Vegetable-Based Diets for Chronic Kidney Disease? It Is Time to Reconsider
Abstract
1. Introduction
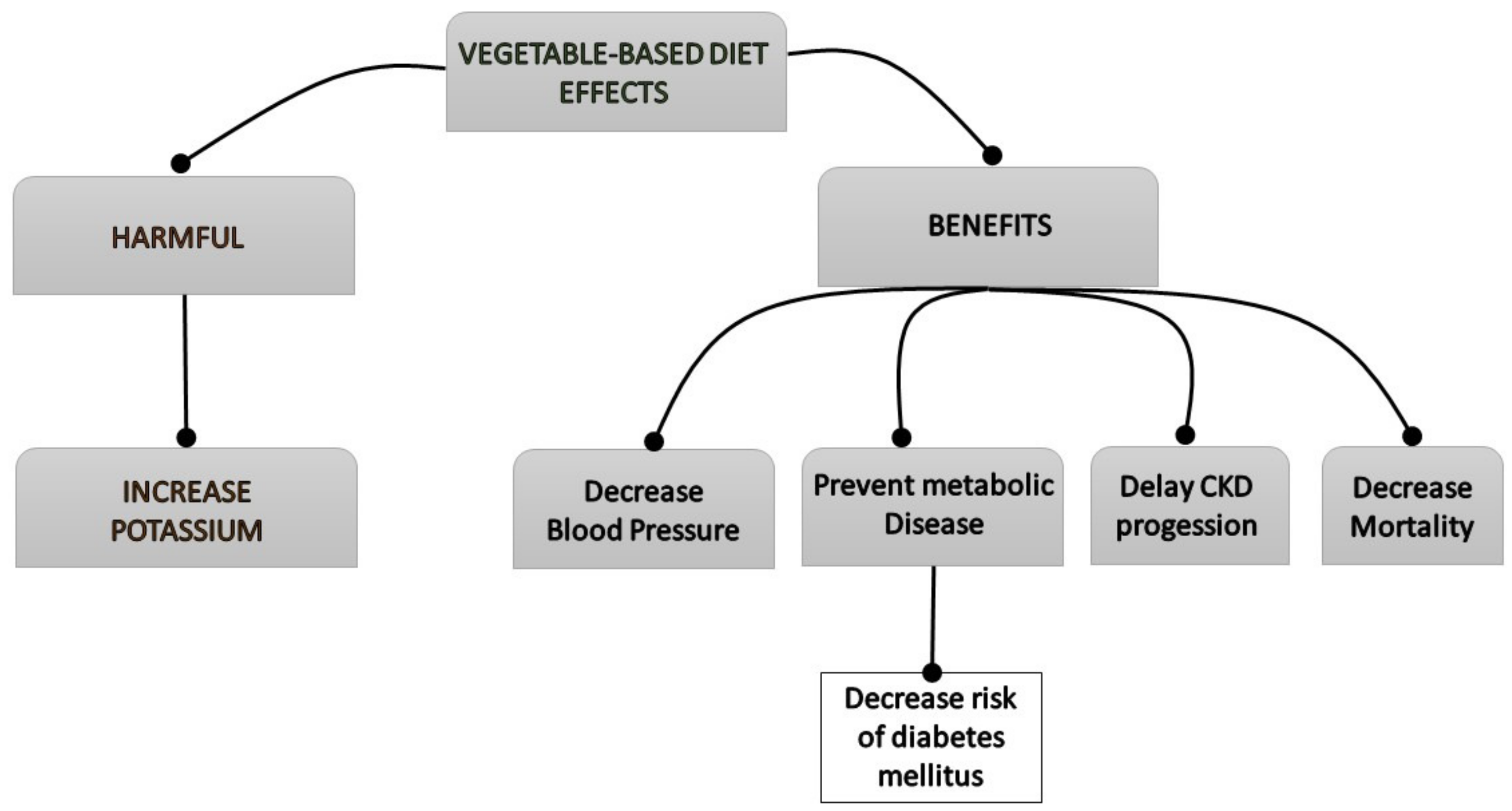
2. Effects of Vegetable-Based Diets
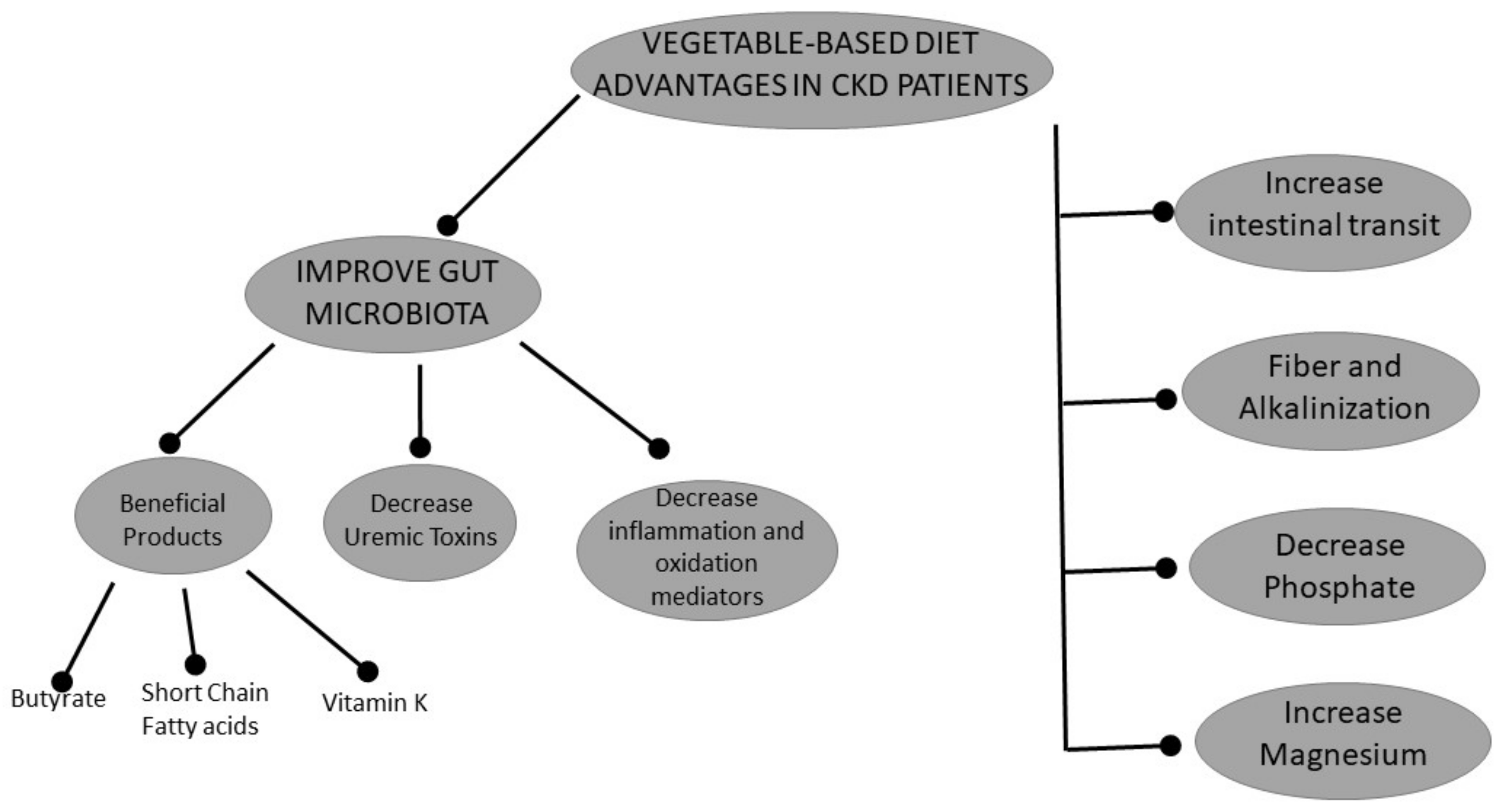
2.1. Vegetarian Diets and Gut Microbiota
2.2. Vegetarian Diet and Metabolic Acidosis
2.3. Phosphorus and a Vegetarian Diet
2.4. Microbiota-Derived Uremic Toxins and a Vegetarian Diet
2.5. Magnesium and a Vegetarian Diet
2.6. Vitamin K and a Vegetarian Diet
2.7. Effects of a Vegetarian Diet on Inflammation and Oxidative Stress
2.8. Vegetarian-Diet and Intestinal Motility
2.9. Risk of Hyperkaliemia
3. Effect of Vegetable-Based Diets on Renal Patient Complications
3.1. Vegetarian Diet in Hypertension and Diabetes Mellitus
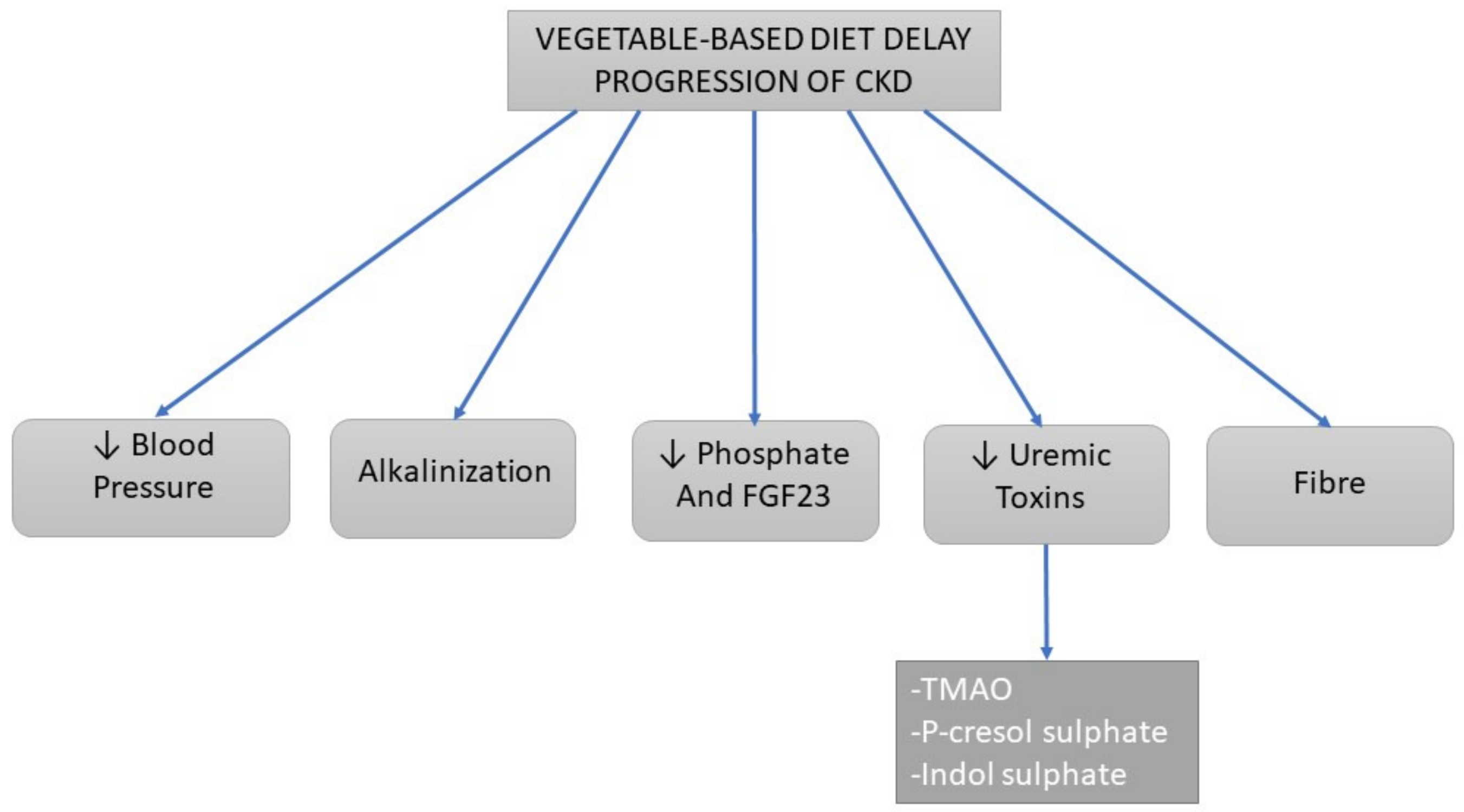
3.2. Progression of Chronic Kidney Disease
3.2.1. Alkalinizing Effects
3.2.2. Dietary Sodium and Blood Pressure
3.2.3. Uremic Toxins
3.2.4. Phosphate
3.2.5. Fiber Content
3.3. Mortality
4. Practical Tips
4.1. Role of the Mediterranean Diet
4.2. Practical Cooking Counselling
4.3. Potential Risks of a Vegan Diet in CKD
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tharrey, M.; Mariotti, F.; Mashchak, A.; Barbillon, P.; Delattre, M.; Fraser, G.E. Patterns of plant and animal protein intake are strongly associated with cardiovascular mortality: The Adventist Health Study-2 cohort. Int. J. Epidemiol. 2018, 47, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Appleby, P.N.; Travis, R.C.; Key, T.J. Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: Results from the EPIC-Oxford cohort study. Am. J. Clin. Nutr. 2013, 97, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Ramarao, P.B.; Norton, H.W.; Johnson, B.C. The amino acids composition and nutritive value of proteins. V. Amino acid requirements as pattern for protein evaluation. J. Nutr. 1964, 82, 88–92. [Google Scholar] [PubMed]
- Rosell, M.; Appleby, P.; Key, T. Height, age at menarche, body weight and body mass index in life-long vegetarians. Public Health Nutr. 2005, 8, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Barsotti, G.; Morelli, E.; Cupisti, A.; Meola, M.; Dani, L.; Giovannetti, S. A low-nitrogen low-phosphorus Vegan diet for patients with chronic renal failure. Nephron 1996, 74, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Vigotti, F.N.; Leone, F.; Capizzi, I.; Daidola, G.; Cabiddu, G.; Avagnina, P. Low-protein diets in CKD: How can we achieve them? A narrative, pragmatic review. Clin. Kidney J. 2015, 8, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Capizzi, I.; Vigotti, F.N.; Leone, F.; D’Alessandro, C.; Giuffrida, D.; Nazha, M.; Roggero, S.; Colombi, N.; Mauro, G.; et al. Low protein diets in patients with chronic kidney disease: A bridge between mainstream and complementary-alternative medicines? BMC Nephrol. 2016, 17, 76. [Google Scholar] [CrossRef][Green Version]
