High-Dose Astaxanthin Supplementation Suppresses Antioxidant Enzyme Activity during Moderate-Intensity Swimming Training in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Astaxanthin Source, Animals, and Experimental Design
2.2. Sample Collection and Preparation
2.3. Antioxidant Enzymes and Malondialdehyde Assays
2.4. Nitric Oxide Synthase and Creatine Kinase Assays
2.5. Analysis of Nrf2 and Nrf2-Dependent Gene Transcription in Gastrocnemius and Heart
2.6. Statistical Analysis
3. Results
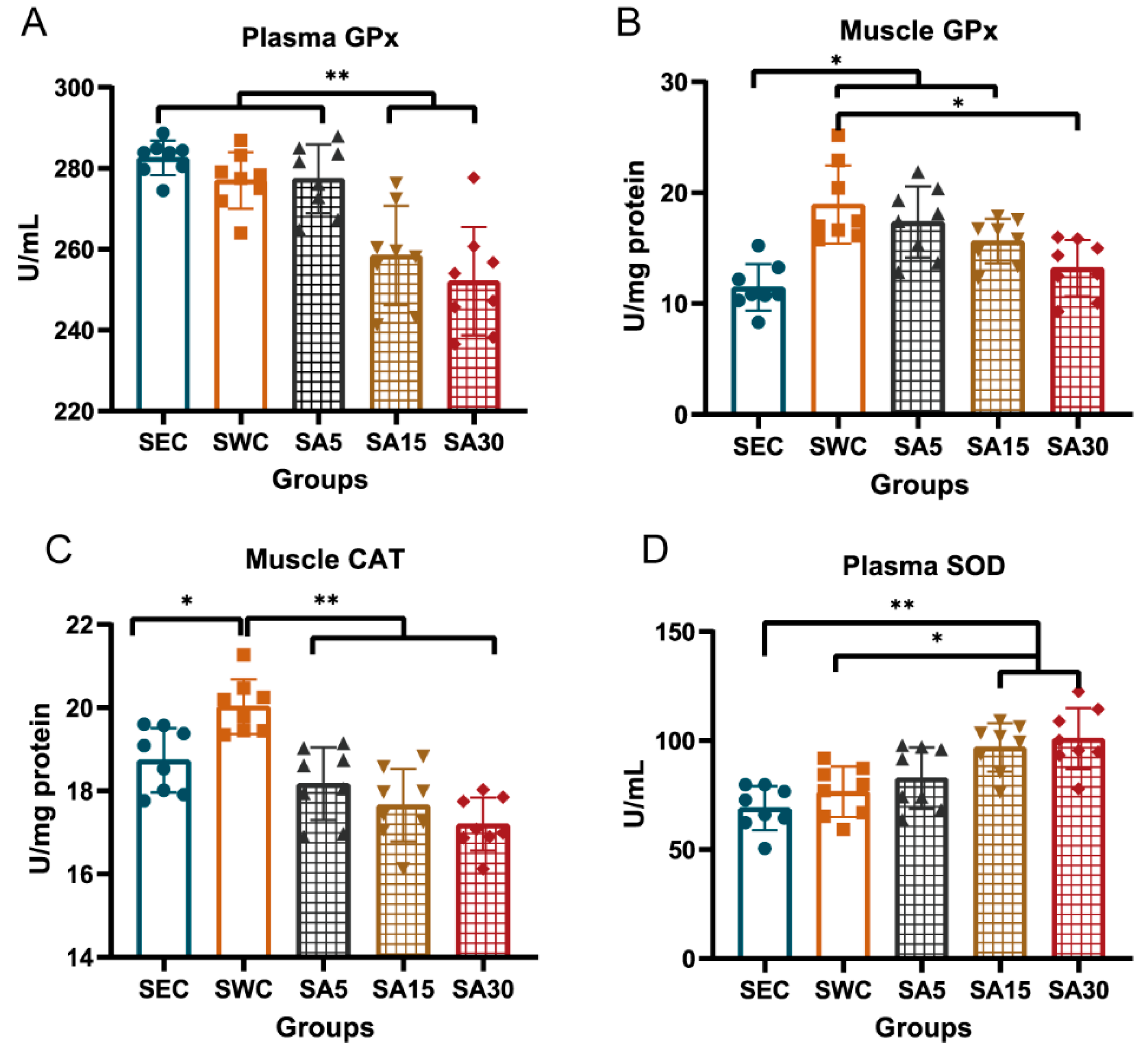
3.1. Antioxidant Enzymes in Plasma or Muscle
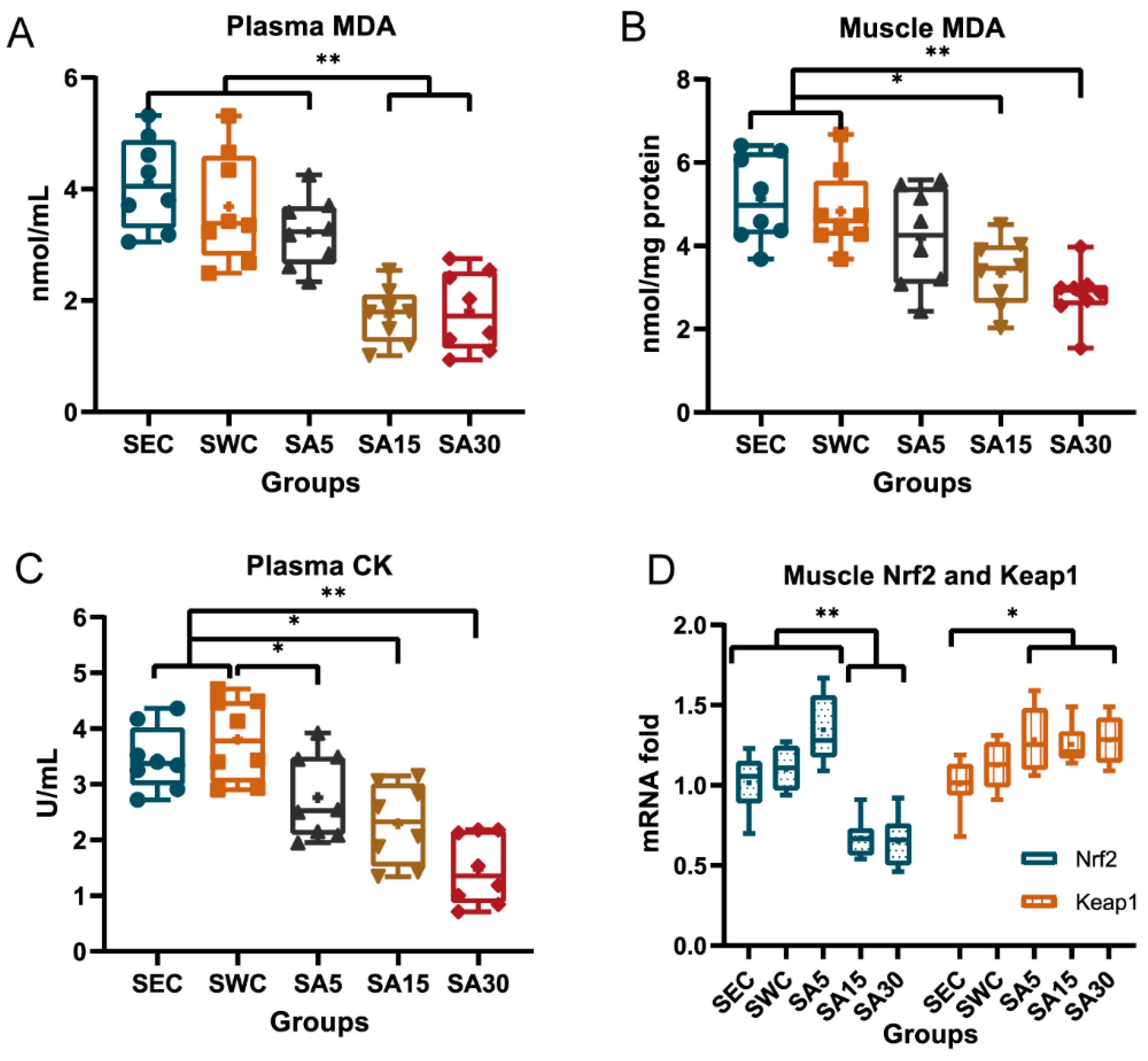
3.2. Plasma Malondialdehyde and Creatine Kinase and Muscle Nrf2-Keap1 Transcription
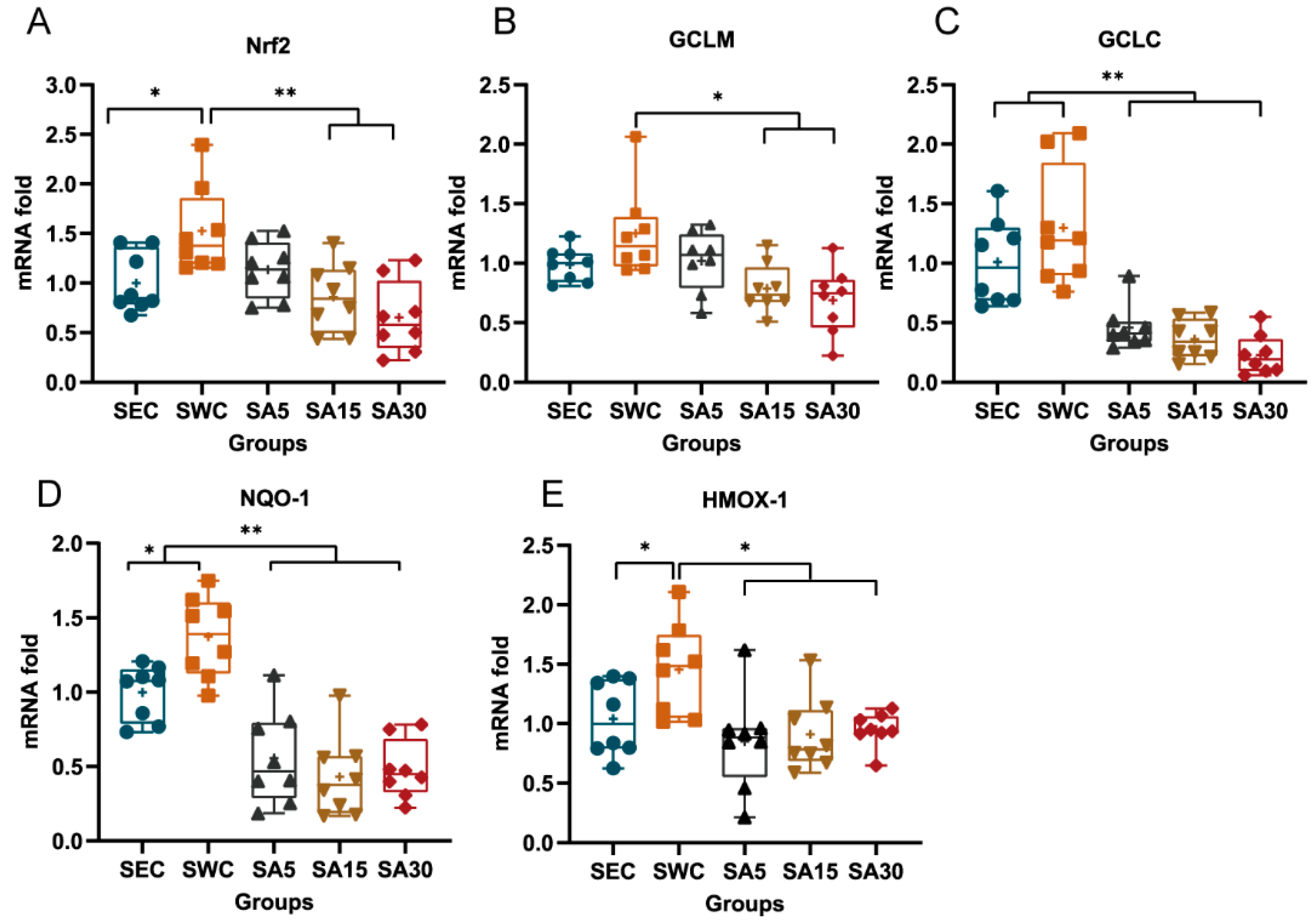
3.3. mRNA of Nrf2 and Nrf2-Dependent Enzymes in Heart
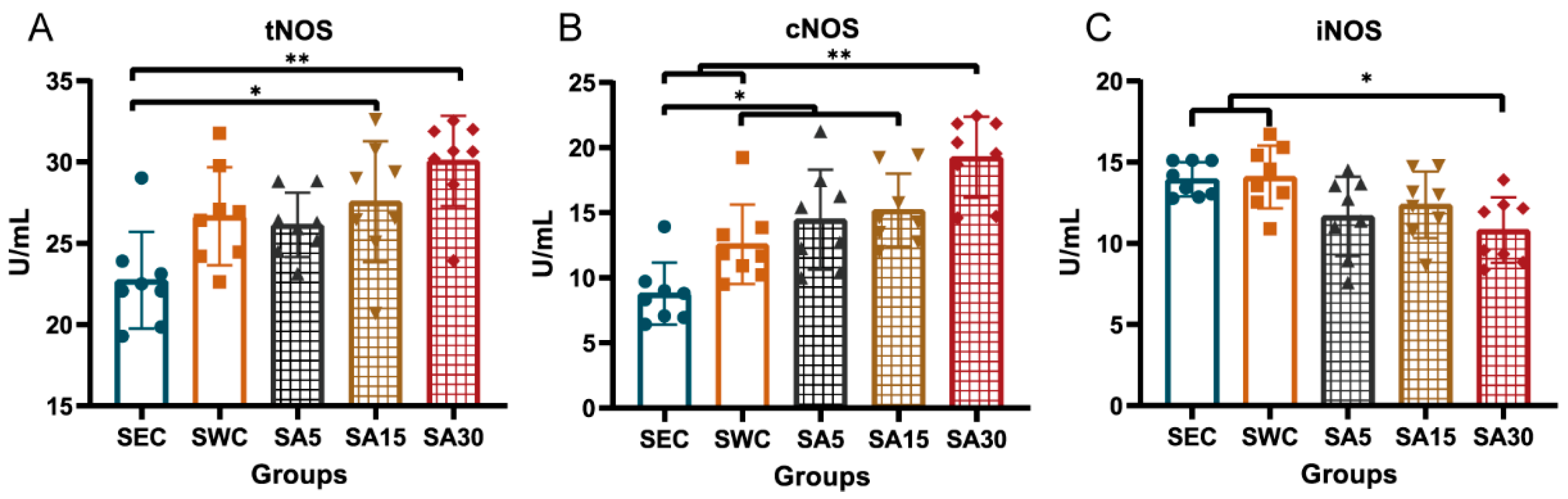
3.4. Plasma Nitric Oxide Synthase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Powers, S.K.; Nelson, W.B.; Hudson, M.B. Exercise-induced oxidative stress in humans: Cause and consequences. Free Radic. Biol. Med. 2011, 51, 942–950. [Google Scholar] [CrossRef]
- Cobley, J.N.; Close, G.L.; Bailey, D.M.; Davison, G.W. Exercise redox biochemistry: Conceptual, methodological and technical recommendations. Redox. Biol. 2017, 12, 540–548. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- Osburn, W.O.; Kensler, T.W. Nrf2 signaling: An adaptive response pathway for protection against environmental toxic insults. Mutat. Res. 2008, 659, 31–39. [Google Scholar] [CrossRef]
- Yang, H.; Magilnick, N.; Lee, C.; Kalmaz, D.; Ou, X.; Chan, J.Y.; Lu, S.C. Nrf1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-kappaB and AP-1. Mol. Cell Biol. 2005, 25, 5933–5946. [Google Scholar] [CrossRef] [PubMed]
- Franklin, C.C.; Backos, D.S.; Mohar, I.; White, C.C.; Forman, H.J.; Kavanagh, T.J. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol. Aspects Med. 2009, 30, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.; Siegel, D. Functions of NQO1 in Cellular Protection and CoQ10 Metabolism and its Potential Role as a Redox Sensitive Molecular Switch. Front Physiol. 2017, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef]
