Chemopreventive Effects of Strawberry and Black Raspberry on Colorectal Cancer in Inflammatory Bowel Disease
Abstract
1. Introduction
2. Animal Models of Inflammatory Bowel Disease and IBD-Related Colorectal Cancer
3. Molecular Mechanisms Associated with Chronic Inflammation and Colorectal Cancer
3.1. Inflammation-Dependent Oxidative Stress
3.2. Genomic Instability
3.3. Cytokines
3.4. NFκB
3.5. Microbiome
4. Chemoprevention of Colorectal Cancer in Inflammatory Bowel Disease with Anti-Inflammation Pharmaceuticals
5. Chemoprevention of Colorectal Cancer in Inflammatory Bowel Disease with Berries
5.1. Efficiency of Strawberry and Black Raspberry in Cell Lines and Animal Models
5.2. Efficiency of Black Raspberry in Clinical Studies
5.3. Major Bioactive Components in Berries
5.4. Mechanisms Associated with Preventative Effects of Berries on Colon Cancer
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ananthakrishnan, A.N. Epidemiology and risk factors for ibd. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, R.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Feagins, L.A.; Souza, R.F.; Spechler, S.J. Carcinogenesis in ibd: Potential targets for the prevention of colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Mattar, M.C.; Lough, D.; Pishvaian, M.J.; Charabaty, A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest. Cancer Res. 2011, 4, 53–61. [Google Scholar]
- Subramanian, V.; Logan, R.F. Chemoprevention of colorectal cancer in inflammatory bowel disease. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 593–606. [Google Scholar] [CrossRef]
- Jess, T.; Rungoe, C.; Peyrin-Biroulet, L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 2012, 10, 639–645. [Google Scholar] [CrossRef]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef]
- Liao, J.; Seril, D.N.; Lu, G.G.; Zhang, M.; Toyokuni, S.; Yang, A.L.; Yang, G.Y. Increased susceptibility of chronic ulcerative colitis-induced carcinoma development in DNA repair enzyme ogg1 deficient mice. Mol. Carcinog. 2008, 47, 638–646. [Google Scholar] [CrossRef]
- Kornbluth, A.; Sachar, D.B. Ulcerative colitis practice guidelines in adults (update): American college of gastroenterology, practice parameters committee. Am. J. Gastroenterol. 2004, 99, 1371–1385. [Google Scholar] [CrossRef]
- Lewis, J.D.; Abreu, M.T. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology 2017, 152, 398–414.e6. [Google Scholar] [CrossRef]
- Khalili, H.; Chan, S.S.M.; Lochhead, P.; Ananthakrishnan, A.N.; Hart, A.R.; Chan, A.T. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.D.; Kim, J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World J. Gastroenterol. 2013, 19, 1011–1019. [Google Scholar] [CrossRef]
- Arts, I.C.; Jacobs, D.R., Jr.; Gross, M.; Harnack, L.J.; Folsom, A.R. Dietary catechins and cancer incidence among postmenopausal women: The iowa women’s health study (united states). Cancer Causes Control 2002, 13, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Simons, C.C.; Hughes, L.A.; Arts, I.C.; Goldbohm, R.A.; van den Brandt, P.A.; Weijenberg, M.P. Dietary flavonol, flavone and catechin intake and risk of colorectal cancer in the netherlands cohort study. Int. J. Cancer 2009, 125, 2945–2952. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.D. Foodstuffs for preventing cancer: The preclinical and clinical development of berries. Cancer Prev. Res. 2009, 2, 187–194. [Google Scholar] [CrossRef]
- Zhu, X.R.; Xiong, L.F.; Zhang, X.Y.; Shi, N.; Zhang, Y.T.; Ke, J.; Sun, Z.; Chen, T. Lyophilized strawberries prevent 7,12-dimethylbenz[alpha]anthracene (dmba)-induced oral squamous cell carcinogenesis in hamsters. J. Funct. Foods 2015, 15, 476–486. [Google Scholar] [CrossRef]
- Chen, T.; Yan, F.; Qian, J.; Guo, M.; Zhang, H.; Tang, X.; Chen, F.; Stoner, G.D.; Wang, X. Randomized phase ii trial of lyophilized strawberries in patients with dysplastic precancerous lesions of the esophagus. Cancer Prev. Res. 2012, 5, 41–50. [Google Scholar] [CrossRef]
- Shi, N.; Clinton, S.K.; Liu, Z.; Wang, Y.; Riedl, K.M.; Schwartz, S.J.; Zhang, X.; Pan, Z.; Chen, T. Strawberry phytochemicals inhibit azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in crj: Cd-1 mice. Nutrients 2015, 7, 1696–1715. [Google Scholar] [CrossRef]
- Duncan, F.J.; Martin, J.R.; Wulff, B.C.; Stoner, G.D.; Tober, K.L.; Oberyszyn, T.M.; Kusewitt, D.F.; Van Buskirk, A.M. Topical treatment with black raspberry extract reduces cutaneous uvb-induced carcinogenesis and inflammation. Cancer Prev. Res. 2009, 2, 665–672. [Google Scholar] [CrossRef]