- Kandouz, S.; Mohamed, A.S.; Zheng, Y.; Sandeman, S.; Davenport, A. Reduced protein bound uraemic toxins in vegetarian kidney failure patients treated by haemodiafiltration. Hemodial. Int. 2016, 20, 610–617. [Google Scholar] [CrossRef]
- Gifford, J.D.; Rutsky, E.A.; Kirk, K.A.; McDaniel, H.G. Control of serum potassium during fasting in patients with end-stage renal disease. Kidney Int. 1989, 35, 90–94. [Google Scholar] [CrossRef]
- Cigarran Guldris, S.; González Parra, E.; Cases Amenós, A. Gut microbiota in chronic kidney disease. Nefrologia 2017, 37, 9–19. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, D.S.; Modersitzki, F.; Asplin, J.R. A randomized, controlled trial of lactic acid bacteria for idiopathic hyperoxaluria. Clin. J. Am. Soc. Nephrol. 2007, 2, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Johnson, D.W.; Xu, H.; Carrero, J.J.; Pascoe, E.; French, C.; Campbell, K.L. Dietary protein-fiber ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Salmean, Y.A.; Segal, M.S.; Langkamp-Henken, B.; Canales, M.T.; Zello, G.A.; Dahl, W.J. Foods with added fiber lower serum creatinine levels in patients with chronic kidney disease. J. Ren. Nutr. 2013, 23, e29–e32. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Esgalhado, M.; Kemp, J.A.; Damasceno, N.R.; Fouque, D.; Mafra, D. Short-chain fatty acids: A link between prebiotics and microbiota in chronic kidney disease. Future Microbiol. 2017, 12, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Fazeli, G.; Di Micco, L.; Autore, G.; Adesso, S.; Dal Piaz, F.; Heidland, A.; Di Iorio, B. Supplementation of Short-Chain Fatty Acid, Sodium Propionate, in Patients on Maintenance Hemodialysis: Beneficial Effects on Inflammatory Parameters and Gut-Derived Uremic Toxins, A Pilot Study (PLAN Study). J. Clin. Med. 2018, 7, 315. [Google Scholar] [CrossRef]
- Khosroshahi, H.T.; Abedi, B.; Ghojazadeh, M.; Samadi, A.; Jouyban, A. Effects of fermentable high fiber diet supplementation on gut derived and conventional nitrogenous product in patients on maintenance hemodialysis: A randomized controlled trial. Nutr. Metab. (Lond). 2019, 16, 18. [Google Scholar] [CrossRef]
- St-Jules, D.E.; Goldfarb, D.S.; Sevick, M.A. Nutrient Non-equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J. Ren. Nutr. 2016, 26, 282–287. [Google Scholar] [CrossRef]
- Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Thomas, F.; Lu, J.L.; Matsushita, K.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Constipation and Incident CKD. J. Am. Soc. Nephrol. 2017, 28, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xie, S.; Lv, D.; Zhang, Y.; Deng, J.; Zeng, L.; Chen, Y. A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression. Antonie Van Leeuwenhoek 2016, 109, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, A.; Mathew, A.V.; Byun, J.; Gadegbeku, C.A.; Gipson, D.S.; Afshinnia, F.; Pennathur, S.; for the Michigan Kidney Translational Core CPROBE Investigator Group. Gut Microbial Product Predicts Cardiovascular Risk in Chronic Kidney Disease Patients. Am. J. Nephrol. 2018, 48, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Windey, K.; De Preter, V.; Verbeke, K. Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 2012, 56, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; Lange, B.; Frick, J.-S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, K.; Klosterhalfen, S.; Enck, P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2012, 66, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, D.A.; Piccolo, B.D.; Vaziri, N.D.; Liu, S.; Lau, W.L.; Khazaeli, M.; Nazertehrani, S.; Moore, M.E.; Marco, M.L.; Martin, R.J.; et al. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am. J. Physiol. Physiol. 2016, 310, F857–F871. [Google Scholar] [CrossRef]
- Laffin, M.R.; Tayebi Khosroshahi, H.; Park, H.; Laffin, L.J.; Madsen, K.; Kafil, H.S.; Abedi, B.; Shiralizadeh, S.; Vaziri, N.D. Amylose resistant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: Microbial analysis from a randomized placebo-controlled trial. Hemodial. Int. 2019, hdi.12753. [Google Scholar] [CrossRef] [PubMed]
- Tayebi Khosroshahi, H.; Vaziri, N.D.; Abedi, B.; Asl, B.H.; Ghojazadeh, M.; Jing, W.; Vatankhah, A.M. Effect of high amylose resistant starch (HAM-RS2) supplementation on biomarkers of inflammation and oxidative stress in hemodialysis patients: A randomized clinical trial. Hemodial. Int. 2018, 22, 492–500. [Google Scholar] [CrossRef]
- Zybailov, B.L.; Glazko, G.V.; Rahmatallah, Y.; Andreyev, D.S.; McElroy, T.; Karaduta, O.; Byrum, S.D.; Orr, L.; Tackett, A.J.; Mackintosh, S.G.; et al. Metaproteomics reveals potential mechanisms by which dietary resistant starch supplementation attenuates chronic kidney disease progression in rats. PLoS One 2019, 14, e0199274. [Google Scholar] [CrossRef]
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am. J. Kidney Dis. 2000, 35, S1–S140.