- Busso, T. Variable dose-response relationship between exercise training and performance. Med. Sci. Sports Exerc. 2003, 35, 1188–1195. [Google Scholar] [CrossRef]
- Radak, Z.; Ishihara, K.; Tekus, E.; Varga, C.; Posa, A.; Balogh, L.; Boldogh, I.; Koltai, E. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox. Biol. 2017, 12, 285–290. [Google Scholar] [CrossRef]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox. Signal 2013, 18, 1208–1246. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Cabo, H.; Ferrando, B.; Vina, J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic. Biol. Med. 2015, 86, 37–46. [Google Scholar] [CrossRef]
- Morrison, D.; Hughes, J.; Della Gatta, P.A.; Mason, S.; Lamon, S.; Russell, A.P.; Wadley, G.D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015, 89, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallen, J.; Ronnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Ostgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Vina, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef]
- Meier, P.; Renga, M.; Hoppeler, H.; Baum, O. The impact of antioxidant supplements and endurance exercise on genes of the carbohydrate and lipid metabolism in skeletal muscle of mice. Cell Biochem. Funct. 2013, 31, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Bell, O.; West, D.J.; Howatson, G.; Stevenson, E.J. Antioxidant-rich beetroot juice does not adversely affect acute neuromuscular adaptation following eccentric exercise. J. Sports Sci. 2017, 35, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Higashida, K.; Kim, S.H.; Higuchi, M.; Holloszy, J.O.; Han, D.H. Normal adaptations to exercise despite protection against oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E779–E784. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Lin, J.C.; Bernard, J.R.; Liao, Y.H. Green tea extract supplementation does not hamper endurance-training adaptation but improves antioxidant capacity in sedentary men. Appl. Physiol. Nutr. Metab. 2015, 40, 990–996. [Google Scholar] [CrossRef]