- Mallery, S.R.; Tong, M.; Shumway, B.S.; Curran, A.E.; Larsen, P.E.; Ness, G.M.; Kennedy, K.S.; Blakey, G.H.; Kushner, G.M.; Vickers, A.M.; et al. Topical application of a mucoadhesive freeze-dried black raspberry gel induces clinical and histologic regression and reduces loss of heterozygosity events in premalignant oral intraepithelial lesions: Results from a multicentered, placebo-controlled clinical trial. Clin. Cancer Res. 2014, 20, 1910–1924. [Google Scholar]
- Shi, N.; Chen, F.; Zhang, X.; Clinton, S.K.; Tang, X.; Sun, Z.; Chen, T. Suppression of oxidative stress and nfkappab/mapk signaling by lyophilized black raspberries for esophageal cancer prevention in rats. Nutrients 2017, 9, 413. [Google Scholar] [CrossRef]
- Shi, N.; Riedl, K.M.; Schwartz, S.J.; Zhang, X.L.; Clinton, S.K.; Chen, T. Efficacy comparison of lyophilised black raspberries and combination of celecoxib and pbit in prevention of carcinogen-induced oesophageal cancer in rats. J. Funct. Foods 2016, 27, 84–94. [Google Scholar] [CrossRef]
- Fu, S.K.; Lawrance, I.C. Anima model of IBD-associated CRC and colorectal cancer tumorigenesis. Clin. Med. Insight 2015, 7, 1–9. [Google Scholar]
- De Robertis, M.; Massi, E.; Poeta, M.L.; Carotti, S.; Morini, S.; Cecchetelli, L.; Signori, E.; Fazio, V.M. The aom/dss murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 2011, 10, 9. [Google Scholar]
- Pozza, A.; Scarpa, M.; Ruffolo, C.; Polese, L.; Erroi, F.; Bridda, A.; Norberto, L.; Frego, M. Colonic carcinogenesis in ibd: Molecular events. Ann. Ital. Chir. 2011, 82, 19–28. [Google Scholar]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Saadatdoust, Z.; Esa, N.M.; Hamzah, H.; Ismail, A. Dietary cocoa protects against colitis-associated cancer by activating the nrf2/keap1 pathway. Biofactors 2015, 41, 1–14. [Google Scholar] [CrossRef]
- Osburn, W.O.; Karim, B.; Dolan, P.M.; Liu, G.; Yamamoto, M.; Huso, D.L.; Kensler, T.W. Increased colonic inflammatory injury and formation of aberrant crypt foci in nrf2-deficient mice upon dextran sulfate treatment. Int. J. Cancer 2007, 121, 1883–1891. [Google Scholar] [CrossRef]
- Barrett, C.W.; Ning, W.; Chen, X.; Smith, J.J.; Washington, M.K.; Hill, K.E.; Coburn, L.A.; Peek, R.M.; Chaturvedi, R.; Wilson, K.T.; et al. Tumor suppressor function of the plasma glutathione peroxidase gpx3 in colitis-associated carcinoma. Cancer Res. 2013, 73, 1245–1255. [Google Scholar] [CrossRef]
- Kawanishi, S.; Hiraku, Y.; Pinlaor, S.; Ma, N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol. Chem. 2006, 387, 365–372. [Google Scholar] [CrossRef]
- Sohn, J.J.; Schetter, A.J.; Yfantis, H.G.; Ridnour, L.A.; Horikawa, I.; Khan, M.A.; Robles, A.I.; Hussain, S.P.; Goto, A.; Bowman, E.D.; et al. Macrophages, nitric oxide and micrornas are associated with DNA damage response pathway and senescence in inflammatory bowel disease. PLoS ONE 2012, 7, e44156. [Google Scholar] [CrossRef]
- Grasso, F.; Di Meo, S.; De Luca, G.; Pasquini, L.; Rossi, S.; Boirivant, M.; Biffoni, M.; Bignami, M.; Di Carlo, E. The mutyh base excision repair gene protects against inflammation-associated colorectal carcinogenesis. Oncotarget 2015, 6, 19671–19684. [Google Scholar] [CrossRef]
- Yaeger, R.; Shah, M.A.; Miller, V.A.; Kelsen, J.R.; Wang, K.; Heins, Z.J.; Ross, J.S.; He, Y.; Sanford, E.; Yantiss, R.K.; et al. Genomic alterations observed in colitis-associated cancers are distinct from those found in sporadic colorectal cancers and vary by type of inflammatory bowel disease. Gastroenterology 2016, 151, 278–287. [Google Scholar] [CrossRef]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073–2087 e2073. [Google Scholar] [CrossRef]
- van Dieren, J.M.; Wink, J.C.; Vissers, K.J.; van Marion, R.; Hoogmans, M.M.; Dinjens, W.N.; Schouten, W.R.; Tanke, H.J.; Szuhai, K.; Kuipers, E.J.; et al. Chromosomal and microsatellite instability of adenocarcinomas and dysplastic lesions (dalm) in ulcerative colitis. Diagn. Mol. Pathol. 2006, 15, 216–222. [Google Scholar] [CrossRef]
- Willenbucher, R.F.; Aust, D.E.; Chang, C.G.; Zelman, S.J.; Ferrell, L.D.; Moore, D.H., 2nd; Waldman, F.M. Genomic instability is an early event during the progression pathway of ulcerative-colitis-related neoplasia. Am. J. Pathol. 1999, 154, 1825–1830. [Google Scholar] [CrossRef]
- Tsai, J.H.; Rabinovitch, P.S.; Huang, D.; Small, T.; Mattis, A.N.; Kakar, S.; Choi, W.T. Association of aneuploidy and flat dysplasia with development of high-grade dysplasia or colorectal cancer in patients with inflammatory bowel disease. Gastroenterology 2017, 153, 1492–1495. [Google Scholar] [CrossRef]
- Itzkowitz, S.H. Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2006, 35, 553–571. [Google Scholar] [CrossRef]
- Hussain, S.P.; Amstad, P.; Raja, K.; Ambs, S.; Nagashima, M.; Bennett, W.P.; Shields, P.G.; Ham, A.J.; Swenberg, J.A.; Marrogi, A.J.; et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: A cancer-prone chronic inflammatory disease. Cancer Res. 2000, 60, 3333–3337. [Google Scholar]