- Mandel, E.I.; Forman, J.P.; Curhan, G.C.; Taylor, E.N. Plasma Bicarbonate and Odds of Incident Hypertension. Am. J. Hypertens. 2013, 26, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Dobre, M.; Yang, W.; Chen, J.; Drawz, P.; Hamm, L.L.; Horwitz, E.; Hostetter, T.; Jaar, B.; Lora, C.M.; Nessel, L.; et al. Association of Serum Bicarbonate With Risk of Renal and Cardiovascular Outcomes in CKD: A Report From the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2013, 62, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J.A.; Madias, N.E. Metabolic Acidosis of CKD: An Update. Am. J. Kidney Dis. 2016, 67, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Schold, J.D.; Arrigain, S.; Jolly, S.E.; Wehbe, E.; Raina, R.; Simon, J.F.; Srinivas, T.R.; Jain, A.; Schreiber, M.J.; et al. Serum Bicarbonate and Mortality in Stage 3 and Stage 4 Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- de Brito-Ashurst, I.; Varagunam, M.; Raftery, M.J.; Yaqoob, M.M. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J. Am. Soc. Nephrol. 2009, 20, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Adeva, M.M.; Souto, G. Diet-induced metabolic acidosis. Clin. Nutr. 2011, 30, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.A.; Todd, K.M.; Morris, R.C.; Sebastian, A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am. J. Clin. Nutr. 1998, 68, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Oh, M.S. The key to halting progression of CKD might be in the produce market, not in the pharmacy. Kidney Int. 2012, 81, 7–9. [Google Scholar] [CrossRef][Green Version]
- Hsu, C.-Y.; Chertow, G.M. Elevations of serum phosphorus and potassium in mild to moderate chronic renal insufficiency. Nephrol. Dial. Transplant 2002, 17, 1419–1425. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. A Comparison of Treating Metabolic Acidosis in CKD Stage 4 Hypertensive Kidney Disease with Fruits and Vegetables or Sodium Bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.; Wesson, D.E. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012, 81, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, P.; Combe, C.; Fouque, D.; Aparicio, M. Vegetarianism: Advantages and drawbacks in patients with chronic kidney diseases. J. Ren. Nutr. 2013, 23, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.; Sayre, S.S.; Leon, J.B.; Machekano, R.; Love, T.E.; Porter, D.; Marbury, M.; Sehgal, A.R. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: A randomized controlled trial. JAMA 2009, 301, 629–635. [Google Scholar] [CrossRef] [PubMed]
- González-Parra, E.; Gracia-Iguacel, C.; Egido, J.; Ortiz, A. Phosphorus and Nutrition in Chronic Kidney Disease. Int. J. Nephrol. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, M.; Komaba, H.; Miyamoto, K. Source Matters: From Phosphorus Load to Bioavailability. Clin. J. Am. Soc. Nephrol. 2011, 6, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.M.; Zidehsarai, M.P.; Chambers, M.A.; Jackman, L.A.; Radcliffe, J.S.; Trevino, L.L.; Donahue, S.E.; Asplin, J.R. Vegetarian Compared with Meat Dietary Protein Source and Phosphorus Homeostasis in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 257–264. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Mannstadt, M.; Isakova, T.; Rauh-Hain, J.A.; Tamez, H.; Shah, A.; Smith, K.; Lee, H.; Thadhani, R.; Jüppner, H.; et al. Fibroblast Growth Factor 23 and Mortality among Patients Undergoing Hemodialysis. N. Engl. J. Med. 2008, 359, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Glorieux, G. Gut-Derived Metabolites and Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2018, 13, 1311–1313. [Google Scholar] [CrossRef]
- Lau, W.L.; Savoj, J.; Nakata, M.B.; Vaziri, N.D. Altered microbiome in chronic kidney disease: Systemic effects of gut-derived uremic toxins. Clin. Sci. (Lond). 2018, 132, 509–522. [Google Scholar] [CrossRef]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Vaziri, N.D. Urea, a true uremic toxin: The empire strikes back. Clin. Sci. (Lond). 2017, 131, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Koguchi, T.; Tadokoro, T. Beneficial Effect of Dietary Fiber on Hyperuricemia in Rats and Humans: A Review. Int. J. Vitam. Nutr. Res. 2019, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Tortorici, A.R.; Chen, J.L.T.; Kamgar, M.; Lau, W.-L.; Moradi, H.; Rhee, C.M.; Streja, E.; Kovesdy, C.P. Dietary restrictions in dialysis patients: Is there anything left to eat? Semin. Dial. 2015, 28, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.P.; Luo, F.J.-G.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. The production of p-cresol sulfate and indoxyl sulfate in vegetarians versus omnivores. Clin. J. Am. Soc. Nephrol. 2012, 7, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Dal Piaz, F.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Tartaglia, D.; Autore, G.; Di Iorio, B. Very low protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purif. 2013, 35, 196–201. [Google Scholar] [CrossRef]
- Wu, T.-T.; Chang, C.-Y.; Hsu, W.-M.; Wang, I.-K.; Hsu, C.-H.; Cheng, S.-H.; Liang, C.-C.; Chang, C.-T.; Huang, C.-C. Nutritional status of vegetarians on maintenance haemodialysis. Nephrology (Carlton). 2011, 16, 582–587. [Google Scholar] [CrossRef]
- Rampton, D.S.; Cohen, S.L.; Crammond, V.D.; Gibbons, J.; Lilburn, M.F.; Rabet, J.Y.; Vince, A.J.; Wager, J.D.; Wrong, O.M. Treatment of chronic renal failure with dietary fiber. Clin. Nephrol. 1984, 21, 159–163. [Google Scholar]
- Chiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.A.; Darling, P.B. Dietary fiber effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2015, 69, 761–768. [Google Scholar] [CrossRef]
- Salmean, Y.A.; Segal, M.S.; Palii, S.P.; Dahl, W.J. Fiber supplementation lowers plasma p-cresol in chronic kidney disease patients. J. Ren. Nutr. 2015, 25, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Cai, X.; Lin, J.; Zhang, X.; Scott, E.M.; Li, X. Association between fibre intake and indoxyl sulphate/P-cresyl sulphate in patients with chronic kidney disease: Meta-analysis and systematic review of experimental studies. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Birkett, A.; Muir, J.; Phillips, J.; Jones, G.; O’Dea, K. Resistant starch lowers fecal concentrations of ammonia and phenols in humans. Am. J. Clin. Nutr. 1996, 63, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Geboes, K.P.; De Hertogh, G.; De Preter, V.; Luypaerts, A.; Bammens, B.; Evenepoel, P.; Ghoos, Y.; Geboes, K.; Rutgeerts, P.; Verbeke, K. The influence of inulin on the absorption of nitrogen and the production of metabolites of protein fermentation in the colon. Br. J. Nutr. 2006, 96, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Younes, H.; Egret, N.; Hadj-Abdelkader, M.; Rémésy, C.; Demigné, C.; Gueret, C.; Deteix, P.; Alphonse, J.-C. Fermentable Carbohydrate Supplementation Alters Nitrogen Excretion in Chronic Renal Failure. J. Ren. Nutr. 2006, 16, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef]
- Koeth, R.A.; Lam-Galvez, B.R.; Kirsop, J.; Wang, Z.; Levison, B.S.; Gu, X.; Copeland, M.F.; Bartlett, D.; Cody, D.B.; Dai, H.J.; et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Invest. 2019, 129, 373–387. [Google Scholar] [CrossRef]
- Chen, M.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.; Zhang, Q.; Mi, M. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. MBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef]
- Hughes, J.; Chiu, D.Y.Y.; Kalra, P.A.; Green, D. Prevalence and outcomes of proton pump inhibitor associated hypomagnesemia in chronic kidney disease. PLoS One 2018, 13, e0197400. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Hamano, T.; Sakaguchi, Y.; Yamaguchi, S.; Kubota, K.; Senda, M.; Yonemoto, S.; Shimada, K.; Matsumoto, A.; Hashimoto, N.; et al. Proteinuria-associated renal magnesium wasting leads to hypomagnesemia: A common electrolyte abnormality in chronic kidney disease. Nephrol. Dial. Transplant 2018. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Tocados, J.M.; Peralta-Ramirez, A.; Rodríguez-Ortiz, M.E.; Raya, A.I.; Lopez, I.; Pineda, C.; Herencia, C.; Montes de Oca, A.; Vergara, N.; Steppan, S.; et al. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int. 2017, 92, 1084–1099. [Google Scholar] [CrossRef] [PubMed]
- Ter Braake, A.D.; Tinnemans, P.T.; Shanahan, C.M.; Hoenderop, J.G.J.; de Baaij, J.H.F. Magnesium prevents vascular calcification in vitro by inhibition of hydroxyapatite crystal formation. Sci. Rep. 2018, 8, 2069. [Google Scholar] [CrossRef] [PubMed]