- Shill, D.D.; Southern, W.M.; Willingham, T.B.; Lansford, K.A.; McCully, K.K.; Jenkins, N.T. Mitochondria-specific antioxidant supplementation does not influence endurance exercise training-induced adaptations in circulating angiogenic cells, skeletal muscle oxidative capacity or maximal oxygen uptake. J. Physiol. 2016, 594, 7005–7014. [Google Scholar] [CrossRef] [PubMed]
- Yfanti, C.; Akerstrom, T.; Nielsen, S.; Nielsen, A.R.; Mounier, R.; Mortensen, O.H.; Lykkesfeldt, J.; Rose, A.J.; Fischer, C.P.; Pedersen, B.K. Antioxidant supplementation does not alter endurance training adaptation. Med. Sci. Sports Exerc. 2010, 42, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Dose, J.; Matsugo, S.; Yokokawa, H.; Koshida, Y.; Okazaki, S.; Seidel, U.; Eggersdorfer, M.; Rimbach, G.; Esatbeyoglu, T. Free Radical Scavenging and Cellular Antioxidant Properties of Astaxanthin. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011, 16, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Takanami, Y.; Ishii, T.; Kawai, Y.; Akagiri, S.; Kato, Y.; Osawa, T.; Yoshikawa, T. Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biochem. Biophys Res. Commun. 2008, 366, 892–897. [Google Scholar] [CrossRef]
- Earnest, C.P.; Lupo, M.; White, K.M.; Church, T.S. Effect of astaxanthin on cycling time trial performance. Int. J. Sports Med. 2011, 32, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of astaxanthin supplementation on exercise-induced fatigue in mice. Biol. Pharm. Bull. 2006, 29, 2106–2110. [Google Scholar] [CrossRef] [PubMed]