- Garrett, W.S.; Punit, S.; Gallini, C.A.; Michaud, M.; Zhang, D.; Sigrist, K.S.; Lord, G.M.; Glickman, J.N.; Glimcher, L.H. Colitis-associated colorectal cancer driven by t-bet deficiency in dendritic cells. Cancer Cell 2009, 16, 208–219. [Google Scholar] [CrossRef][Green Version]
- Neufert, C.; Becker, C.; Tureci, O.; Waldner, M.J.; Backert, I.; Floh, K.; Atreya, I.; Leppkes, M.; Jefremow, A.; Vieth, M.; et al. Tumor fibroblast-derived epiregulin promotes growth of colitis-associated neoplasms through erk. J. Clin. Investig. 2013, 123, 1428–1443. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Han, G.C.; Wang, R.X.; Xiao, H.; Hou, C.M.; Guo, R.F.; Dou, Y.; Shen, B.F.; Li, Y.; et al. Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (il-1)/il-6 axis. Mucosal. Immunol. 2014, 7, 1106–1115. [Google Scholar] [CrossRef]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Cytokines, ibd, and colitis-associated cancer. Inflamm. Bowel. Dis. 2015, 21, 409–418. [Google Scholar] [CrossRef]
- Popivanova, B.K.; Kitamura, K.; Wu, Y.; Kondo, T.; Kagaya, T.; Kaneko, S.; Oshima, M.; Fujii, C.; Mukaida, N. Blocking tnf-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Investig. 2008, 118, 560–570. [Google Scholar]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. Il-6 and stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef]
- Matsumoto, S.; Hara, T.; Mitsuyama, K.; Yamamoto, M.; Tsuruta, O.; Sata, M.; Scheller, J.; Rose-John, S.; Kado, S.; Takada, T. Essential roles of il-6 trans-signaling in colonic epithelial cells, induced by the il-6/soluble-il-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J. Immunol. 2010, 184, 1543–1551. [Google Scholar] [CrossRef]
- McGovern, D.P.; Rotter, J.I.; Mei, L.; Haritunians, T.; Landers, C.; Derkowski, C.; Dutridge, D.; Dubinsky, M.; Ippoliti, A.; Vasiliauskas, E.; et al. Genetic epistasis of il23/il17 pathway genes in crohn’s disease. Inflamm. Bowel. Dis. 2009, 15, 883–889. [Google Scholar] [CrossRef]
- Zenewicz, L.A.; Antov, A.; Flavell, R.A. Cd4 t-cell differentiation and inflammatory bowel disease. Trends Mol. Med. 2009, 15, 199–207. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Zhou, M.; He, J.; Shen, Y.; Zhang, C.; Wang, J.; Chen, Y. New Frontiers in Genetics, Gut Microbiota, and Immunity: A Rosetta Stone for the Pathogenesis of Inflammatory Bowel Disease. Biomed. Res. Int. 2017, 2017, 8201672. [Google Scholar] [CrossRef]
- Velayos, F.S.; Terdiman, J.P.; Walsh, J.M. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: A systematic review and metaanalysis of observational studies. Am. J. Gastroenterol. 2005, 100, 1345–1353. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Santino, A.; Scarano, A.; De Santis, S.; De Benedictis, M.; Giovinazzo, G.; Chieppa, M. Gut Microbiota Modulation and Anti-Inflammatory Properties of Dietary Polyphenolsin IBD: New and Consolidated Perspectives. Curr. Pharm. Des. 2017, 23, 2344–2351. [Google Scholar] [CrossRef]
- Duricova, D.; Burisch, J.; Jess, T.; Gower-Rousseau, C.; Lakatos, P.L.; ECCO-EpiCom. Age-related differences in presentation and course of inflammatory bowel disease: An update on the population-based literature. J. Crohns Colitis 2014, 8, 1351–1361. [Google Scholar] [CrossRef]
- Cho, H.; Jung, H.; Lee, H.; Yi, H.C.; Kwak, H.K.; Hwang, K.T. Chemopreventive activity of ellagitannins and their derivatives from black raspberry seeds on ht-29 colon cancer cells. Food Funct. 2015, 6, 1675–1683. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef]
- Montrose, D.C.; Horelik, N.A.; Madigan, J.P.; Stoner, G.D.; Wang, L.S.; Bruno, R.S.; Park, H.J.; Giardina, C.; Rosenberg, D.W. Anti-inflammatory effects of freeze-dried black raspberry powder in ulcerative colitis. Carcinogenesis 2011, 32, 343–350. [Google Scholar] [CrossRef]
- Wang, L.S.; Kuo, C.T.; Stoner, K.; Yearsley, M.; Oshima, K.; Yu, J.; Huang, T.H.; Rosenberg, D.; Peiffer, D.; Stoner, G.; et al. Dietary black raspberries modulate DNA methylation in dextran sodium sulfate (dss)-induced ulcerative colitis. Carcinogenesis 2013, 34, 2842–2850. [Google Scholar] [CrossRef]
- Wang, L.S.; Kuo, C.T.; Huang, T.H.; Yearsley, M.; Oshima, K.; Stoner, G.D.; Yu, J.; Lechner, J.F.; Huang, Y.W. Black raspberries protectively regulate methylation of wnt pathway genes in precancerous colon tissue. Cancer Prev. Res. 2013, 6, 1317–1327. [Google Scholar] [CrossRef]
- Huang, C.; Huang, Y.; Li, J.; Hu, W.; Aziz, R.; Tang, M.S.; Sun, N.; Cassady, J.; Stoner, G.D. Inhibition of benzo(a)pyrene diol-epoxide-induced transactivation of activated protein 1 and nuclear factor kappab by black raspberry extracts. Cancer Res. 2002, 62, 6857–6863. [Google Scholar]