- Massy, Z.A.; Drüeke, T.B. Magnesium and cardiovascular complications of chronic kidney disease. Nat. Rev. Nephrol. 2015, 11, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.M. Endothelial cells and magnesium: Implications in atherosclerosis. Clin. Sci. 2012, 122, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Fujii, N.; Shoji, T.; Hayashi, T.; Rakugi, H.; Isaka, Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014, 85, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Fein, P.; Weiss, S.; Ramos, F.; Singh, P.; Chattopadhyay, J.; Avram, M.M. Serum magnesium concentration is a significant predictor of mortality in peritoneal dialysis patients. Adv. Perit. Dial. 2014, 30, 90–93. [Google Scholar]
- Kanbay, M.; Yilmaz, M.I.; Apetrii, M.; Saglam, M.; Yaman, H.; Unal, H.U.; Gok, M.; Caglar, K.; Oguz, Y.; Yenicesu, M.; et al. Relationship between Serum Magnesium Levels and Cardiovascular Events in Chronic Kidney Disease Patients. Am. J. Nephrol. 2012, 36, 228–237. [Google Scholar] [CrossRef]
- Xiong, J.; He, T.; Wang, M.; Nie, L.; Zhang, Y.; Wang, Y.; Huang, Y.; Feng, B.; Zhang, J.; Zhao, J. Serum magnesium, mortality, and cardiovascular disease in chronic kidney disease and end-stage renal disease patients: A systematic review and meta-analysis. J. Nephrol. 2019. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Fujii, N.; Shoji, T.; Hayashi, T.; Rakugi, H.; Iseki, K.; Tsubakihara, Y.; Isaka, Y. Committee of Renal Data Registry of the Japanese Society for Dialysis Therapy Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: A cohort study. PLoS One 2014, 9, e116273. [Google Scholar] [CrossRef] [PubMed]
- Tin, A.; Grams, M.E.; Maruthur, N.M.; Astor, B.C.; Couper, D.; Mosley, T.H.; Selvin, E.; Coresh, J.; Kao, W.H.L. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int. 2015, 87, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Van Laecke, S.; Nagler, E.V.; Verbeke, F.; Van Biesen, W.; Vanholder, R. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am. J. Med. 2013, 126, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Turgut, F.; Kanbay, M.; Metin, M.R.; Uz, E.; Akcay, A.; Covic, A. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int. Urol. Nephrol. 2008, 40, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Tzanakis, I.P.; Stamataki, E.E.; Papadaki, A.N.; Giannakis, N.; Damianakis, N.E.; Oreopoulos, D.G. Magnesium retards the progress of the arterial calcifications in hemodialysis patients: A pilot study. Int. Urol. Nephrol. 2014, 46, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Bressendorff, I.; Hansen, D.; Schou, M.; Silver, B.; Pasch, A.; Bouchelouche, P.; Pedersen, L.; Rasmussen, L.M.; Brandi, L. Oral Magnesium Supplementation in Chronic Kidney Disease Stages 3 and 4: Efficacy, Safety, and Effect on Serum Calcification Propensity-A Prospective Randomized Double-Blinded Placebo-Controlled Clinical Trial. Kidney Int. reports 2017, 2, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Plebani, M.; Iervasi, G.; Gallieni, M. Vitamin K Deficiency in Chronic Kidney Disease: Evidence Is Building Up. Am. J. Nephrol. 2017, 45, 1–3. [Google Scholar] [CrossRef]
- Fusaro, M.; D’Alessandro, C.; Noale, M.; Tripepi, G.; Plebani, M.; Veronese, N.; Iervasi, G.; Giannini, S.; Rossini, M.; Tarroni, G.; et al. Low vitamin K1 intake in haemodialysis patients. Clin. Nutr. 2017, 36, 601–607. [Google Scholar] [CrossRef]
- Cranenburg, E.C.M.; Schurgers, L.J.; Uiterwijk, H.H.; Beulens, J.W.J.; Dalmeijer, G.W.; Westerhuis, R.; Magdeleyns, E.J.; Herfs, M.; Vermeer, C.; Laverman, G.D. Vitamin K intake and status are low in hemodialysis patients. Kidney Int. 2012, 82, 605–610. [Google Scholar] [CrossRef]
- Holden, R.M.; Morton, A.R.; Garland, J.S.; Pavlov, A.; Day, A.G.; Booth, S.L. Vitamins K and D status in stages 3-5 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 590–597. [Google Scholar] [CrossRef]
- Cupisti, A.; D’Alessandro, C.; Gesualdo, L.; Cosola, C.; Gallieni, M.; Egidi, M.F.; Fusaro, M. Non-Traditional Aspects of Renal Diets: Focus on Fiber, Alkali and Vitamin K1 Intake. Nutrients 2017, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Caluwé, R.; Verbeke, F.; De Vriese, A.S. Evaluation of vitamin K status and rationale for vitamin K supplementation in dialysis patients. Nephrol. Dial. Transplant 2018. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Mangano, M.; Galassi, A.; Ciceri, P.; Messa, P.; Nigwekar, S. Vitamin K in Chronic Kidney Disease. Nutrients 2019, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qureshi, A.R.; Parini, P.; Hurt-Camejo, E.; Ripsweden, J.; Brismar, T.B.; Barany, P.; Jaminon, A.M.; Schurgers, L.J.; Heimbürger, O.; et al. Does statins promote vascular calcification in chronic kidney disease? Eur. J. Clin. Invest. 2017, 47, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Mac-Way, F.; Poulin, A.; Utescu, M.S.; De Serres, S.A.; Marquis, K.; Douville, P.; Desmeules, S.; Larivière, R.; Lebel, M.; Agharazii, M. The impact of warfarin on the rate of progression of aortic stiffness in hemodialysis patients: A longitudinal study. Nephrol. Dial. Transplant 2014, 29, 2113–2120. [Google Scholar] [CrossRef]
- Schlieper, G.; Westenfeld, R.; Krüger, T.; Cranenburg, E.C.; Magdeleyns, E.J.; Brandenburg, V.M.; Djuric, Z.; Damjanovic, T.; Ketteler, M.; Vermeer, C.; et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J. Am. Soc. Nephrol. 2011, 22, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Claes, K.; Meijers, B.; Laurent, M.; Bammens, B.; Naesens, M.; Sprangers, B.; Pottel, H.; Cavalier, E.; Kuypers, D. Poor Vitamin K Status Is Associated With Low Bone Mineral Density and Increased Fracture Risk in End-Stage Renal Disease. J. Bone Miner. Res. 2019, 34, 262–269. [Google Scholar] [CrossRef]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefańczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3-5. Pol. Arch. Med. Wewn. 2015, 125, 631–640. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Hanna, D.L.; Zhang, W.; Baba, H.; Lenz, H.-J. Molecular Pathways: Cachexia Signaling—A Targeted Approach to Cancer Treatment. Clin. Cancer Res. 2016, 22, 3999–4004. [Google Scholar] [CrossRef]
- Lau, W.L.; Kalantar-Zadeh, K.; Vaziri, N.D. The Gut as a Source of Inflammation in Chronic Kidney Disease. Nephron 2015, 130, 92–98. [Google Scholar] [CrossRef]
- Mafra, D.; Lobo, J.C.; Barros, A.F.; Koppe, L.; Vaziri, N.D.; Fouque, D. Role of altered intestinal microbiota in systemic inflammation and cardiovascular disease in chronic kidney disease. Future Microbiol. 2014, 9, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hébert, J.R. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Kim, C.H.; Dang, B.; Zhan, C.-D.; Liang, K. Downregulation of hepatic acyl-CoA:diglycerol acyltransferase in chronic renal failure. Am. J. Physiol. Renal Physiol. 2004, 287, F90–F94. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Huang, Y.-F.; Wang, M.-Q.; Chen, D.-X.; Wan, H.; Wei, L.-B.; Xiao, W. Dietary fiber intake is associated with chronic kidney disease (CKD) progression and cardiovascular risk, but not protein nutritional status, in adults with CKD. Asia Pac. J. Clin. Nutr. 2017, 26, 598–605. [Google Scholar] [PubMed]
- Krishnamurthy, V.M.R.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Haghighatdoost, F.; Bellissimo, N.; Totosy de Zepetnek, J.O.; Rouhani, M.H. Association of vegetarian diet with inflammatory biomarkers: A systematic review and meta-analysis of observational studies. Public Health Nutr. 2017, 20, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Knauf, C. How gut microbes talk to organs: The role of endocrine and nervous routes. Mol. Metab. 2016, 5, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Massy, Z.A.; Stenvinkel, P.; Drueke, T.B. The Role of Oxidative Stress in Chronic Kidney Disease. Semin. Dial. 2009, 22, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Cho, S.W.; Park, Y.K. Long-term vegetarians have low oxidative stress, body fat, and cholesterol levels. Nutr. Res. Pract. 2012, 6, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Torramade-Moix, S.; Martin-Rodriguez, S.; Cases, A.; Cruzado, J.M.; Rivera, J.; Escolar, G.; Palomo, M.; Diaz-Ricart, M. Antioxidant and Anti-Inflammatory Strategies Based on the Potentiation of Glutathione Peroxidase Activity Prevent Endothelial Dysfunction in Chronic Kidney Disease. Cell. Physiol. Biochem. 2018, 51, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.; Birkett, A.; Hulshof, T.; Verbeke, K.; Gibes, K. Effects of Cereal, Fruit and Vegetable Fibers on Human Fecal Weight and Transit Time: A Comprehensive Review of Intervention Trials. Nutrients 2016, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on Dietary Reference Values for Carbohydrates and Dietary Fibre. Available online: http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/1462.pdf (accessed on 30 May 2019).