- Polotow, T.G.; Vardaris, C.V.; Mihaliuc, A.R.; Goncalves, M.S.; Pereira, B.; Ganini, D.; Barros, M.P. Astaxanthin supplementation delays physical exhaustion and prevents redox imbalances in plasma and soleus muscles of Wistar rats. Nutrients 2014, 6, 5819–5838. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Maoka, T.; Abe, R.; Fujishita, M.; Tominaga, K. Comparison of the effect of non-esterified and esterified astaxanthins on endurance performance in mice. J. Clin. Biochem. Nutr. 2018, 62, 161–166. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Avila, L.C.; Bruggemann, T.R.; Bobinski, F.; da Silva, M.D.; Oliveira, R.C.; Martins, D.F.; Mazzardo-Martins, L.; Duarte, M.M.; de Souza, L.F.; Dafre, A.; et al. Effects of High-Intensity Swimming on Lung Inflammation and Oxidative Stress in a Murine Model of DEP-Induced Injury. PLoS ONE 2015, 10, e0137273. [Google Scholar] [CrossRef]
- Casquero, A.C.; Berti, J.A.; Teixeira, L.L.S.; de Oliveira, H.C.F. Chronic Exercise Reduces CETP and Mesterolone Treatment Counteracts Exercise Benefits on Plasma Lipoproteins Profile: Studies in Transgenic Mice. Lipids 2017, 52, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Dimauro, I.; Mercatelli, N.; Caporossi, D. Exercise-induced ROS in heat shock proteins response. Free Radic. Biol. Med. 2016, 98, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Olguin, C.; Diaz-Vegas, A.; Utreras-Mendoza, Y.; Campos, C.; Arias-Calderon, M.; Llanos, P.; Contreras-Ferrat, A.; Espinosa, A.; Altamirano, F.; Jaimovich, E.; et al. NOX2 Inhibition Impairs Early Muscle Gene Expression Induced by a Single Exercise Bout. Front Physiol. 2016, 7, 282. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Morrison, D.; McConell, G.K.; Wadley, G.D. Muscle redox signalling pathways in exercise. Role of antioxidants. Free Radic. Biol. Med. 2016, 98, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.; Shaw, C.S.; Stepto, N.K.; Levinger, I. Exercise and Glycemic Control: Focus on Redox Homeostasis and Redox-Sensitive Protein Signaling. Front Endocrinol. 2017, 8, 87. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Theodorou, A.A.; Paschalis, V.; Veskoukis, A.S.