- Lu, H.; Li, J.; Zhang, D.; Stoner, G.D.; Huang, C. Molecular mechanisms involved in chemoprevention of black raspberry extracts: From transcription factors to their target genes. Nutr. Cancer 2006, 54, 69–78. [Google Scholar] [CrossRef]
- Harris, G.K.; Gupta, A.; Nines, R.G.; Kresty, L.A.; Habib, S.G.; Frankel, W.L.; LaPerle, K.; Gallaher, D.D.; Schwartz, S.J.; Stoner, G.D. Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2′-deoxyguanosine levels in the fischer 344 rat. Nutr. Cancer 2001, 40, 125–133. [Google Scholar] [CrossRef]
- Bi, X.; Fang, W.; Wang, L.S.; Stoner, G.D.; Yang, W. Black raspberries inhibit intestinal tumorigenesis in apc1638+/− and muc2−/− mouse models of colorectal cancer. Cancer Prev. Res. 2010, 3, 1443–1450. [Google Scholar] [CrossRef]
- Wang, L.S.; Arnold, M.; Huang, Y.W.; Sardo, C.; Seguin, C.; Martin, E.; Huang, T.H.; Riedl, K.; Schwartz, S.; Frankel, W.; et al. Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: A phase i pilot study. Clin. Cancer Res. 2011, 17, 598–610. [Google Scholar] [CrossRef]
- Wang, L.S.; Kuo, C.T.; Cho, S.J.; Seguin, C.; Siddiqui, J.; Stoner, K.; Weng, Y.I.; Huang, T.H.; Tichelaar, J.; Yearsley, M.; et al. Black raspberry-derived anthocyanins demethylate tumor suppressor genes through the inhibition of dnmt1 and dnmt3b in colon cancer cells. Nutr. Cancer 2013, 65, 118–125. [Google Scholar] [CrossRef]
- Wu, Q.K.; Koponen, J.M.; Mykkanen, H.M.; Torronen, A.R. Berry phenolic extracts modulate the expression of p21(waf1) and bax but not bcl-2 in ht-29 colon cancer cells. J. Agric. Food Chem. 2007, 55, 1156–1163. [Google Scholar] [CrossRef]
- McDougall, G.J.; Ross, H.A.; Ikeji, M.; Stewart, D. Berry extracts exert different antiproliferative effects against cervical and colon cancer cells grown in vitro. J. Agric. Food Chem. 2008, 56, 3016–3023. [Google Scholar] [CrossRef]
- Zhang, Y.; Seeram, N.P.; Lee, R.; Feng, L.; Heber, D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J. Agric. Food Chem. 2008, 56, 670–675. [Google Scholar] [CrossRef]
- Kanodia, L.; Borgohain, M.; Das, S. Effect of fruit extract of Fragaria vesca L. on experimentally induced inflammatory bowel disease in albino rats. Indian J. Pharm. 2011, 43, 18–21. [Google Scholar] [CrossRef]
- Mentor-Marcel, R.A.; Bobe, G.; Sardo, C.; Wang, L.S.; Kuo, C.T.; Stoner, G.; Colburn, N.H. Plasma cytokines as potential response indicators to dietary freeze-dried black raspberries in colorectal cancer patients. Nutr. Cancer 2012, 64, 820–825. [Google Scholar] [CrossRef]
- Duthie, S.J.; Gardner, P.T.; Morrice, P.C.; Wood, S.G.; Pirie, L.; Bestwick, C.C.; Milne, L.; Duthie, G.G. DNA stability and lipid peroxidation in vitamin e-deficient rats in vivo and colon cells in vitro--modulation by the dietary anthocyanin, cyanidin-3-glycoside. Eur. J. Nutr. 2005, 44, 195–203. [Google Scholar] [CrossRef]
- Elisia, I.; Kitts, D.D. Anthocyanins inhibit peroxyl radical-induced apoptosis in caco-2 cells. Mol. Cell. Biochem. 2008, 312, 139–145. [Google Scholar] [CrossRef]
- Renis, M.; Calandra, L.; Scifo, C.; Tomasello, B.; Cardile, V.; Vanella, L.; Bei, R.; La Fauci, L.; Galvano, F. Response of cell cycle/stress-related protein expression and DNA damage upon treatment of CaCO2 cells with anthocyanins. Br. J. Nutr. 2008, 100, 27–35. [Google Scholar] [CrossRef]
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.; Gescher, A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1163–1170. [Google Scholar]
- Bornsek, S.M.; Ziberna, L.; Polak, T.; Vanzo, A.; Ulrih, N.P.; Abram, V.; Tramer, F.; Passamonti, S. Bilberry and blueberry anthocyanins act as powerful intracellular antioxidants in mammalian cells. Food Chem. 2012, 134, 1878–1884. [Google Scholar] [CrossRef]
- Katsube, N.; Iwashita, K.; Tsushida, T.; Yamaki, K.; Kobori, M. Induction of apoptosis in cancer cells by bilberry (vaccinium myrtillus) and the anthocyanins. J. Agric. Food Chem. 2003, 51, 68–75. [Google Scholar] [CrossRef]
- Lala, G.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson, B.A. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr. Cancer 2006, 54, 84–93. [Google Scholar] [CrossRef]
- Kangawa, Y.; Yoshida, T.; Maruyama, K.; Okamoto, M.; Kihara, T.; Nakamura, M.; Ochiai, M.; Hippo, Y.; Hayashi, S.M.; Shibutani, M. Cilostazol and enzymatically modified isoquercitrin attenuate experimental colitis and colon cancer in mice by inhibiting cell proliferation and inflammation. Food Chem. Toxicol. 2017, 100, 103–114. [Google Scholar] [CrossRef]
- Charepalli, V.; Reddivari, L.; Radhakrishnan, S.; Vadde, R.; Agarwal, R.; Vanamala, J.K. Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J. Nutr. Biochem. 2015, 26, 1641–1649. [Google Scholar] [CrossRef]