- Degen, L.P.; Phillips, S.F. Variability of gastrointestinal transit in healthy women and men. Gut 1996, 39, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, Flavones, Flavanones, and Human Health: Epidemiological Evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Saha, S.; Nordstrom, J.; Gerdtham, U.-G.; Mattisson, I.; Nilsson, P.M.; Scarborough, P. Prevention of Cardiovascular Disease and Cancer Mortality by Achieving Healthy Dietary Goals for the Swedish Population: A Macro-Simulation Modelling Study. Int. J. Environ. Res. Public Health 2019, 16, 890. [Google Scholar] [CrossRef]
- Reboul, E.; Thap, S.; Perrot, E.; Amiot, M.-J.; Lairon, D.; Borel, P. Effect of the main dietary antioxidants (carotenoids, γ-tocopherol, polyphenols and vitamin C) on α-tocopherol absorption. Eur. J. Clin. Nutr. 2007, 61, 1167–1173. [Google Scholar] [CrossRef]
- Claudie, D.; Alexandrine, D.; Bertrand, C.; Franck, T.; Marie-Josephe, A. Citrus flavanones enhance carotenoid uptake by intestinal Caco-2 cells. Food Funct. 2013, 4, 1625–1631. [Google Scholar] [CrossRef]
- Phan, M.A.T.; Paterson, J.; Bucknall, M.; Arcot, J. Interactions between phytochemicals from fruits and vegetables: Effects on bioactivities and bioavailability. Crit. Rev. Food Sci. Nutr. 2018, 58, 1310–1329. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Blacklock, R.; Wood, E.; Rumjon, A.; Simmonds, S.; Fletcher-Rogers, J.; Ariyanayagam, R.; Al-Yassin, A.; Sharpe, C.; Vinen, K. Prevalence and factors associated with hyperkalemia in predialysis patients followed in a low-clearance clinic. Clin. J. Am. Soc. Nephrol. 2012, 7, 1234–1241. [Google Scholar] [CrossRef]
- Clegg, D.J.; Hill Gallant, K.M. Plant-Based Diets in CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Shah, S.; Kalantar-Zadeh, K. Adequacy of Plant-Based Proteins in Chronic Kidney Disease. J. Ren. Nutr. 2019, 29, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, P.; Aparicio, M.; Bellizzi, V.; Campbell, K.; Hong, X.; Johansson, L.; Kolko, A.; Molina, P.; Sezer, S.; Wanner, C.; et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol. Dial. Transplant. 2018, 33, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.T.; Palmer, S.C.; Wai, S.N.; Ruospo, M.; Carrero, J.-J.; Campbell, K.L.; Strippoli, G.F.M. Healthy Dietary Patterns and Risk of Mortality and ESRD in CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2017, 12, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Saglimbene, V.M.; Wong, G.; Craig, J.C.; Ruospo, M.; Palmer, S.C.; Campbell, K.; Garcia-Larsen, V.; Natale, P.; Teixeira-Pinto, A.; Carrero, J.-J.; et al. The Association of Mediterranean and DASH Diets with Mortality in Adults on Hemodialysis: The DIET-HD Multinational Cohort Study. J. Am. Soc. Nephrol. 2018, 29, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Kass, E.H. Low blood pressure in vegetarians: Effects of specific foods and nutrients. Am. J. Clin. Nutr. 1988, 48, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Fraser, G.E. Vegetarian diets: What do we know of their effects on common chronic diseases? Am. J. Clin. Nutr. 2009, 89, 1607S–1612S. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef]
- Barnard, N.D.; Levin, S.M.; Yokoyama, Y. A Systematic Review and Meta-Analysis of Changes in Body Weight in Clinical Trials of Vegetarian Diets. J. Acad. Nutr. Diet. 2015, 115, 954–969. [Google Scholar] [CrossRef]
- Chauveau, P.; Koppe, L.; Combe, C.; Lasseur, C.; Trolonge, S.; Aparicio, M. Vegetarian diets and chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-S.; Lai, N.-S.; Ho, L.-T.; Lin, C.-L. Insulin sensitivity in Chinese ovo-lactovegetarians compared with omnivores. Eur. J. Clin. Nutr. 2004, 58, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Butler, T.; Yan, R.; Fraser, G.E. Type of Vegetarian Diet, Body Weight, and Prevalence of Type 2 Diabetes. Diabetes Care 2009, 32, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Barnard, N.D.; Levin, S.M.; Watanabe, M. Vegetarian diets and glycemic control in diabetes: A systematic review and meta-analysis. Cardiovasc. Diagn. Ther. 2014, 4, 373–382. [Google Scholar] [PubMed]
- Kontessis, P.A.; Bossinakou, I.; Sarika, L.; Iliopoulou, E.; Papantoniou, A.; Trevisan, R.; Roussi, D.; Stipsanelli, K.; Grigorakis, S.; Souvatzoglou, A. Renal, metabolic, and hormonal responses to proteins of different origin in normotensive, nonproteinuric type I diabetic patients. Diabetes Care 1995, 18, 1233. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E.; Simoni, J.; Broglio, K.; Sheather, S. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am. J. Physiol. Renal Physiol. 2011, 300, F830–F837. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Retarding progression of chronic kidney disease: Use of modalities that counter acid retention. Curr. Opin. Nephrol. Hypertens. 2018, 27, 94–101. [Google Scholar] [CrossRef]
- Scialla, J.J.; Appel, L.J.; Astor, B.C.; Miller, E.R.; Beddhu, S.; Woodward, M.; Parekh, R.S.; Anderson, C.A.M. Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int. 2012, 82, 106–112. [Google Scholar] [CrossRef]
- Scialla, J.J.; Anderson, C.A.M. Dietary Acid Load: A Novel Nutritional Target in Chronic Kidney Disease? Adv. Chronic Kidney Dis. 2013, 20, 141–149. [Google Scholar] [CrossRef]
- Khanna, A.; Simoni, J.; Hacker, C.; Duran, M.-J.; Wesson, D.E. Increased endothelin activity mediates augmented distal nephron acidification induced by dietary protein. Trans. Am. Clin. Climatol. Assoc. 2005, 116, 239–256; discussion 257-8. [Google Scholar] [CrossRef]
- Wesson, D.E. Endothelins and Kidney Acidification. In Endothelin in Renal Physiology and Disease; Barton, M., Kohan, D., Eds.; Karger Publishers: Basel, Switzerland, 2011; pp. 84–93. [Google Scholar]
