Dietary Diversity and Nutritional Adequacy among an Older Spanish Population with Metabolic Syndrome in the PREDIMED-Plus Study: A Cross-Sectional Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of the Study
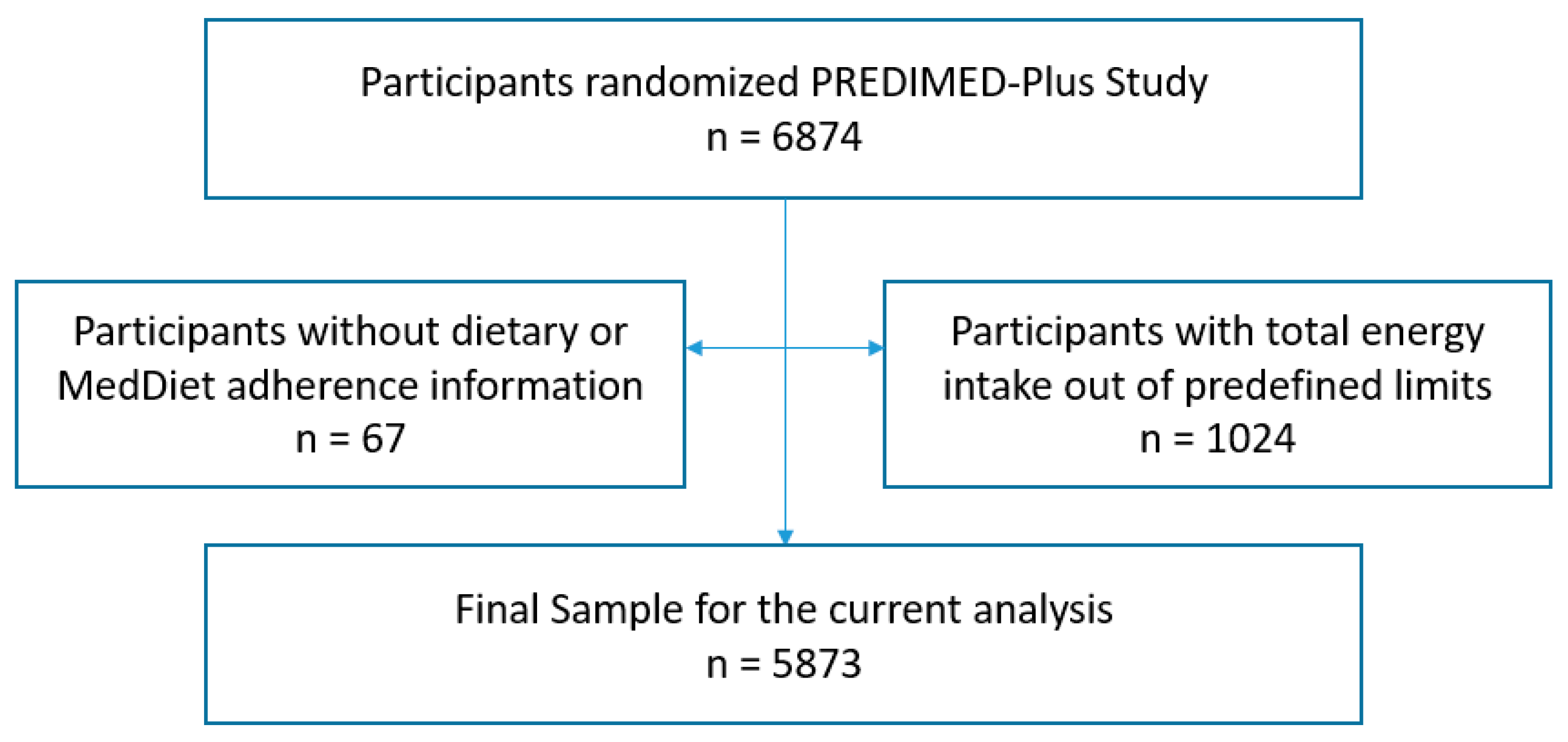
2.2. Study Population
2.3. Dietary Intake Assessment
2.4. Dietary Diversity Score Construction
2.5. Nutrient Adequate Intake
2.6. Assessment of Non-Dietary Variables
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of PREDIMED-Plus Participants by Quartiles of DDS
3.2. Associations Between Demographic and Lifestyle Variables with DDS
3.3. Adherence to MedDiet and Dietary Intake of PREDIMED-Plus Participants by Quartiles of DDS Adjusted by Energy
3.4. Distribution of Participants ny Number of Nutrients below Adequate Intake According to the DDS by Age and Sex
3.5. Multivariable Logistic Regression Model for Inadequate Intake of Four or More out Eight Micronutrients According to Food Group’s Diversity Intake and Total DDS Quartiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeBoer, M.D.; Gurka, M.J.; Golden, S.H.; Musani, S.K.; Sims, M.; Vishnu, A.; Guo, Y.; Pearson, T.A. Independent associations between metabolic syndrome severity and future coronary heart disease by sex and race. J. Am. Cardiol. 2017, 69, 1204–1205. [Google Scholar] [CrossRef]
- Micucci, C.; Valli, D.; Matacchione, G.; Catalano, A. Current perspectives between metabolic syndrome and cancer. Oncotarget 2016, 7, 38959–38972. [Google Scholar] [CrossRef]
- Zhang, P.; Tian, B. Metabolic syndrome: An important risk factor for parkinson’s disease. Oxid. Med. Cell. Longev. 2014, 2014, 729194. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guallar-Castillon, P.; Pérez, R.F.; García, E.L.; León-Muñoz, L.M.; Aguilera, M.T.; Graciani, A.; Gutiérrez-Fisac, J.L.; Banegas, J.R.; Rodríguez-Artalejo, F. Magnitude and management of metabolic syndrome in Spain in 2008–2010: The ENRICA Study. Rev. Española Cardiol. (Engl. Ed.) 2014, 67, 367–373. [Google Scholar] [CrossRef]
- Boudreau, D.; Malone, D.; Raebel, M.; Fishman, P.; Nichols, G.; Feldstein, A.; Boscoe, A.; Ben-Joseph, R.; Magid, D.; Okamoto, L. Health care utilization and costs by metabolic syndrome risk factors. Metab. Syndr. Relat. Disord. 2009, 7, 305–314. [Google Scholar] [CrossRef] [PubMed]
- De La Iglesia, R.; Loria-Kohen, V.; Zulet, M.A.; Martínez, J.A.; Reglero, G.; De Molina, A.R. Dietary strategies implicated in the prevention and treatment of metabolic syndrome. Int. J. Mol. Sci. 2016, 17, 1877. [Google Scholar] [CrossRef] [PubMed]
