Blackcurrant (Ribes nigrum) Extract Exerts an Anti-Inflammatory Action by Modulating Macrophage Phenotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Diet
2.2. Cell Culture and Treatments
2.3. Cytokine Secretion Analysis
2.4. Reverse Transcription and Quantitative Real-Time PCR (qRT-PCR)
2.5. Statistical Analysis
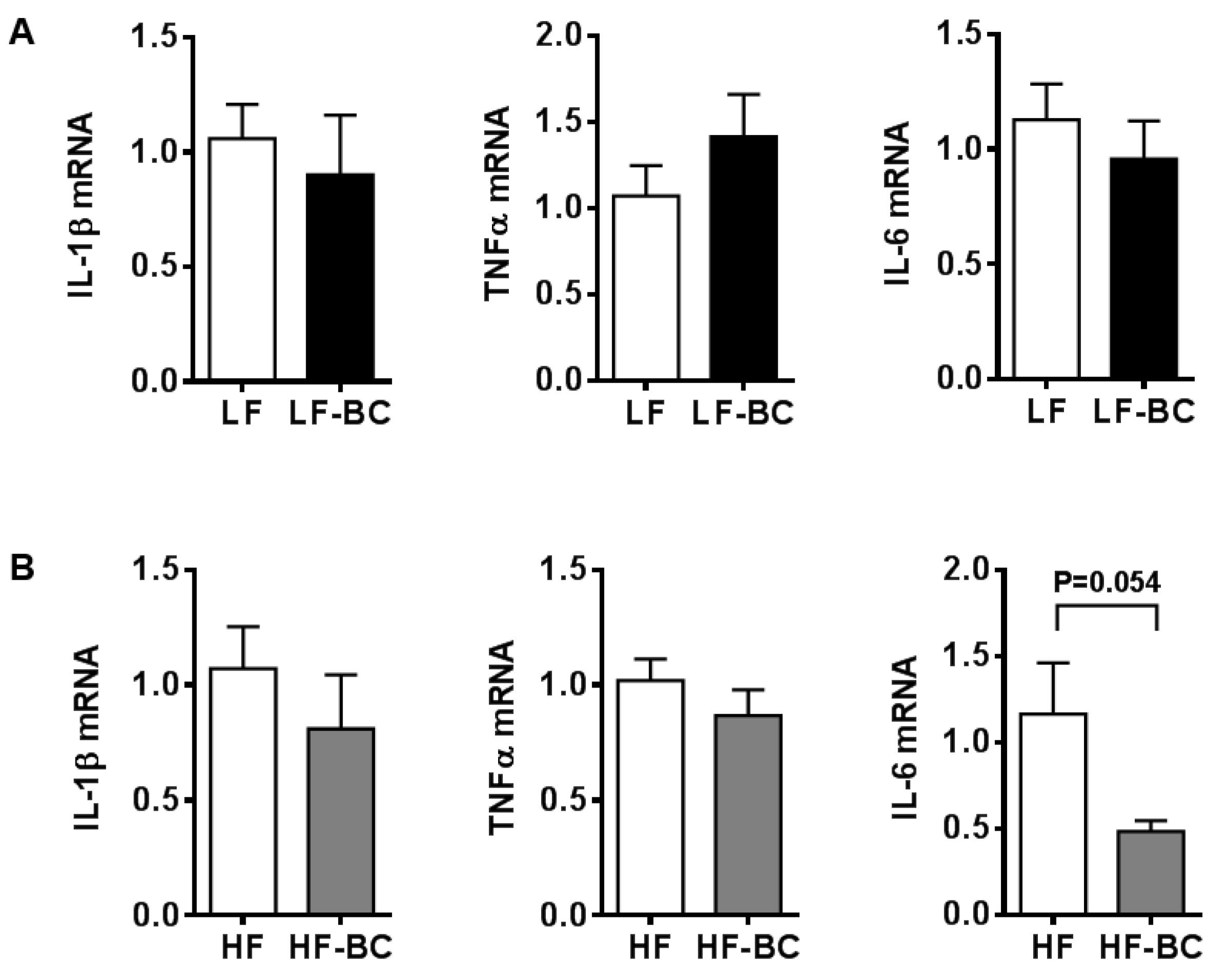
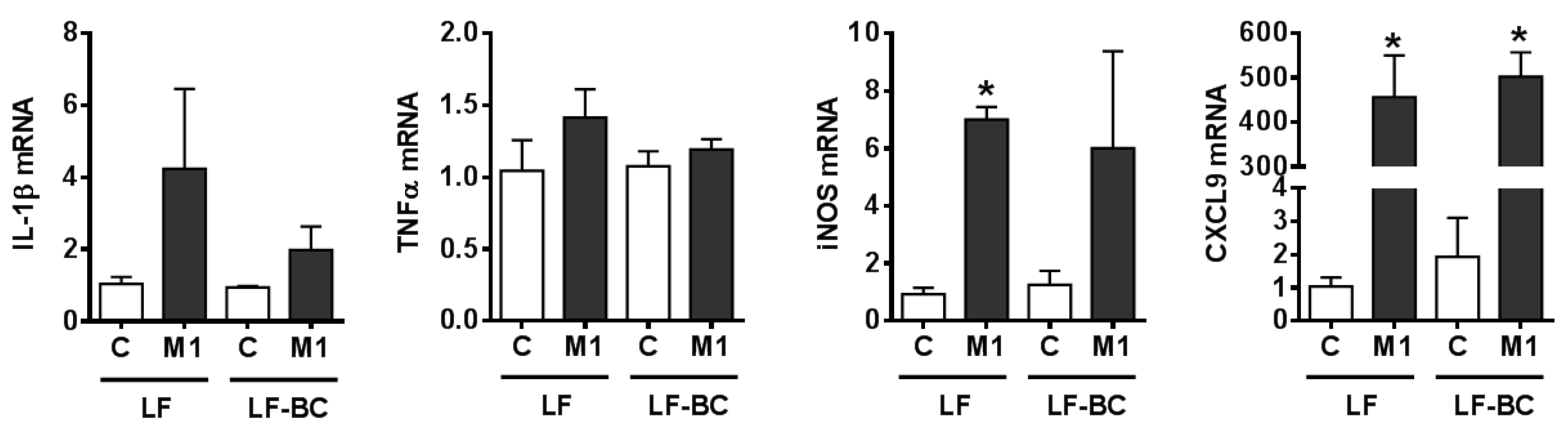
3. Results
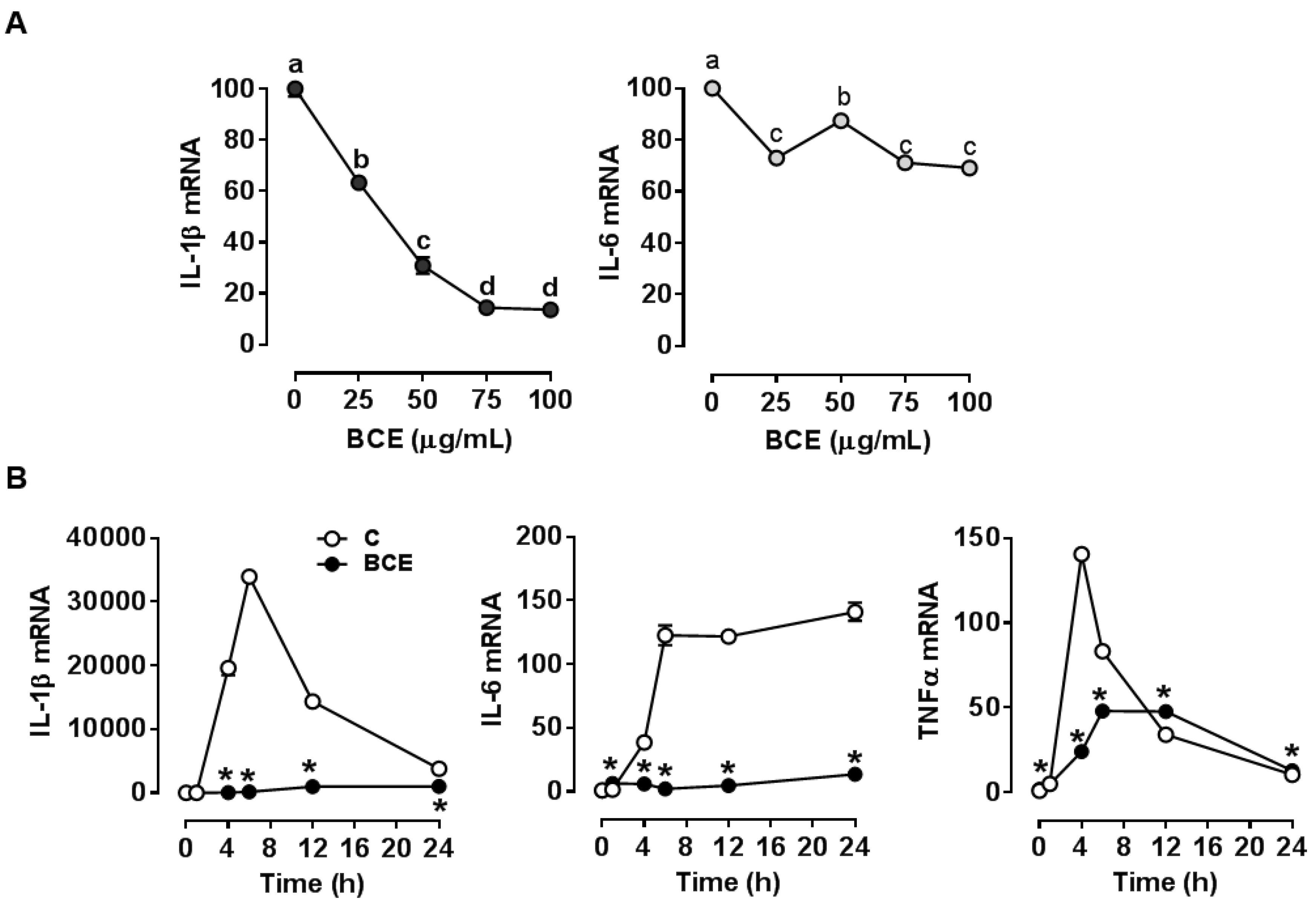
3.1. Repression of LPS Stimulated-Inflammatory Responses by BCE in RAW 264.7 Macrophages
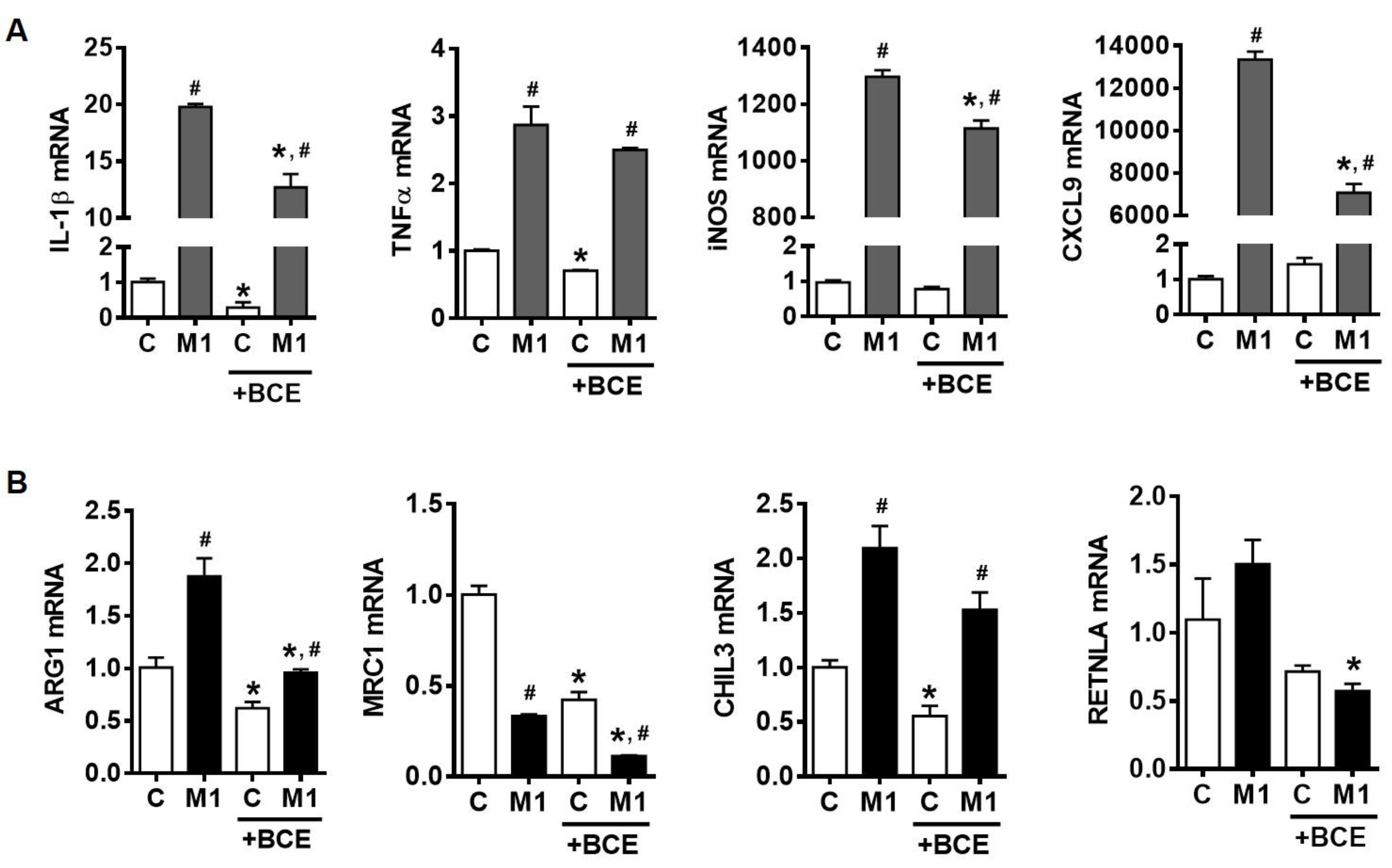
3.2. Suppression of Macrophage Polarization into M1 by BCE in BMDM
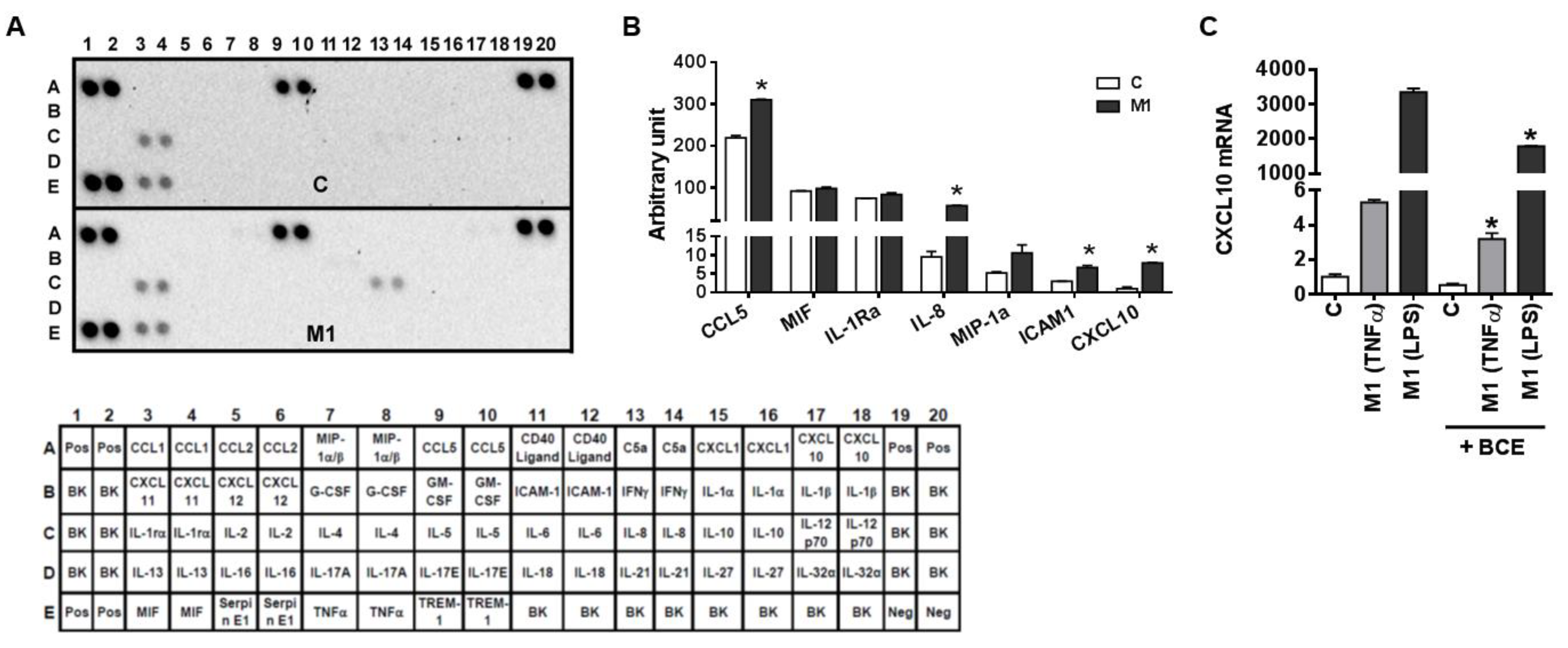
3.3. Characterization of M1 Polarization in Human THP-1 Macrophages and the Role of BCE in Macrophage M1 Polarization
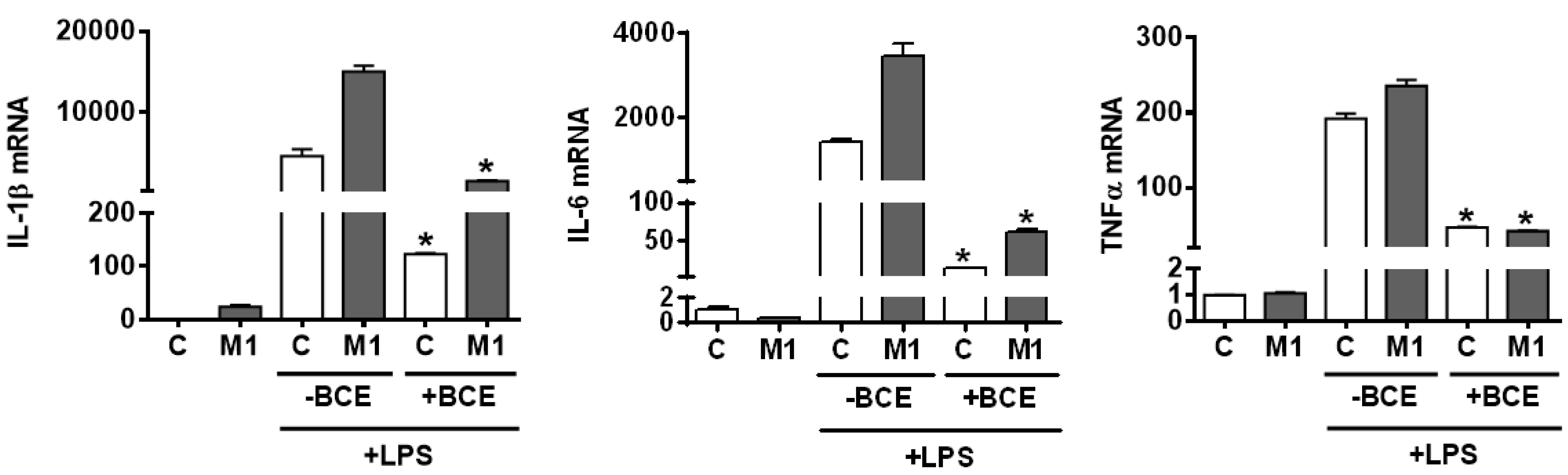
3.4. Attenuation of Inflammatory Responses to LPS Stimulation in both Resting and Polarized BMDM
3.5. Effect of Mouse Serum in BMDM and Its Polarization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Krenkel, O.; Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017, 17, 306. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Zimmermann, H.W. Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 2014, 60, 1090–1096. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015, 2015, 16. [Google Scholar] [CrossRef]