- Banerjee, T.; Tucker, K.; Griswold, M.; Wyatt, S.B.; Harman, J.; Young, B.; Taylor, H.; Powe, N.R. Dietary Potential Renal Acid Load and Risk of Albuminuria and Reduced Kidney Function in the Jackson Heart Study. J. Ren. Nutr. 2018, 28, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Fung, T.T.; Hu, F.B.; Curhan, G.C. Association of dietary patterns with albuminuria and kidney function decline in older white women: A subgroup analysis from the Nurses’ Health Study. Am. J. Kidney Dis. 2011, 57, 245–254. [Google Scholar] [CrossRef]
- Buendia, J.R.; Bradlee, M.L.; Daniels, S.R.; Singer, M.R.; Moore, L.L. Longitudinal Effects of Dietary Sodium and Potassium on Blood Pressure in Adolescent Girls. JAMA Pediatr. 2015, 169, 560. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hébert, J.R.; Li, W.; Bertone-Johnson, E.R.; Olendzki, B.; Pagoto, S.L.; Tinker, L.; Rosal, M.C.; Ockene, I.S.; Ockene, J.K.; et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition 2008, 24, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C. Nitric oxide synthase derangements and hypertension in kidney disease. Curr. Opin. Nephrol. Hypertens. 2012, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Golzarand, M.; Asghari, G.; Azizi, F. Consumption of nitrate containing vegetables and the risk of chronic kidney disease: Tehran Lipid and Glucose Study. Ren. Fail. 2016, 38, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Demigné, C.; Sabboh, H.; Puel, C.; Rémésy, C.; Coxam, V. Organic anions and potassium salts in nutrition and metabolism. Nutr. Res. Rev. 2004, 17, 249. [Google Scholar] [CrossRef]
- Ramezani, A.; Raj, D.S. The Gut Microbiome, Kidney Disease, and Targeted Interventions. J. Am. Soc. Nephrol. 2014, 25, 657–670. [Google Scholar] [CrossRef]
- Kestenbaum, B. Phosphate metabolism in the setting of chronic kidney disease: Significance and recommendations for treatment. Semin. Dial. 2007, 20, 286–294. [Google Scholar] [CrossRef]
- Selamet, U.; Tighiouart, H.; Sarnak, M.J.; Beck, G.; Levey, A.S.; Block, G.; Ix, J.H. Relationship of dietary phosphate intake with risk of end-stage renal disease and mortality in chronic kidney disease stages 3-5: The Modification of Diet in Renal Disease Study. Kidney Int. 2016, 89, 176–184. [Google Scholar] [CrossRef]
- Santamaría, R.; Díaz-Tocados, J.M.; Pendón-Ruiz de Mier, M.V.; Robles, A.; Salmerón-Rodríguez, M.D.; Ruiz, E.; Vergara, N.; Aguilera-Tejero, E.; Raya, A.; Ortega, R.; et al. Increased Phosphaturia Accelerates The Decline in Renal Function: A Search for Mechanisms. Sci. Rep. 2018, 8, 13701. [Google Scholar] [CrossRef]
- Asghari, G.; Yuzbashian, E.; Mirmiran, P.; Azizi, F. The association between Dietary Approaches to Stop Hypertension and incidence of chronic kidney disease in adults: The Tehran Lipid and Glucose Study. Nephrol. Dial. Transplant 2017, 32, ii224–ii230. [Google Scholar] [CrossRef]
- Mirmiran, P.; Yuzbashian, E.; Asghari, G.; Sarverzadeh, S.; Azizi, F. Dietary fibre intake in relation to the risk of incident chronic kidney disease. Br. J. Nutr. 2018, 119, 479–485. [Google Scholar] [CrossRef]
- Orlich, M.J.; Singh, P.N.; Sabaté, J.; Jaceldo-Siegl, K.; Fan, J.; Knutsen, S.; Beeson, W.L.; Fraser, G.E. Vegetarian Dietary Patterns and Mortality in Adventist Health Study 2. JAMA Intern. Med. 2013, 173, 1230. [Google Scholar] [CrossRef]
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of Animal and Plant Protein Intake With All-Cause and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef]
- Eddington, H.; Hoefield, R.; Sinha, S.; Chrysochou, C.; Lane, B.; Foley, R.N.; Hegarty, J.; New, J.; O’Donoghue, D.J.; Middleton, R.J.; et al. Serum Phosphate and Mortality in Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 2251–2257. [Google Scholar] [CrossRef]
- Isakova, T.; Barchi-Chung, A.; Enfield, G.; Smith, K.; Vargas, G.; Houston, J.; Xie, H.; Wahl, P.; Schiavenato, E.; Dosch, A.; et al. Effects of Dietary Phosphate Restriction and Phosphate Binders on FGF23 Levels in CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 1009–1018. [Google Scholar] [CrossRef]
- Chen, X.; Wei, G.; Jalili, T.; Metos, J.; Giri, A.; Cho, M.E.; Boucher, R.; Greene, T.; Beddhu, S. The Associations of Plant Protein Intake with All-Cause Mortality in CKD. Am. J. Kidney Dis. 2016, 67, 423–430. [Google Scholar] [CrossRef]
- Mayne, S.T.; Risch, H.A.; Dubrow, R.; Chow, W.H.; Gammon, M.D.; Vaughan, T.L.; Farrow, D.C.; Schoenberg, J.B.; Stanford, J.L.; Ahsan, H.; et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 1055–1062. [Google Scholar]
- Preis, S.R.; Stampfer, M.J.; Spiegelman, D.; Willett, W.C.; Rimm, E.B. Dietary protein and risk of ischemic heart disease in middle-aged men. Am. J. Clin. Nutr. 2010, 92, 1265–1272. [Google Scholar] [CrossRef]
- Alonso, A.; Beunza, J.J.; Bes-Rastrollo, M.; Pajares, R.M.; Martínez-González, M.A. Vegetable protein and fiber from cereal are inversely associated with the risk of hypertension in a Spanish cohort. Arch. Med. Res. 2006, 37, 778–786. [Google Scholar] [CrossRef]
- Halton, T.L.; Liu, S.; Manson, J.E.; Hu, F.B. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2008, 87, 339–346. [Google Scholar] [CrossRef]
- Goraya, N.; Wesson, D.E. Management of the Metabolic Acidosis of Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2017, 24, 298–304. [Google Scholar] [CrossRef]
- Scialla, J.J.; Appel, L.J.; Astor, B.C.; Miller, E.R.; Beddhu, S.; Woodward, M.; Parekh, R.S.; Anderson, C.A.M. Estimated net endogenous acid production and serum bicarbonate in African Americans with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 1526–1532. [Google Scholar] [CrossRef]