- Calton, E.K.; James, A.P.; Pannu, P.K.; Soares, M.J. Certain dietary patterns are beneficial for the metabolic syndrome: Reviewing the evidence. Nutr. Res. 2014, 34, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Guasch-Ferre, M.; Lee, C.-H.; Estruch, R.; Clish, C.B.; Ros, E. Protective effects of the mediterranean diet on type 2 diabetes and metabolic syndrome123. J. Nutr. 2016, 146, 920S–927S. [Google Scholar] [CrossRef] [PubMed]
- Castro-Quezada, I.; Román-Viñas, B.; Serra-Majem, L. The mediterranean diet and nutritional adequacy: A review. Nutrients 2014, 6, 231–248. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Martín-Calvo, N. The major European dietary patterns and metabolic syndrome. Rev. Endocr. Metab. Disord. 2013, 14, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Aranceta Bartrina, J.; Arija Val, V.V.; Maíz Aldalur, E.; Martínez de Victoria Muñoz, E.; Ortega Anta, R.M.; Pérez-Rodrigo, C.; Quiles Izquierdo, J.; Rodríguez Martín, A.; Román Viñas, B.; Salvador Castell, G.; et al. Dietary guidelines for the Spanish population (SENC; diciembre 2016); the new graphic icon of healthy food. Nutr. Hosp. 2016, 33, 1–48. [Google Scholar] [CrossRef]
- Jayawardena, R.; Byrne, N.M.; Soares, M.J.; Katulanda, P.; Yadav, B.; Hills, A.P. High dietary diversity is associated with obesity in Sri Lankan adults: An evaluation of three dietary scores. BMC Public Health 2013, 13, 314. [Google Scholar] [CrossRef]
- Kennedy, E. Dietary diversity, diet quality, and body weight regulation. Nutr. Rev. 2004, 62, 78–81. [Google Scholar] [CrossRef]
- Tian, X.; Xu, X.; Zhang, K.; Wang, H. Gender difference of metabolic syndrome and its association with dietary diversity at different ages. Oncotarget 2017, 8, 73568–73578. [Google Scholar] [CrossRef]
- Karimbeiki, R.; Pourmasoumi, M.; Feizi, A.; Abbasi, B.; Hadi, A.; Rafie, N.; Safavi, S. Higher dietary diversity score is associated with obesity: A case–control study. Public Health 2018, 157, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Wilkens, L.R.; Basiotis, P.P.; Foote, J.A.; Carlson, A. Dietary variety increases the probability of nutrient adequacy among adults. J. Nutr. 2004, 134, 1779–1785. [Google Scholar]
- Azadbakht, L.; Mirmiran, P.; Esmaillzadeh, A.; Azizi, F. Dietary diversity score and cardiovascular risk factors in Tehranian adults. Public Health Nutr. 2006, 9, 728–736. [Google Scholar] [CrossRef]
- Gholizadeh, F.; Moludi, J.; Yagin, N.L.; Alizadeh, M.; Nachvak, S.M.; Abdollahzad, H.; Mirzaei, K.; Mostafazadeh, M. The relation of Dietary diversity score and food insecurity to metabolic syndrome features and glucose level among pre-diabetes subjects. Prim. Care Diabetes 2018, 12, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Barba, L.R.; Cavelaars, A.; Veer, P.V.; Viñas, B.R.; Ngo, J.; Gurinovic, M.; Novakovic, R.; De Groot, L.C.; Matthys, C.; Majem, L.S. Projected Prevalence of Inadequate Nutrient Intakes in Europe. Ann. Nutr. Metab. 2011, 59, 84–95. [Google Scholar]