- Urias-Lugo, D.A.; Heredia, J.B.; Muy-Rangel, M.D.; Valdez-Torres, J.B.; Serna-Saldivar, S.O.; Gutierrez-Uribe, J.A. Anthocyanins and phenolic acids of hybrid and native blue maize (Zea mays L.) extracts and their antiproliferative activity in mammary (MCF7), liver (HepG2), colon (Caco2 and HT29) and prostate (PC3) cancer cells. Plant Foods Hum. Nutr. 2015, 70, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Qian, B.; Zhao, S.; Qi, X.; Ye, L.; Monica Giusti, M.; Wang, X. Effect of glycosylation patterns of chinese eggplant anthocyanins and other derivatives on antioxidant effectiveness in human colon cell lines. Food Chem. 2015, 172, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Xu, J.; Kim, J.; Chen, T.Y.; Su, X.; Standard, J.; Carey, E.; Griffin, J.; Herndon, B.; Katz, B.; et al. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013, 57, 1908–1917. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Lee, W.S.; Kim, M.J.; Lu, J.N.; Kang, M.H.; Kim, H.G.; Kim, D.C.; Choi, E.J.; Choi, J.Y.; Lee, Y.K.; et al. Characterization of a profile of the anthocyanins isolated from vitis coignetiae pulliat and their anti-invasive activity on ht-29 human colon cancer cells. Food Chem. Toxicol. 2010, 48, 903–909. [Google Scholar] [CrossRef]
- Dai, J.; Patel, J.D.; Mumper, R.J. Characterization of blackberry extract and its antiproliferative and anti-inflammatory properties. J. Med. Food 2007, 10, 258–265. [Google Scholar] [CrossRef]
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agric. Food Chem. 2005, 53, 7320–7329. [Google Scholar] [CrossRef]
- Kang, S.Y.; Seeram, N.P.; Nair, M.G.; Bourquin, L.D. Tart cherry anthocyanins inhibit tumor development in apc(min) mice and reduce proliferation of human colon cancer cells. Cancer Lett. 2003, 194, 13–19. [Google Scholar] [CrossRef]
- Kawamori, T.; Tanaka, T.; Kojima, T.; Suzui, M.; Ohnishi, M.; Mori, H. Suppression of azoxymethane-induced rat colon aberrant crypt foci by dietary protocatechuic acid. Jpn. J. Cancer Res. 1994, 85, 686–691. [Google Scholar] [CrossRef]
- Tanaka, T.; Kojima, T.; Suzui, M.; Mori, H. Chemoprevention of colon carcinogenesis by the natural product of a simple phenolic compound protocatechuic acid: Suppressing effects on tumor development and biomarkers expression of colon tumorigenesis. Cancer Res. 1993, 53, 3908–3913. [Google Scholar]
- Farombi, E.O.; Adedara, I.A.; Awoyemi, O.V.; Njoku, C.R.; Micah, G.O.; Esogwa, C.U.; Owumi, S.E.; Olopade, J.O. Dietary protocatechuic acid ameliorates dextran sulphate sodium-induced ulcerative colitis and hepatotoxicity in rats. Food Funct. 2016, 7, 913–921. [Google Scholar] [CrossRef]
- Venancio, V.P.; Cipriano, P.A.; Kim, H.; Antunes, L.M.; Talcott, S.T.; Mertens-Talcott, S.U. Cocoplum (chrysobalanus icaco l.) anthocyanins exert anti-inflammatory activity in human colon cancer and non-malignant colon cells. Food Funct. 2017, 8, 307–314. [Google Scholar] [CrossRef]
- Charepalli, V.; Reddivari, L.; Vadde, R.; Walia, S.; Radhakrishnan, S.; Vanamala, J.K. Eugenia jambolana (java plum) fruit extract exhibits anti-cancer activity against early stage human hct-116 colon cancer cells and colon cancer stem cells. Cancers (Basel) 2016, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Butelli, E.; De Santis, S.; Cavalcanti, E.; Hill, L.; De Angelis, M.; Giovinazzo, G.; Chieppa, M.; Martin, C.; Santino, A. Combined Dietary Anthocyanins, Flavonols, and Stilbenoids Alleviate Inflammatory Bowel Disease Symptoms in Mice. Front. Nutr. 2018, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health. Compr. Rev. Food Sci. Food Saf. 2013, 12, 24. [Google Scholar]
- Knobloch, T.J.; Uhrig, L.K.; Pearl, D.K.; Casto, B.C.; Warner, B.M.; Clinton, S.K.; Sardo-Molmenti, C.L.; Ferguson, J.M.; Daly, B.T.; Riedl, K.; et al. Suppression of proinflammatory and prosurvival biomarkers in oral cancer patients consuming a black raspberry phytochemical-rich troche. Cancer Prev. Res. 2016, 9, 159–171. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Tan, H.P.; Ling, S.K.; Abdul Wahab, N.; Sim, K.S. Phytochemical investigation of gynura bicolor leaves and cytotoxicity evaluation of the chemical constituents against hct 116 cells. Nat. Prod. Res. 2016, 30, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Femia, A.P.; Caderni, G.; Buzzigoli, C.; Cocca, E.; Salvadori, M.; Dolara, P. Effect of simple phenolic compounds on azoxymethane-induced aberrant crypt foci in rat colon. Nutr. Cancer 2001, 41, 107–110. [Google Scholar]