- Raphael, K.L.; Wei, G.; Baird, B.C.; Greene, T.; Beddhu, S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2011, 79, 356–362. [Google Scholar] [CrossRef]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef]
- Moorthi, R.N.; Armstrong, C.L.H.; Janda, K.; Ponsler-Sipes, K.; Asplin, J.R.; Moe, S.M. The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. Am. J. Nephrol. 2014, 40, 582–591. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Mediterranean diets: What is so special about the diet of Greece? The scientific evidence. J. Nutr. 2001, 131, 3065S–3073S. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Xu, H.; Rossi, M.; Campbell, K.L.; Sencion, G.L.; Ärnlöv, J.; Cederholm, T.; Sjögren, P.; Risérus, U.; Lindholm, B.; Carrero, J.J. Excess protein intake relative to fiber and cardiovascular events in elderly men with chronic kidney disease. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 597–602. [Google Scholar] [CrossRef]
- Sabatino, A.; Regolisti, G.; Brusasco, I.; Cabassi, A.; Morabito, S.; Fiaccadori, E. Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol. Dial. Transplant. 2015, 30, 924–933. [Google Scholar] [CrossRef]
- Frassetto, L.; Banerjee, T.; Powe, N.; Sebastian, A. Acid Balance, Dietary Acid Load, and Bone Effects-A Controversial Subject. Nutrients 2018, 10, 517. [Google Scholar] [CrossRef]
- Bethke, P.C.; Jansky, S.H. The effects of boiling and leaching on the content of potassium and other minerals in potatoes. J. Food Sci. 2008, 73, H80–H85. [Google Scholar] [CrossRef]
- Acal, I.; Cigarran, S.; Sanjurjo, A.; Latorre, J.; Menéndez, N.; Rodriguez, B.; Vazquez-Oderiz, L.R.A. Influence of culinary techniques of different vegetables products. Implications for chronic kidney disease patients. J. Renal Nutr. 2019. (under review). [Google Scholar]
- Ho-Pham, L.T.; Nguyen, N.D.; Nguyen, T. V Effect of vegetarian diets on bone mineral density: A Bayesian meta-analysis. Am. J. Clin. Nutr. 2009, 90, 943–950. [Google Scholar] [CrossRef]
- Craig, W.J. Health effects of vegan diets. Am. J. Clin. Nutr. 2009, 89, 1627S–1633S. [Google Scholar] [CrossRef]
| Mediterranean Diet Characteristics |
|---|
| 1.-Fruits and vegetables in every meal day |
| 2.-Dairy products, preferably low fat: Every day |
| 3.-Bread, Pasta or Rice: Every day |
| 4.-Cereals and olive oils: Every day |
| 5.-Nuts and olives: Every day |
| 6.-Potatoes, White meat, Fish, Legumes and Eggs: Every week |
| 7.-Reduced: Sweets, red and processed meat. |
| Biodiversity, fresh, seasonal, unprocessed and traditional culinary activity. |
| Authors | Population | Dietary Intervention | Outcomes and Measurements | Reference |
|---|---|---|---|---|
| Barsotti et al. | 22 stage III/IV CKD patients | Special vegan diet (SVD) vs. Conventional low-peotein diet (CLPD) vsunrestricted protein diet (UPD) | Urea ↓, Pi ↓, H+ ↓ and serum proteins (=) | [5] |
| Kandouz et al. | 138 patients in hemodiafiltration (HDF) | Vegan vs. non-vegan diet | Serum Indoxyl sulfate (IS) ↓ and p-cresyl sulfate (PCS) ↓ | [8] |
| Rossi et al. | 22 stage IV/V CKD patients | Symbiotic therapy | IS ↓, PCS ↓, renal parameters | [12] |
| Salmean at al. | 13 CKD patients (≥50 mL/min/1.73 m2) | Cross-over low-fiber diet vs. high fiber diet | Renal parameters (↑ eGFR, BUN ↓, SCr ↓) | [13] |
| Khosroshahi et al. | 50 ESRD patients on hemodialysis | Diet containing resistant stach vs. placebo | IS ↓, PCS ↓, Renal parameters (Urea ↓, Cr ↓, Uric acid ↓) | [18] |
| Goraya et al. | 76 stage IV CKD patients | NaHCO3 vs. vegetable-based diet | Cystatin C =, UNAG↓, TGFβ =, aldoresterona ↑, tetrahidrocortisol/ tetrahidrocortisone ratio ↑, PTCO2 ↑ | [35] |
| Goraya et al. | Macroalbuminuric CKD: Stage 1 (26 patients) and stage 2 (40 patients) | NaHCO3 vs. vegetable-based diet | Ualb ↑; UNAG =; TGFβ =; ET-1 ↓; Aldo ↑ | [36] |
| Moe et al. | 9 stage III/IV CKD patients | Vegetable-based diet vs. meat based diet for 7 days. | FGF-23(↑, PTH =, Ca =, Serum and urinary phosphate↓ | [41] |
| Wu et al. | 318 ESRD on hemodialysis | Vegetarians vs. non-vegetarians | nPCR ↓, Albumin =, antropometry (BMI↓, MACM↓) and hand grip = | [55] |
| Sirich et al. | 56 ESRD on hemodialysis | Diet containing resistant stach vs. control starch | IS ↓, PCS ↓ | [57] |
| Younes et al. | 9 chronic renal failure patients | Fermentable carbohydrates suppementation (crossover) | Nutritional status and biochemistry (Urea ↓, Albumin =, pre-alb =) | [61] |
| Lu et al. | 157 stage IV CKD patients | Dietary fiber correlation | ΔeGFR (slow), IL6 ↓, CRP ↓, IS ↓, SCh ↓ | [99] |
| Saglinbene et al. | 9757 ESRD patients on hemodialysis | Mediterranean and DASH diet scores | CV and total mortality (=) | [122] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cases, A.; Cigarrán-Guldrís, S.; Mas, S.; Gonzalez-Parra, E. Vegetable-Based Diets for Chronic Kidney Disease? It Is Time to Reconsider. Nutrients 2019, 11, 1263. https://doi.org/10.3390/nu11061263
Cases A, Cigarrán-Guldrís S, Mas S, Gonzalez-Parra E. Vegetable-Based Diets for Chronic Kidney Disease? It Is Time to Reconsider. Nutrients. 2019; 11(6):1263. https://doi.org/10.3390/nu11061263
Chicago/Turabian StyleCases, Aleix, Secundino Cigarrán-Guldrís, Sebastián Mas, and Emilio Gonzalez-Parra. 2019. "Vegetable-Based Diets for Chronic Kidney Disease? It Is Time to Reconsider" Nutrients 11, no. 6: 1263. https://doi.org/10.3390/nu11061263
APA StyleCases, A., Cigarrán-Guldrís, S., Mas, S., & Gonzalez-Parra, E. (2019). Vegetable-Based Diets for Chronic Kidney Disease? It Is Time to Reconsider. Nutrients, 11(6), 1263. https://doi.org/10.3390/nu11061263