- Marra, F.; Lotersztajn, S. Pathophysiology of NASH: Perspectives for a targeted treatment. Curr. Pharm. Des. 2013, 19, 5250–5269. [Google Scholar] [CrossRef]
- Fujisaka, S.; Usui, I.; Bukhari, A.; Ikutani, M.; Oya, T.; Kanatani, Y.; Tsuneyama, K.; Nagai, Y.; Takatsu, K.; Urakaze, M.; et al. Regulatory Mechanisms for Adipose Tissue M1 and M2 Macrophages in Diet-Induced Obese Mice. Diabetes 2009, 58, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Wan, J.; Benkdane, M.; Teixeira-Clerc, F.; Bonnafous, S.; Louvet, A.; Lafdil, F.; Pecker, F.; Tran, A.; Gual, P.; Mallat, A.; et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: A protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 2014, 59, 130–142. [Google Scholar] [CrossRef]
- Lee, Y.; Pham, T.X.; Bae, M.; Hu, S.; O’Neill, E.; Chun, O.K.; Han, M.J.; Koo, S.I.; Park, Y.-K.; Lee, J.-Y. Blackcurrant (Ribes nigrum) Prevents Obesity-Induced Nonalcoholic Steatohepatitis in Mice. Obesity 2019, 27, 112–120. [Google Scholar] [CrossRef]