- Dean, M.; Raats, M.M.; Grunert, K.G.; Lumbers, M. The Food in Later Life Team Factors influencing eating a varied diet in old age. Public Health Nutr. 2009, 12, 2421. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Bes-Rastrollo, M.; Román-Viñas, B.; Pfrimer, K.; Sánchez-Villegas, A.; Martinez-Gonzalez, M.A. Dietary patterns and nutritional adequacy in a Mediterranean country. Br. J. Nutr. 2009, 101, S21–S28. [Google Scholar] [CrossRef]
- Buil-Cosiales, P.; Vioque, J.; Romaguera, D.; Martínez, J.A.; López-Miranda, J.; Bueno-Cavanillas, A.; A Tur, J.; Serra-Majem, L.; Martín, V.; Lapetra, J.; et al. Cohort Profile: Design and methods of the PREDIMED-Plus randomized trial. Int. J. Epidemiol. 2018, 48, 387–388. [Google Scholar]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart; lung; and blood institute; American heart association; world heart federation; International atherosclerosis society; and international association for the study of obesity. Circulation 2009, 16, 1640–1645. [Google Scholar] [CrossRef]
- Willet, W. Nutritional Epidemiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Martin-Moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernandez-Rodriguez, J.C.; Salvini, S.; Willett, W.C. Development and validation of a food frequency questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef]
- Moreiras, O.C.A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de alimentos. (Spanish Food Composition Tables), 7th ed.; Pirámide: Madrid, Spain, 2003. [Google Scholar]
- Mataix-Verdú, J.; García diz, L.; Mañas Almendros, M.; Emilio, M.d.V.; Llopis González, J. Tablas de Composición de Alimentos, 4th ed.; Universidad de Granada: Granada, Spain, 2009. [Google Scholar]
- Kant, A.K.; Schatzkin, A.; Harris, T.B.; Ziegler, R.G.; Block, G. Dietary diversity and subsequent mortality in the First National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am. J. Clin. Nutr. 1993, 57, 434–440. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Jahangiry, L. Dietary diversity score is associated with cardiovascular risk factors and serum adiponectin concentrations in patients with metabolic syndrome. BMC Cardiovasc. Disord. 2018, 18, 68. [Google Scholar] [CrossRef]
- Schroder, H.; Fíto, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- IOM Nutrient Recommendations: Dietary Reference Intakes. Available online: https://ods.od.nih.gov/Health_Information/Dietary_Reference_Intakes.aspx (accessed on 4 May 2018).