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Kyparos, A.; Nikolaidis, M.G. Adaptations to endurance training depend on exercise-induced oxidative stress: Exploiting redox interindividual variability. Acta. Physiol. 2018, 222. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Rangasamy, T.; Thimmulappa, R.K.; Lee, H.; Osburn, W.O.; Brigelius-Flohe, R.; Kensler, T.W.; Yamamoto, M.; Biswal, S. Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs, is regulated by Nrf2. Am. J. Respir. Cell Mol. Biol. 2006, 35, 639–650. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef]
- Mattei, R.; Polotow, T.G.; Vardaris, C.V.; Guerra, B.A.; Leite, J.R.; Otton, R.; Barros, M.P. Astaxanthin limits fish oil-related oxidative insult in the anterior forebrain of Wistar rats: Putative anxiolytic effects? Pharmacol. Biochem. Be. 2011, 99, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Stralin, P.; Marklund, S.L. Vasoactive factors and growth factors alter vascular smooth muscle cell EC-SOD expression. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1621–H1629. [Google Scholar] [CrossRef]
- Fukai, T.; Siegfried, M.R.; Ushio-Fukai, M.; Cheng, Y.; Kojda, G.; Harrison, D.G. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J. Clin. Invest. 2000, 105, 1631–1639. [Google Scholar] [CrossRef]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.R.; Dias, I.H.; Willetts, R.S.; Devitt, A. Redox regulation of protein damage in plasma. Redox. Biol. 2014, 2, 430–435. [Google Scholar] [CrossRef]
- Chen, C.A.; De Pascali, F.; Basye, A.; Hemann, C.; Zweier, J.L. Redox modulation of endothelial nitric oxide synthase by glutaredoxin-1 through reversible oxidative post-translational modification. Biochemistry 2013, 52, 6712–6723. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.J.; Higgs, E.A. Nitric Oxide: Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Sessa, W.C.; Pritchard, K.; Seyedi, N.; Wang, J.; Hintze, T.H. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ. Res. 1994, 74, 349–353. [Google Scholar] [CrossRef] [PubMed]