- Han, Y.-H.; Kim, H.-J.; Na, H.; Nam, M.-W.; Kim, J.-Y.; Kim, J.-S.; Koo, S.-H.; Lee, M.-O. RORα Induces KLF4-Mediated M2 Polarization in the Liver Macrophages that Protect against Nonalcoholic Steatohepatitis. Cell Rep. 2017, 20, 124–135. [Google Scholar] [CrossRef]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, B.; Yang, Y.; Pham, T.X.; Park, Y.-K.; Manatou, J.; Koo, S.I.; Chun, O.K.; Lee, J.-Y. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J. Nutr. Biochem. 2014, 25, 404–411. [Google Scholar] [CrossRef]
- Slimestad, R.; Solheim, H. Anthocyanins from black currants (Ribes nigrum L.). J. Agric. Food Chem. 2002, 50, 3228–3231. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, S.G.; Park, Y.-K.; Ku, C.S.; Pham, T.X.; Wegner, C.J.; Yang, Y.; Koo, S.I.; Chun, O.K.; Lee, J.-Y. Blueberry, blackberry, and blackcurrant differentially affect plasma lipids and pro-inflammatory markers in diet-induced obesity mice. Nutr. Res. Pract. 2016, 10, 494–500. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, B.; Soung, D.Y.; Vance, T.; Lee, J.S.; Lee, J.-y.; Koo, S.I.; Kim, D.-O.; Drissi, H.; Chun, O.K. Relationship between oxidative stress and bone mass in obesity and effects of berry supplementation on bone remodeling in obese male mice: An exploratory study. J. Med. Food 2015, 18, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.; Kim, B.; Park, Y.