–1.82) | 0.584 | 0.51 (0.19–1.42) | 0.200 |
| Ca x PO4 ≥ 55 mg2/dL2 | 1.00 (0.52–1.94) | 0.995 | 1.41 (0.62–3.26) | 0.415 | 0.59 (0.20–1.78) | 0.350 |
| iPTH ≥ 300 pg/mL | 0.62 (0.34–1.15) | 0.129 | 0.96 (0.44–2.10) | 0.927 | 0.29 (0.10–0.88) | 0.029 |
| Hcy > 14 µmol/L | 0.76 (0.35–1.62) | 0.473 | 0.80 (0.30–2.12) | 0.650 | 0.64 (0.19–2.18) | 0.471 |
| Albumin, g/dL | 0.37 (0.18–0.74) | 0.005 | 0.29 (0.12–0.74) | 0.009 | 0.43 (0.14–1.32) | 0.138 |
| Pre-BUN, mg/dL | 0.99 (0.98–1.01) | 0.420 | 1.00 (0.98–1.02) | 0.708 | 0.99 (0.97–1.01) | 0.348 |
| Creatinine, mg/dL | 0.81 (0.70–0.94) | 0.005 | 0.76 (0.63–0.93) | 0.006 | 0.83 (0.66–1.05) | 0.114 |
| Hyperkalemia (K ≥ 5.0 mEq/L) | 0.81 (0.44–1.48) | 0.488 | 0.89 (0.41–1.94) | 0.767 | 0.75 (0.27–2.08) | 0.581 |
| Uric acid, mg/dL | 0.75 (0.61–0.92) | 0.005 | 0.83 (0.63–1.09) | 0.182 | 0.63 (0.43–0.91) | 0.015 |
| Overall (N = 365) | HOMA-IR < 5.18 (N = 183) | HOMA-IR ≥ 5.18 (N = 182) | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| PBF, % | 0.95 (0.92–0.99) | 0.006 | 0.96 (0.92–1.01) | 0.090 | 0.94 (0.89–1.00) | 0.033 |
| Hemodialysis vintage, year | - | 0.92 (0.82–1.03) | 0.154 | - | ||
| CCI | 1.20 (0.98–1.48) | 0.082 | 1.11 (0.86–1.44) | 0.411 | 1.20 (0.86–1.67) | 0.289 |
| CTR ≥ 50% | 1.71 (0.94–3.10) | 0.080 | 1.89 (0.68–5.23) | 0.222 | ||
| hs-CRP > 0.5 mg/dL | 3.08 (1.63–5.82) | 0.001 | 4.64 (1.95–11.05) | 0.001 | - | |
| IFG 1 | 2.14 (0.96–4.80) | 0.065 | 1.71 (0.68–4.29) | 0.255 | - | |
| Dyslipidemia 2 | - | - | 0.36 (0.11–1.19) | 0.094 | ||
| iPTH ≥ 300 pg/mL | - | - | 0.19 (0.05–0.66) | 0.009 | ||
| Albumin, g/dL | 0.89 (0.42–1.88) | 0.765 | 1.00 (0.36–2.78) | 0.998 | - | |
| Creatinine, mg/dL | 0.85 (0.72–1.02) | 0.073 | 0.81 (0.64–1.01) | 0.058 | - | |
| Uric acid, mg/dL | 0.82 (0.63–1.07) | 0.138 | - | 0.78 (0.54–1.13) | 0.186 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong, T.V.; Wong, T.-C.; Chen, H.-H.; Chen, T.-H.; Hsu, Y.-H.; Peng, S.-J.; Kuo, K.-L.; Liu, H.-C.; Lin, E.-T.; Yang, S.-H. Impact of Percent Body Fat on All-Cause Mortality among Adequate Dialysis Patients with and without Insulin Resistance: A Multi-Center Prospective Cohort Study. Nutrients 2019, 11, 1304. https://doi.org/10.3390/nu11061304
Duong TV, Wong T-C, Chen H-H, Chen T-H, Hsu Y-H, Peng S-J, Kuo K-L, Liu H-C, Lin E-T, Yang S-H. Impact of Percent Body Fat on All-Cause Mortality among Adequate Dialysis Patients with and without Insulin Resistance: A Multi-Center Prospective Cohort Study. Nutrients. 2019; 11(6):1304. https://doi.org/10.3390/nu11061304
Chicago/Turabian StyleDuong, Tuyen Van, Te-Chih Wong, Hsi-Hsien Chen, Tso-Hsiao Chen, Yung-Ho Hsu, Sheng-Jeng Peng, Ko-Lin Kuo, Hsiang-Chung Liu, En-Tzu Lin, and Shwu-Huey Yang. 2019. "Impact of Percent Body Fat on All-Cause Mortality among Adequate Dialysis Patients with and without Insulin Resistance: A Multi-Center Prospective Cohort Study" Nutrients 11, no. 6: 1304. https://doi.org/10.3390/nu11061304
APA StyleDuong, T. V., Wong, T.-C., Chen, H.-H., Chen, T.-H., Hsu, Y.-H., Peng, S.-J., Kuo, K.-L., Liu, H.-C., Lin, E.-T., & Yang, S.-H. (2019). Impact of Percent Body Fat on All-Cause Mortality among Adequate Dialysis Patients with and without Insulin Resistance: A Multi-Center Prospective Cohort Study. Nutrients, 11(6), 1304. https://doi.org/10.3390/nu11061304