- Mace, T.A.; King, S.A.; Ameen, Z.; Elnaggar, O.; Young, G.; Riedl, K.M.; Schwartz, S.J.; Clinton, S.K.; Knobloch, T.J.; Weghorst, C.M.; et al. Bioactive compounds or metabolites from black raspberries modulate t lymphocyte proliferation, myeloid cell differentiation and jak/stat signaling. Cancer Immunol. Immunother. 2014, 63, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Park, H.C.; Choi, S.; Kim, S.; Bao, C.; Kim, H.W.; Choi, H.K.; Lee, H.J.; Auh, J.H. Metabolomic analysis reveals cyanidins in black raspberry as candidates for suppression of lipopolysaccharide-induced inflammation in murine macrophages. J. Agric. Food Chem. 2015, 63, 5449–5458. [Google Scholar] [CrossRef]
- Wang, L.S.; Hecht, S.; Carmella, S.; Seguin, C.; Rocha, C.; Yu, N.; Stoner, K.; Chiu, S.; Stoner, G. Berry ellagitannins may not be sufficient for prevention of tumors in the rodent esophagus. J. Agric. Food Chem. 2010, 58, 3992–3995. [Google Scholar] [CrossRef] [PubMed]
- Paudel, L.; Wyzgoski, F.J.; Giusti, M.M.; Johnson, J.L.; Rinaldi, P.L.; Scheerens, J.C.; Chanon, A.M.; Bomser, J.A.; Miller, A.R.; Hardy, J.K.; et al. Nmr-based metabolomic investigation of bioactivity of chemical constituents in black raspberry (rubus occidentalis l.) fruit extracts. J. Agric. Food Chem. 2014, 62, 1989–1998. [Google Scholar] [CrossRef]
- Farzaei, M.H.; El-Senduny, F.F.; Momtaz, S.; Parvizi, F.; Iranpanah, A.; Tewari, D.; Naseri, R.; Abdolghaffari, A.H.; Rezaei, N. An update on dietary consideration in inflammatory bowel disease: Anthocyanins and more. Expert Rev. Gastroenterol. Hepatol. 2018, 10, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Sodagari, H.R.; Farzaei, M.H.; Bahramsoltani, R.; Abdolghaffari, A.H.; Mahmoudi, M.; Rezaei, N. Dietary anthocyanins as a complementary medicinal approach for management of inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Burke, C.A.; Hasson, H.; Kuo, C.T.; Molmenti, C.L.; Seguin, C.; Liu, P.; Huang, T.H.; Frankel, W.L.; Stoner, G.D. A phase ib study of the effects of black raspberries on rectal polyps in patients with familial adenomatous polyposis. Cancer Prev. Res. 2014, 7, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Hecht, S.S.; Carmella, S.G.; Yu, N.; Larue, B.; Henry, C.; McIntyre, C.; Rocha, C.; Lechner, J.F.; Stoner, G.D. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev. Res. 2009, 2, 84–93. [Google Scholar] [CrossRef]
- Peiffer, D.S.; Wang, L.S.; Zimmerman, N.P.; Ransom, B.W.; Carmella, S.G.; Kuo, C.T.; Chen, J.H.; Oshima, K.; Huang, Y.W.; Hecht, S.S.; et al. Dietary consumption of black raspberries or their anthocyanin constituents alters innate immune cell trafficking in esophageal cancer. Cancer Immunol. Res. 2016, 4, 72–82. [Google Scholar] [CrossRef]
- Peiffer, D.S.; Zimmerman, N.P.; Wang, L.S.; Ransom, B.W.; Carmella, S.G.; Kuo, C.T.; Siddiqui, J.; Chen, J.H.; Oshima, K.; Huang, Y.W.; et al. Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev. Res. 2014, 7, 574–584. [Google Scholar] [CrossRef]
- Chen, T.; Rose, M.E.; Hwang, H.; Nines, R.G.; Stoner, G.D. Black raspberries inhibit n-nitrosomethylbenzylamine (nmba)-induced angiogenesis in rat esophagus parallel to the suppression of cox-2 and inos. Carcinogenesis 2006, 27, 2301–2307. [Google Scholar] [CrossRef]
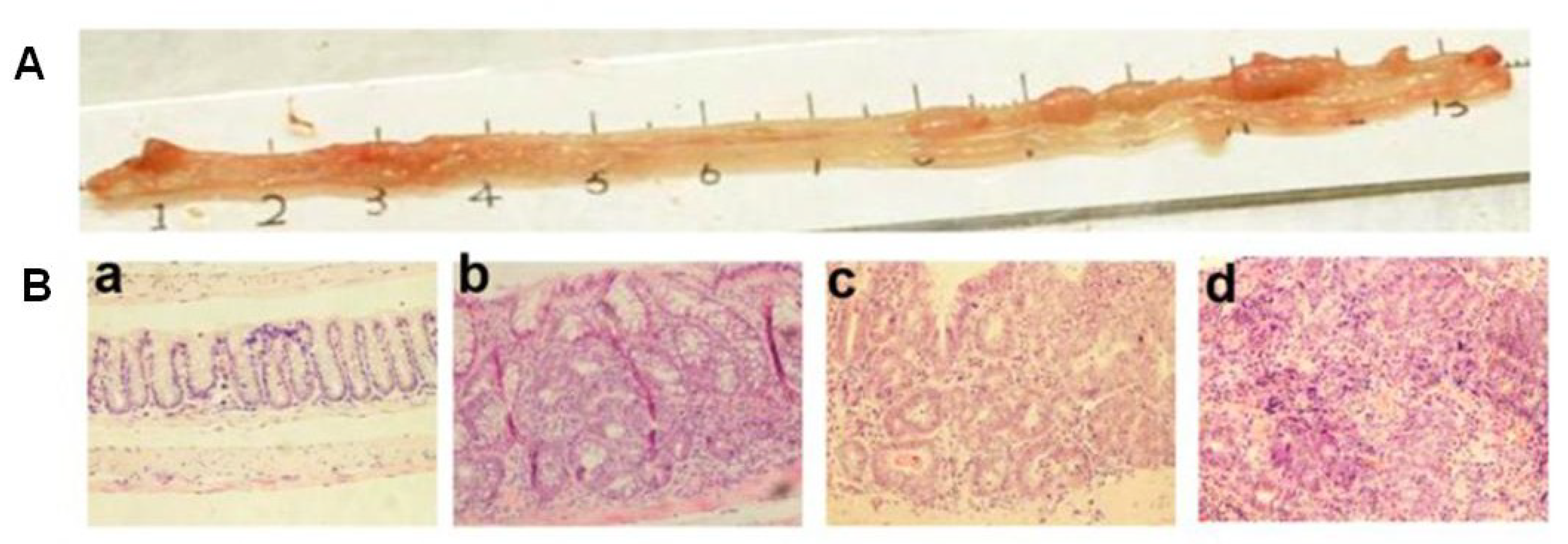
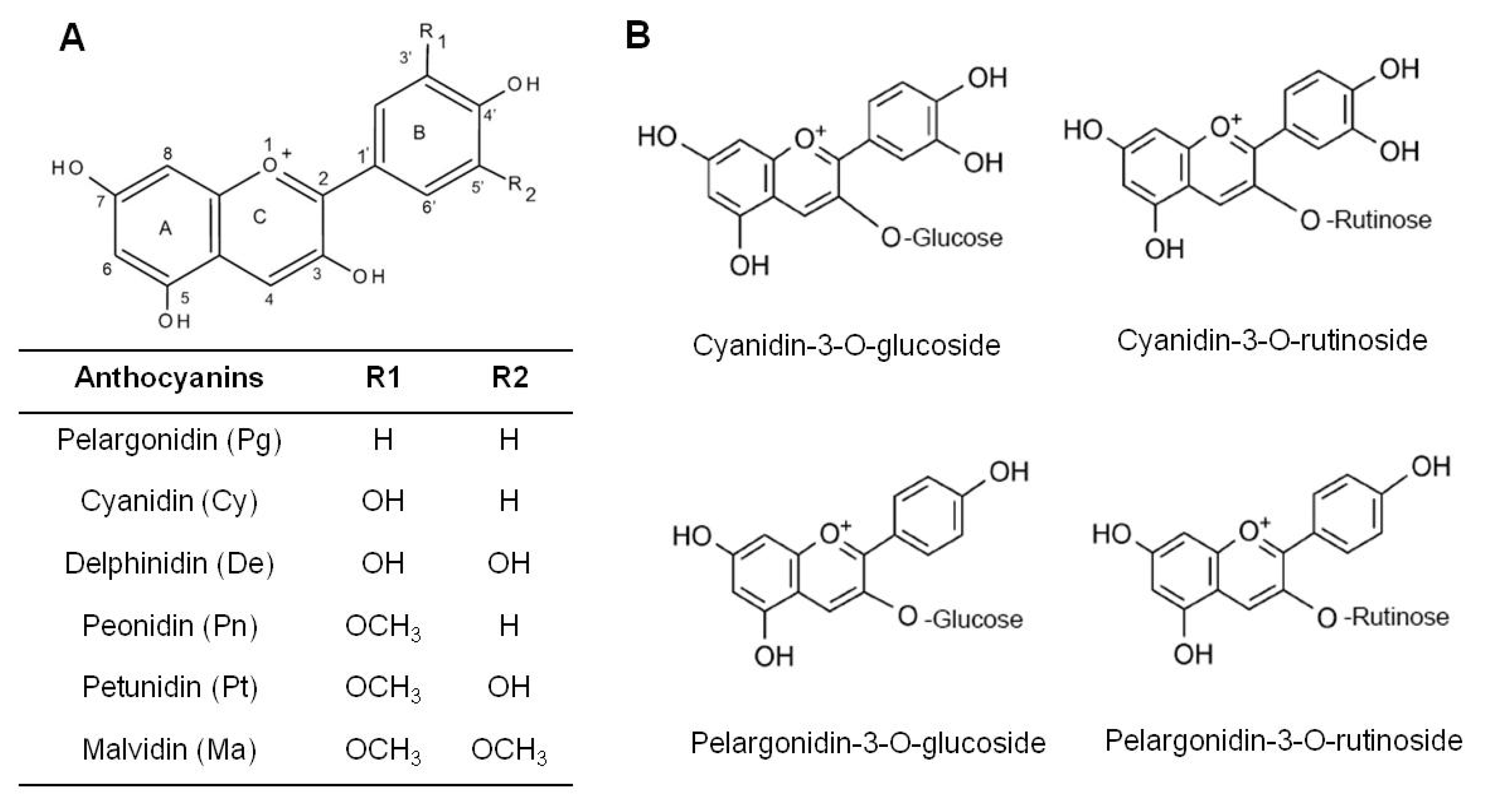
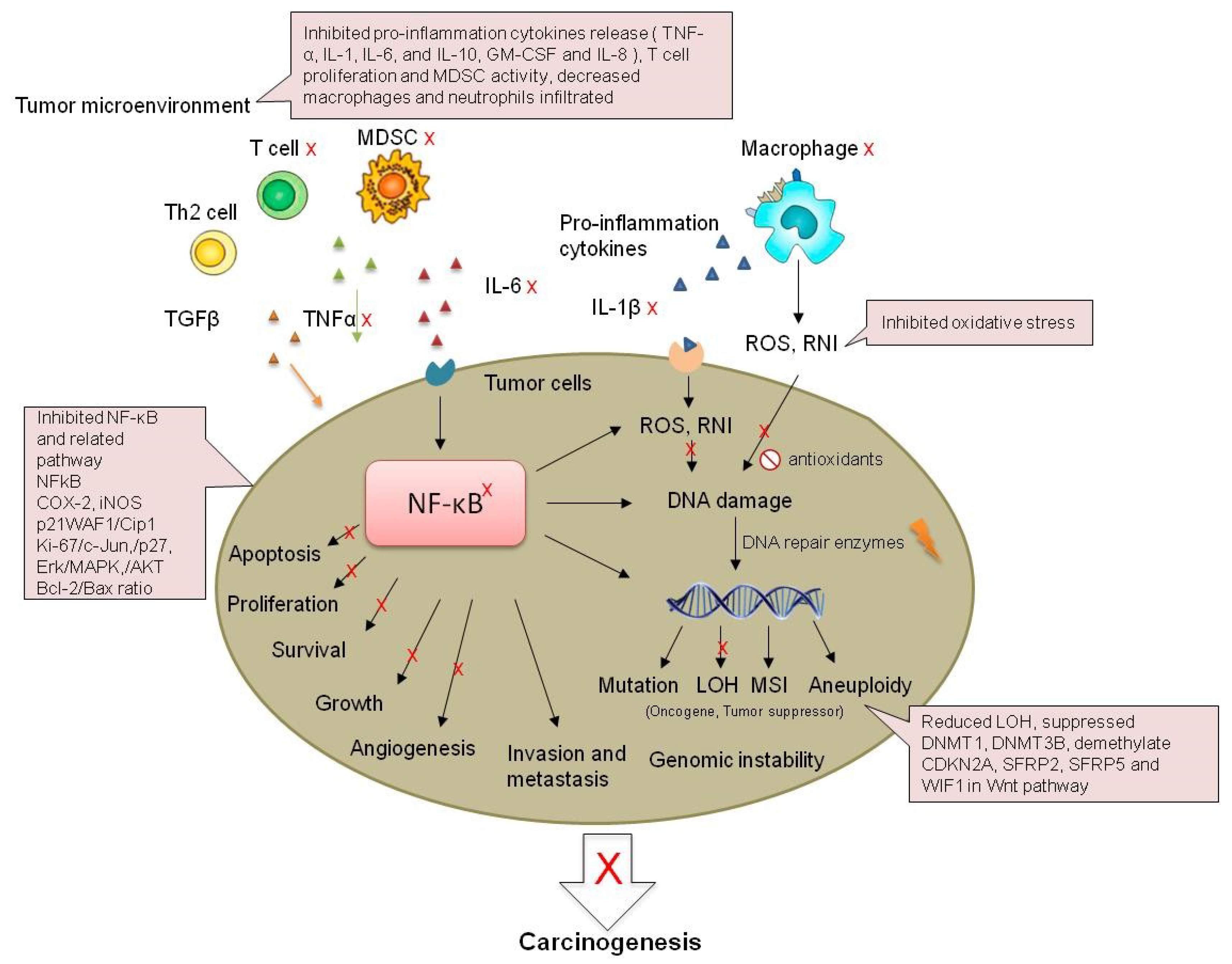
| Systems | Major Findings | Ref. |
|---|---|---|
| Black Raspberry | ||
| 1. CRC Cell Lines | ||
| HT-29 /HT-116 cell lines | Regulating cell cycle and apoptosis | [56,57] |
| 2. Animal Models of IBD | ||
| DSS treated mouse | Colonic epithelium acute injury↓, ulceration↓, TNF-α and IL-1β↓, COX2 and NFκB↓ | [58] |
| DSS treated mouse | Ulceration↓, macrophages and neutrophils infiltrated the colon tissue↓, NFκB↓, Dkk3↑, β-Catenin nuclear localization↓,c-Myc, DNMT3B, HDAC1, HDAC2, MBD2↓ | [59] |
| IL-10 knockout mice | Ulceration↓, Wnt pathway↓, wif1, sox17, and qki↑,dnmt3b, hdac1, hdac2, and mbd2↓ | [60] |
| 3. Animal Models of IBD-Related CRC | ||
| Mouse epidermal JB6Cl41 cells | AP-1, NFκB, and COX-2↓ | [61,62] |
| AOM induced rat model | ACF multiplicity↓, total tumor multiplicity↓, urinary 8-OHdG↓ | [63] |
| Muc2−/− mice | COX-2, TNF-α, IL-1, IL-6, and IL-10 ↓ | [64] |
| 4.Clinical Studies of CRC | ||
| CRC patients | GM-CSF and IL-8↓, Ki-67↓, apoptosis↓ | [65] |
| CRC patients | Wnt pathway↓ (SFRP2, WIF1, β-catenin, E-cadherin), DNMT1↓ | [66] |
| Strawberry | ||
| 1. CRC Cell Lines | ||
| HT-29 cell lines | Proliferation↓, cell apoptosis and p21WAF1↑ | [57,67] |
| CaCo-2 | Proliferation↓ | [68] |
| HCT-116 | Proliferation↓ | [69] |
| 2. Animal Models of IBD | ||
| Gum acacia induced IBD rats | Disease activity index↓, lesion scores↓, antioxidant enzymes myeloperoxidase↑, tissue catalase↑, superoxide dismutase↑ | [70] |
| 3. Animal Models of IBD-Related CRC | ||