-K.; Yang, Y.; Pham, T.X.; Ku, C.S.; Farruggia, C.; Harness, E.; Smyth, J.A.; Lee, J.-Y. Polyphenol-rich blackcurrant extract exerts hypocholesterolaemic and hypoglycaemic effects in mice fed a diet containing high fat and cholesterol. Br. J. Nutr. 2015, 113, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.; Kim, B.; Park, Y.-K.; Wegner, C.J.; Harness, E.; Nam, T.-G.; Kim, D.-O.; Lee, J.S.; Lee, J.-Y. Polyphenol-rich blackcurrant extract prevents inflammation in diet-induced obese mice. J. Nutr. Biochem. 2014, 25, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.B.; Stefanovic-Racic, M.; Dedousis, N.; Sipula, I.; O’Doherty, R.M. Similar degrees of obesity induced by diet or aging cause strikingly different immunologic and metabolic outcomes. Physiol. Rep. 2016, 4, e12708. [Google Scholar] [CrossRef]
- Pham, T.X.; Bae, M.; Lee, Y.; Park, Y.-K.; Lee, J.-Y. Transcriptional and posttranscriptional repression of histone deacetylases by docosahexaenoic acid in macrophages. J. Nutr. Biochem. 2018, 57, 162–169. [Google Scholar] [CrossRef]