- Aranceta, J.; Serra-Majem, L.; Pérez-Rodrigo, C.; Llopis, J.; Mataix, J.; Ribas, L.; Tojo, R.; A Tur, J. Vitamins in Spanish food patterns: The eVe Study. Public Health Nutr. 2001, 4, 1317–1323. [Google Scholar] [CrossRef]
- EFSA Dietary Reference Values and Dietary Guidelines. Available online: https://www.efsa.europa.eu/en/topics/topic/dietary-reference-values (accessed on 1 October 2018).
- Elosua, R.; García, M.; Aguilar, A.; Molina, L.; Covas, M.-I.; Marrugat, J. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish Women. Med. Sci. Sports Exerc. 2000, 32, 1431–1437. [Google Scholar] [CrossRef]
- Elosua, R.; Marrugat, J.; Molina, L.; Pons, S.; Pujol, E. Validation of the Minnesota leisure time physical activity questionnaire in Spanish men. Am. J. Epidemiol. 1994, 139, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, S.; Letenneur, L.; Berr, C.; Dartigues, J.F.; Ritchie, K.; Alperovitch, A.; Tavernier, B.; Barberger-Gateau, P. Sociodemographic differences in dietary habits in a population-based sample of elderly subjects: The 3C study. J. Nutr. Health Aging 2004, 8, 497–502. [Google Scholar]
- Conklin, A.I.; Forouhi, N.G.; Surtees, P.; Wareham, N.J.; Monsivais, P. Gender and the double burden of economic and social disadvantages on healthy eating: Cross-sectional study of older adults in the EPIC-Norfolk cohort. BMC Public Health 2015, 15, 692. [Google Scholar] [CrossRef]
- Westenhoefer, J. Age and gender dependent profile of food choice. Nutr. Oppor. Asia 2005, 57, 44–51. [Google Scholar]
- Hanna, K.L.; Collins, P.F. Relationship between living alone and food and nutrient intake. Nutr. Rev. 2015, 73, 594–611. [Google Scholar] [CrossRef] [PubMed]
- Vinther, J.L.; Conklin, A.I.; Wareham, N.J.; Monsivais, P. Marital transitions and associated changes in fruit and vegetable intake: Findings from the population-based prospective EPIC-Norfolk cohort; UK. Soc. Sci. Med. 2016, 157, 120–126. [Google Scholar] [CrossRef]
- Ochieng, J.; Afari-Sefa, V.; Lukumay, P.J.; Dubois, T. Determinants of dietary diversity and the potential role of men in improving household nutrition in Tanzania. PLoS ONE 2017, 12, e0189022. [Google Scholar] [CrossRef]
- Fukuda, Y.; Ishikawa, M.; Yokoyama, T.; Hayashi, T.; Nakaya, T.; Takemi, Y.; Kusama, K.; Yoshiike, N.; Nozue, M.; Yoshiba, K.; et al. Physical and social determinants of dietary variety among older adults living alone in Japan. Geriatr. Gerontol. Int. 2017, 17, 2232–2238. [Google Scholar] [CrossRef]
- Hampl, J.S.; Ma, J.; Betts, N.M. Clustering of Lifestyle Behaviors: The Relationship between Cigarette Smoking, Alcohol Consumption, and Dietary Intake. Am. J. Health Promot. 2000, 15, 107–117. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, T.I.; Previdelli, Á.N.; Ferreira, M.P.N.; Marques, K.M.; Goulart, R.M.M.; de Aquino, R.C. Factors associated with diet quality of older adults. Rev. Nutr. 2017, 30, 297–306. [Google Scholar] [CrossRef]
- Kobayashi, M.; Iwasaki, M.; Tsugane, S. Relationship between the variety of food consumed and sufficient nutrition in middle—Aged Japanese. Nutr. Food Sci. 2013, 43, 344–355. [Google Scholar] [CrossRef]