| AOM/DSS mouse | Tumor incidence↓, nitrosative stress↓, TNF-α, IL-1β, IL-6, COX-2 and iNOS ↓, PI3K, Akt, ERK and NFκB↓ | [18] |
| Strawberry | Black Raspberry | ||||
|---|---|---|---|---|---|
| mg/ 100 mg | % by mg | mg/ 100 mg | % by mg | ||
| Anthocyanins | Anthocyanins | ||||
| pelargonidin glucoside | 367.7 | 41.1 | cyanidin rutinoside | 2924.7 | 58.2 |
| pelargonidin malonyl glucoside | 83.9 | 9.4 | cyanidin xylorutinoside | 916.3 | 18.2 |
| pelargonidin rutinoside | 55.3 | 6.2 | cyanidin glucoside | 245.1 | 4.9 |
| cyanidin glucoside | 14.9 | 1.7 | cyanidin sambubioside | 103.5 | 2.1 |
| pelargonidin rutinoside | 38.3 | 0.8 | |||
| 130.4 | 58.4% | 4227.9 | 84.2% | ||
| Ellagitannins | Ellagitannins | ||||
| ellagitannin | 64.1 | 7.2 | sanguiin H6 | 173.2 | 3.4 |
| ellagitannin | 11.4 | 1.3 | ellagitannin 783-1 | 101.1 | 2.0 |
| ellagitannin | 23.1 | 2.6 | ellagitannin 933-2 | 75.8 | 1.5 |
| Lambertianin | 20.3 | 2.3 | elagitannin 783-2 | 75.8 | 1.5 |
| ellagitannin 935-1 | 62.8 | 1.3 | |||
| ellagitannin 933-1 | 50.5 | 1.0 | |||
| Lambertiannin | 31.3 | 0.6 | |||
| ellagitannin 935-2 | 7.9 | 0.2 | |||
| 118.9 | 13.3% | 578.5 | 11.5% | ||
| Ellagic acid and derivatives | Ellagic acid and derivatives | ||||
| Agrimoniin | 144.5 | 16.2 | methyl ellagic acid pentoside | 16.0 | 0.3 |
| ellagic acid rhamnoside | 23.1 | 2.6 | ellagic acid | 9.3 | 0.2 |
| ellagic acid | 7.3 | 0.8 | ellagic acid rhamnoside | 5.8 | 0.1 |
| myricetin hexoside, EA derivative (coelution) | 3.8 | 0.1 | |||
| 174.9 | 19.5% | 34.8 | 0.7% | ||
| Flavonols | Flavonols | ||||
| quercetin hexuronide | 58.8 | 6.6 | quercetin hexuronide | 82.2 | 1.6 |
| kaempferol glucoside/hexuronide | 14.5 | 1.6 | rutin (quercetin rutinoside) | 75.4 | 1.5 |
| kaempferol malonyl hexoside | 5.1 | 0.6 | quercetin xylorutinoside | 24.2 | 0.5 |
| 78.4 | 8.8% | 181.8 | 3.6% | ||
| Original Sources | Models | Major Findings | Anthocyanin Profiles | Ref. |
|---|---|---|---|---|
| Cell Lines | ||||
| 1. Black Raspberry | ||||
| Black raspberry extract | Cell line HT-29 HCT-116 | Inhibited cell growth HT-29 IC50 = 89.11, HCT-116, IC50 = 89.00 | cyanidin-3-sophoroside rhamnoside, cyanidin-3-sambubioside rhamnoside, cyanidin-3-rutinoside | [57] |
| Black raspberry anthocyanin-enriched extract | Cell line HCT116 | Decreased DNMT activity; decreased methylation of CDKN2A, SFRP5, SFRP2 and WIF1; suppressed cell proliferation; induced apoptosis | cyanidin-3-O-glucoside, cyanidin- 3-O-rutinoside, cyanidin-3-O-xylosylrutinoside, cyanidin-3-O-sambubioside | [58] |
| 2. Strawberry | ||||
| Strawberry extract | Cell line HT-29 HCT-116 | Inhibited cell growth HT-29 IC50 = 114.30, HCT-116, IC50 = 62.00 | cyanidin-3-glucoside, pelargonidin-3-glucoside, pelargonidin-3-rutinoside | [57] |
| Strawberry extract | Cell line HT29 HCT-116 | Antioxidative effects. | cyanidin-3-glucoside, pelargonidin, cyanidin-3-glucoside, pelargonidin, pelargonidin-3-rutinoside | [66] |
| Strawberry extract | Cell line HT29 | Inhibited proliferation; reduced expression of p21WAF1 | cyanidin derivative; pelargonidin derivative | [67] |
| 3. Anthocyanins | ||||
| cyanidin-3-glycoside | Cell line HCEC | Decreased DNA strand breakage | cyanidin-3-glycoside | [72] |
| Cyanidin-3-glycoside | Cell line Caoco-2 | Reduced cytotoxicity induced by AAPH; suppressed apoptosis; decreased sub-G1 phase cell population | cyanidin-3-glycoside | [73] |
| Cyanidin-3-O-beta glucopyranoside, cyanidin chloride | Cell line Caoco-2 | Inhibited cell growth and proliferation; decreased reactive oxygen species (ROS) level | cyanidin-3-O-beta glucopyranoside, cyanidin chloride | [74] |
| 4. Protocatechuic Acid (PCA) | ||||
| Brown rice extracted PCA | Cell line SW480 | Inhibited cell growth and colony formation | PCA | [75] |
| 5. Other Fruit and Vegetables | ||||
| Blueberry | Cell line Caoco-2 | IC50 0.53 ± 0.04 | delphinidin 3-galactoside, delphinidin 3-glucoside, cyanidin 3-galactoside, delphinidin 3-arabinoside, cyanidin 3-glucoside, petunidin 3-galactoside, cyanidin 3-arabinoside, petunidin 3-glucoside, peonidin 3-galactoside, petunidin 3-arabinoside, peonidin 3-glucoside, malvidin 3-galactoside, peonidin 3-arabinoside, malvidin 3-glucoside, malvidin 3-arabinoside | [76] |
| Blueberry extract | Cell lines HT-29 | Inhibited cell growth; induced apoptosis | delphinidin 3-O-β-glucopyranoside; cyanidin 3-O-β-galactopyranoside; cyanidin 3-O-β-glucopyranoside; petunidin 3-O-β-glucopyranoside; peonidin 3-O-β-galactopyranoside; peonidin 3-O-β-glucopyranoside; malvidin 3-O-β-glucopyranoside. | [77] |
| Bilberry purified anthocyanins | Cell line HCT-116 | Decreased cell viability | pelargonidin, cyanidin, peonidin, delphinidin, and malvidin | [78] |