- Pham, T.X.; Park, Y.K.; Bae, M.; Lee, J.Y. The Potential Role of an Endotoxin Tolerance-Like Mechanism for the Anti-Inflammatory Effect of Spirulina platensis Organic Extract in Macrophages. J. Med. Food 2017, 20, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kitade, H.; Sawamoto, K.; Nagashimada, M.; Inoue, H.; Yamamoto, Y.; Sai, Y.; Takamura, T.; Yamamoto, H.; Miyamoto, K.-i.; Ginsberg, H.N.; et al. CCR5 Plays a Critical Role in Obesity-Induced Adipose Tissue Inflammation and Insulin Resistance by Regulating Both Macrophage Recruitment and M1/M2 Status. Diabetes 2012, 61, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, M.; Schoeden, G. Macrophage biology and immunology: Man is not a mouse. J. Leukoc. Biol. 2007, 81, 579. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Schoedon, G.; Schneemann, M.; Hofer, S.; Guerrero, L.; Blau, N.; Schaffner, A. Regulation of the L-arginine-dependent and tetrahydrobiopterin-dependent biosynthesis of nitric oxide in murine macrophages. Eur. J. Biochem. 1993, 213, 833–839. [Google Scholar] [CrossRef]
- Hribar, U.; Ulrih, N.P. The metabolism of anthocyanins. Curr. Drug Metab. 2014, 15, 3–13. [Google Scholar] [CrossRef]