- Vadiveloo, M.K.; Parekh, N. Dietary variety: An overlooked strategy for obesity and chronic disease control. Am. J. Prev. Med. 2015, 49, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Adebukola, C.O.; Ugwunna, U.M.; Ekerette, N.N. Dietary diversity and nutrient intake adequacy among women in Iwo Local Government Area; Osun State Nigeria. Afr. J. Food Agr. Nutr. Dev. 2017, 17, 12641–12656. [Google Scholar] [CrossRef]
- Wirt, A.; E Collins, C. Diet quality—What is it and does it matter? Public Health Nutr. 2009, 12, 2473. [Google Scholar] [CrossRef]
- De Oliveira Otto, M.C.; Anderson, C.A.M.; Dearborn, J.L.; Ferranti, E.P.; Mozaffarian, D.; Rao, G.; Wylie-Rosett, J.; Lichtenstein, A.H.; On behalf of the American Heart Association Behavioral Change for Improving Health Factors Committee of the Council on Lifestyle and Cardiometabolic Health and Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; et al. Dietary diversity: Implications for obesity prevention in adult populations: A science advisory from the American Heart Association. Circulation 2018, 138, e160–e168. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Dorosty-Motlagh, A.R.; Hoshiar-Rad, A.; Eshraghian, M.R.; Sotoudeh, G.; Azadbakht, L.; Karimi, M.; Jalali-Farahani, S. Is dietary diversity a proxy measurement of nutrient adequacy in Iranian elderly women? Appetite 2016, 105, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Marshall, T.A.; Stumbo, P.J.; Warren, J.J.; Xie, X.J. Inadequate nutrient intakes are common and are associated with low diet variety in rural; community-dwelling elderly. J. Nutr. 2001, 131, 2192–2196. [Google Scholar] [CrossRef] [PubMed]
- Masset, G.; Scarborough, P.; Rayner, M.; Mishra, G.; Brunner, E.J. Can nutrient profiling help to identify foods which diet variety should be encouraged? Results from the Whitehall II cohort. Br. J. Nutr. 2015, 113, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wang, Y.; Li, T.; Loo-Bouwman, C.A.V.; Zhang, Y.; Man-Yau Szeto, I. Dietary diversity and food variety in chinese children aged 3–17 years: Are they negatively associated with dietary micronutrient inadequacy? Nutrients 2018, 10, 1674. [Google Scholar] [CrossRef]
- De Gavelle, E.; Huneau, J.F.; Mariotti, F. Patterns of protein food intake are associated with nutrient adequacy in the general french adult population. Nutrients 2018, 10, 226. [Google Scholar] [CrossRef]
- Azadbakht, L.; Mirmiran, P.; Azizi, F. Variety scores of food groups contribute to the specific nutrient adequacy in Tehranian men. Eur. J. Clin. Nutr. 2005, 59, 1233–1240. [Google Scholar] [CrossRef]
- Ortiz-Andrellucchi, A.; Sánchez-Villegas, A.; Doreste-Alonso, J.; De Vries, J.; De Groot, L.; Serra-Majem, L. Dietary assessment methods for micronutrient intake in elderly people: A systematic review. Br. J. Nutr. 2009, 102, S118. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K. Consumption of energy-dense, nutrient-poor foods by adult Americans: Nutritional and health implications. The third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Clin. Nutr. 2000, 72, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Ávila, J.M.; Valero, T.; Del Pozo, S.; Rodriguez, P.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; et al. Macronutrient Distribution and Dietary Sources in the Spanish Population: Findings from the ANIBES Study. Nutrients 2016, 8, 177. [Google Scholar] [CrossRef] [PubMed]
| Q1 (n = 1647) | Q2 (n = 1647) | Q3 (n = 1647) | Q4 (n = 1646) | p Value | |