| Cocoplum anthocyanins exert | Cell line HT-29 | Cell proliferation was suppressed; increased intracellular ROS production; increased intracellular ROS production | delphinidin-3-glucoside, cyanidin 3-glucoside, petunidin 3-glucoside, delphinidin 3-(6-acetoyl) galactoside, delphinidin 3-(6-oxaloyl) arabinoside, peonidin 3-glucoside, petunidin 3-(6-acetoyl) galactoside, petunidin 3-(6-oxaloyl) arabinoside, peonidin 3-(6-acetoyl) glucoside, peonidin 3-(6-oxaloyl) arabinoside | [79] |
| Eugenia jambolana (Java Plum) fruit extract | Cell lines HCT-116 colon cancer stem cells | Inhibited proliferation; induced apoptosis | delphinidin-3,5-diglucoside, cyanidin-3,5-diglucoside, ptunidin-3,5-diglucosid, dtunidin-3,5-diglucosid, peonidin-3,5-diglucoside, monidin-3,5-diglucosid, cyanidin-3-glucoside, petunidin-3-glucoside, etunidin-3-glucosi | [80] |
| Anthocyanin-containing baked purple-fleshed potato extract | Colon cancer stem cells | Suppressed proliferation; elevated apoptosis; decreased β-catenin, c-Myc and Cyclin D1 | pet-3-rut-5-glc, mal-3-rut-5-glc, cya-3-O(6-O-malonyl- β-d-glc), peo-3-(p-coum)-isophoro-5-glc, peo-3-rut-5-glc, pet-3-(p-coum)-rut-5-glc, peo-3-caffeyl-rut-5-glc, pel-3-(p-coum)-rut-5-glc, pel-3-(4-ferul-rut)-5-glc, peo-3-(p-coum)-rut-5-glc, mal-3-(p-coum)-rut-5-glc | [81] |
| Blue maize extract | Cell line Caco2 and HT29 | Suppressed proliferation | cyanidin 3-glucoside, cyanidin 3-glucoside, cyanidin malonyl-glucoside, cyanidin succinyl-glucoside, pelargonidin 3-glucoside, pelargonidin malonyl-glucoside | [82] |
| Eggplant extract | Cell lines HT-29 | Decreased DNA damage | delphinidin-3-rhamnosyl-glucoside-5-glucoside, delphinidin-3-rutinoside-5-glucoside | [83] |
| anthocyanin-enriched purple-fleshed sweet potato | Cell line SW480 | Decreased cell number, G1 phase arrest | peonidin-3-glucoside | [84] |
| Vitis coignetiae Pullia extract | Inhibited cell invasion; suppressed MMP-2, MMP-9, NFkB | delphinidin-3,5-diglucoside, cyanidin-3,5-diglucoside, petunidin-3,5-diglucoside, delphinidin-3-glucoside, malvdin-3,5-diglucoside, peonidin-3,5-diglucoside, cyanidin-3-glucoside, petunidin-3-glucoside, peonidin-3-glucoside, malvidin-3-glucoside | [85] | |
| Blackberry extract | Cell lines HT-29 | Inhibited cell growth; inhibited IL-12 release | cyanidin-3-glucoside, cyanidin-3-arabinoside, delphinidin-3-xyloside, cyanidin-3-xyloside, cyanidin-3-malonylglucoside, cyanidin-3-dioxalylglucoside | [86] |
| Tart cherry anthocyanin | Cell line HT 29, HCT16 | Inhibited cell growth | 3-cyanidin 2″-O-β-d-glucopyranosyl-6″-O-α-l-rhamnopyransyl-β-d-glucopyranoside | [87] |
| Animal Models | ||||
| 1. PCA | ||||
| PCA | AOM-treated rat | Decreased the number of aberrant crypt foci, ornithine decarboxylase activity and AgNOR | PCA | [88,89] |
| PCA | DSS-treated rat | Prevented diarrhea and bleeding; decreased pro-inflammatory cytokines; nitric oxide concentration, oxidative damage, and expression of COX-2 and iNOS | PCA | [90] |
| 2. Other fruit and vegetables | ||||
| Anthocyanin-rich extracts from bilberry, chokeberry, and grape | AOM-treated rat | Reduced total ACF and the number of large ACF Suppressed cell proliferation reduces COX-2 | [91] | |
| Anthocyanin derived from purple sweet potato color in their basal diet | AOM/DSS rats | Decreased MDF, colon weight, low-grade dysplasia and total histopathology changes; decreased the expression of β-catenin, Ki67and Cyclin D1 | [92] | |
| anthocyanin-enriched purple-fleshed sweet potato | Animal AOM mice | Suppressed formation of aberrant crypt foci; decreased PCNA; increased caspase-3 | peonidin-3-glucoside | [84] |
| Tart cherry anthocyanin | ApcMin mice | Decreased the number and volume of adenomas | 3-cyanidin 6″-O-α-l-rhamnopyranosyl-β-d-glucopyranoside | [87] |
| Tomato | DSS mice | Increased bacterial Parabacteroides and Lactobacilli | [93] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Shi, N.; Afzali, A. Chemopreventive Effects of Strawberry and Black Raspberry on Colorectal Cancer in Inflammatory Bowel Disease. Nutrients 2019, 11, 1261. https://doi.org/10.3390/nu11061261
Chen T, Shi N, Afzali A. Chemopreventive Effects of Strawberry and Black Raspberry on Colorectal Cancer in Inflammatory Bowel Disease. Nutrients. 2019; 11(6):1261. https://doi.org/10.3390/nu11061261
Chicago/Turabian StyleChen, Tong, Ni Shi, and Anita Afzali. 2019. "Chemopreventive Effects of Strawberry and Black Raspberry on Colorectal Cancer in Inflammatory Bowel Disease" Nutrients 11, no. 6: 1261. https://doi.org/10.3390/nu11061261
APA StyleChen, T., Shi, N., & Afzali, A. (2019). Chemopreventive Effects of Strawberry and Black Raspberry on Colorectal Cancer in Inflammatory Bowel Disease. Nutrients, 11(6), 1261. https://doi.org/10.3390/nu11061261