- McGuire, T.R.; Brusnahan, S.K.; Bilek, L.D.; Jackson, J.D.; Kessinger, M.A.; Berger, A.M.; Garvin, K.L.; O’Kane, B.J.; Tuljapurkar, S.R.; Sharp, J.G. Inflammation Associated With Obesity: Relationship With Blood and Bone Marrow Endothelial Cells. Obesity 2011, 19, 2130–2136. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Mercader, J.M.; Catalan, V.; Moreno-Navarrete, J.M.; Pueyo, N.; Sabater, M.; Gomez-Ambrosi, J.; Anglada, R.; Fernandez-Formoso, J.A.; Ricart, W.; et al. Targeting the circulating microRNA signature of obesity. Clin. Chem. 2013, 59, 781–792. [Google Scholar] [CrossRef]
- Hijmans, J.G.; Diehl, K.J.; Bammert, T.D.; Kavlich, P.J.; Lincenberg, G.M.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Influence of Overweight and Obesity on Circulating Inflammation-Related microRNA. Microrna 2018, 7, 148–154. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, M.; Tong, M.; Yang, L.; Pang, L.; Chen, L.; Xu, G.; Chi, X.; Hong, Q.; Ni, Y.; et al. miR-148a is Associated with Obesity and Modulates Adipocyte Differentiation of Mesenchymal Stem Cells through Wnt Signaling. Sci. Rep. 2015, 5, 9930. [Google Scholar] [CrossRef]