- McConell, G.K.; Kingwell, B.A. Does nitric oxide regulate skeletal muscle glucose uptake during exercise? Exerc. Sport Sci. Rev. 2006, 34, 36–41. [Google Scholar] [CrossRef]
- Green, S.J.; Scheller, L.F.; Marletta, M.A.; Seguin, M.C.; Klotz, F.W.; Slayter, M.; Nelson, B.J.; Nacy, C.A. Nitric oxide: Cytokine-regulation of nitric oxide in host resistance to intracellular pathogens. Immunol. Lett. 1994, 43, 87–94. [Google Scholar] [CrossRef]
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I kappa B kinase-dependent NF-kappa B activation. Mol. Cells 2003, 16, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; Stals, E.; Panis, B.; Keulemans, J.; Swennen, R.L. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2005, 347, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Sylviana, N.; Gunawan, H.; Lesmana, R.; Purba, A.; Akbar, I.B. The Effect of Astaxanthin and Regular Training on Dynamic Pattern of Oxidative Stress on Male under Strenuous Exercise. IOP Conf. Ser.: Mater. Sci. Eng. 2017, 180. [Google Scholar] [CrossRef]
- Djordjevic, B.; Baralic, I.; Kotur-Stevuljevic, J.; Stefanovic, A.; Ivanisevic, J.; Radivojevic, N.; Andjelkovic, M.; Dikic, N. Effect of astaxanthin supplementation on muscle damage and oxidative stress markers in elite young soccer players. J. Sports Med. Phys. Fitness 2012, 52, 382–392. [Google Scholar] [PubMed]
- Bloomer, R.J. The role of nutritional supplements in the prevention and treatment of resistance exercise-induced skeletal muscle injury. Sports Med. 2007, 37, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Sakuma, K.; Kuchide, M.; Tokuda, H.; Maoka, T.; Toyokuni, S.; Oka, S.; Yasuhara, M.; Yoshikawa, T. Astaxanthin limits exercise-induced skeletal and cardiac muscle damage in mice. Antioxid. Redox. Signal. 2003, 5, 139–144. [Google Scholar] [CrossRef]