- Huang, F.; Zhao, J.-L.; Wang, L.; Gao, C.-C.; Liang, S.-Q.; An, D.-J.; Bai, J.; Chen, Y.; Han, H.; Qin, H.-Y. miR-148a-3p Mediates Notch Signaling to Promote the Differentiation and M1 Activation of Macrophages. Front. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392. [Google Scholar] [CrossRef] [PubMed]
- Grainger, J.R.; Konkel, J.E.; Zangerle-Murray, T.; Shaw, T.N. Macrophages in gastrointestinal homeostasis and inflammation. Pflugers Arch. 2017, 469, 527–539. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Lee, J.-Y. Blackcurrant (Ribes nigrum) Extract Exerts an Anti-Inflammatory Action by Modulating Macrophage Phenotypes. Nutrients 2019, 11, 975. https://doi.org/10.3390/nu11050975
Lee Y, Lee J-Y. Blackcurrant (Ribes nigrum) Extract Exerts an Anti-Inflammatory Action by Modulating Macrophage Phenotypes. Nutrients. 2019; 11(5):975. https://doi.org/10.3390/nu11050975
Chicago/Turabian StyleLee, Yoojin, and Ji-Young Lee. 2019. "Blackcurrant (Ribes nigrum) Extract Exerts an Anti-Inflammatory Action by Modulating Macrophage Phenotypes" Nutrients 11, no. 5: 975. https://doi.org/10.3390/nu11050975
APA StyleLee, Y., & Lee, J.-Y. (2019). Blackcurrant (Ribes nigrum) Extract Exerts an Anti-Inflammatory Action by Modulating Macrophage Phenotypes. Nutrients, 11(5), 975. https://doi.org/10.3390/nu11050975