- Choi, H.D.; Youn, Y.K.; Shin, W.G. Positive Effects of Astaxanthin on Lipid Profiles and Oxidative Stress in Overweight Subjects. Plant. Food Hum. Nutr. 2011, 66, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Res, P.T.; Cermak, N.M.; Stinkens, R.; Tollakson, T.J.; Haenen, G.R.; Bast, A.; Van Loon, L.J.C. Astaxanthin Supplementation Does Not Augment Fat Use or Improve Endurance Performance. Med. Sci. Sport Exer. 2013, 45, 1158–1165. [Google Scholar] [CrossRef]
- Klinkenberg, L.J.; Res, P.T.; Haenen, G.R.; Bast, A.; van Loon, L.J.; van Dieijen-Visser, M.P.; Meex, S.J. Effect of antioxidant supplementation on exercise-induced cardiac troponin release in cyclists: A randomized trial. PLoS ONE 2013, 8, e79280. [Google Scholar] [CrossRef]
- Brown, D.R.; Gough, L.A.; Deb, S.K.; Sparks, S.A.; McNaughton, L.R. Astaxanthin in Exercise Metabolism, Performance and Recovery: A Review. Front. Nutr. 2017, 4, 76. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Baker, J.S.; Chen, X.; Wang, Y.; Chen, H.; Davison, G.W.; Yan, X. High-Dose Astaxanthin Supplementation Suppresses Antioxidant Enzyme Activity during Moderate-Intensity Swimming Training in Mice. Nutrients 2019, 11, 1244. https://doi.org/10.3390/nu11061244
Zhou Y, Baker JS, Chen X, Wang Y, Chen H, Davison GW, Yan X. High-Dose Astaxanthin Supplementation Suppresses Antioxidant Enzyme Activity during Moderate-Intensity Swimming Training in Mice. Nutrients. 2019; 11(6):1244. https://doi.org/10.3390/nu11061244
Chicago/Turabian StyleZhou, Yingsong, Julien S Baker, Xiaoping Chen, Yajun Wang, Haimin Chen, Gareth W Davison, and Xiaojun Yan. 2019. "High-Dose Astaxanthin Supplementation Suppresses Antioxidant Enzyme Activity during Moderate-Intensity Swimming Training in Mice" Nutrients 11, no. 6: 1244. https://doi.org/10.3390/nu11061244
APA StyleZhou, Y., Baker, J. S., Chen, X., Wang, Y., Chen, H., Davison, G. W., & Yan, X. (2019). High-Dose Astaxanthin Supplementation Suppresses Antioxidant Enzyme Activity during Moderate-Intensity Swimming Training in Mice. Nutrients, 11(6), 1244. https://doi.org/10.3390/nu11061244