|---|---|---|---|---|---|
| Age (Year), n (%) | |||||
| 55–70 years | 1442 (87.6) | 1416 (86.0) | 1403 (85.2) | 1356 (82.4) | <0.001 |
| >70 years | 205 (12.5) | 231 (14.0) | 244 (14.8) | 290 (17.6) | |
| Mean ± SD | 64.1 ± 5.1 | 64.8 ± 4.9 | 65.3 ± 4.8 | 65.8 ± 4.7 | <0.001 |
| Sex, n (%) | |||||
| Male | 1116 (67.8) | 916 (55.6) | 770 (46.8) | 600 (36.5) | <0.001 |
| Female | 531 (32.2) | 731 (44.4) | 877 (53.3) | 1046 (63.6) | |
| Smoking Habits, n (%) | |||||
| Current Smoker | 297 (18.0) | 195 (11.8) | 171 (10.4) | 152 (9.2) | <0.001 |
| Former Smoker | 793 (48.2) | 747 (45.4) | 722 (43.8) | 593 (36.0) | |
| Never Smoker | 548 (33.3) | 699 (42.4) | 749 (45.5) | 893 (54.3) | |
| Without information | 9 (0.6) | 6 (0.4) | 5 (0.3) | 8 (0.5) | |
| Physical Activity, n (%) | |||||
| Less active | 1014 (61.7) | 985 (59.9) | 983 (59.9) | 940 (57.4) | 0.29 |
| Moderately active | 294 (17.9) | 304 (18.5) | 319 (19.4) | 326 (19.9) | |
| Active | 335 (20.4) | 355 (21.6) | 340 (20.7) | 373 (22.8) | |
| Educational Level, n (%) | |||||
| Tertiary level | 421 (25.6) | 360 (21.9) | 340 (20.6) | 320 (19.5) | <0.001 |
| Secondary level | 534 (32.4) | 480 (29.2) | 449 (27.3) | 435 (26.4) | |
| Primary level | 679 (41.3) | 796 (48.4) | 842 (51.1) | 873 (53.1) | |
| Without information | 13 (0.7) | 11 (0.6) | 16 (1.0) | 18 (1.0) | |
| Civil Status, n (%) | |||||
| Married | 1258 (76.7) | 1254 (76.4) | 1243 (75.7) | 1278 (77.8) | 0.030 |
| Widowed | 151 (9.2) | 162 (9.9) | 182 (11.1) | 186 (11.3) | |
| Divorced/Separated | 145 (8.8) | 123 (7.5) | 130 (7.9) | 117 (7.1) | |
| Others a | 93 (5.2) | 108 (6.2) | 92 (5.3) | 65 (3.8) | |
| Living alone, n (%) | 193 (11.8) | 189 (11.5) | 220 (13.4) | 212 (12.9) | 0.29 |
| BMI (kg/m2), Mean ± SD | 32.6 ± 3.4 | 32.5 ± 3.4 | 32.5 ± 3.5 | 32.5 ± 3.5 | 0.82 |
| WC (cm), Mean ± SD | 109.3 ± 9.5 | 108.1 ± 9.5 | 107.0 ± 9.7 | 106.3 ± 9.6 | <0.001 |
| Total DDS | ||
|---|---|---|
| Mean Differences (95% CI) | p Value | |
| Sex | ||
| Men | 0 (ref) | |
| Women | 0.26 (0.18, 0.33) | <0.001 |
| Age | ||
| ≤70 years | 0 (ref) | |
| More 70 years | 0.06 (−0.15, 0.14) | 0.12 |
| Smoking Habits | ||
| Current smoker | 0 (ref) | |
| Former smoker | 0.14 (0.06, 0.23) | 0.001 |
| Never smoker | 0.18 (0.09, 0.27) | <0.001 |
| Physical Activity Status | ||
| Less active | 0 (ref) | |
| Moderate active | 0.06 (−0.01, 0.13) | 0.11 |
| Active | 0.03 (−0.04, 0.10) | 0.34 |
| Educational Status | ||
| Tertiary Level | 0 (ref) | |
| Secondary Level | 0.02 (−0.05, 0.09) | 0.59 |
| Primary Le | 0.07 (0.03, 0.14) | 0.041 |
| MedDiet Adherence | ||
| Low Adherence | 0 (ref) | |
| Medium Adherence | 0.34 (0.28, 0.40) | <0.001 |
| High Adherence | 0.65 (0.58, 0.73) | <0.001 |
| Civil Status | ||
| Married | 0 (ref) | |
| Widowed | −0.15 (−0.26, −0.05) | 0.004 |
| Divorced/Separated | −0.12 (−0.23, −0.02) | 0.026 |
| Others a | −0.21 (−0.34, −0.08) | 0.002 |
| Living alone | −0.31 (−0.14, 0.07) | 0.57 |
| WC (cm) b | −0.01 (−0.01, 0.01) | 0.57 |
| BMI (kg/m2) b | −0.01 (−0.01, 0.01) | 0.75 |
| Alcohol intake (g) b | −0.01 (−0.02, −0.01) | <0.001 |
| Q1 (n = 1647) | Q2 (n = 1647) | Q3 (n = 1647) | Q4 (n = 1646) | p Value | |
|---|---|---|---|---|---|
| MedDiet Adherence, n (%) | |||||
| Low adherence | 857 (52.0) | 638 (38.7) | 507 (30.8) | 372 (22.6) | <0.001 |
| Medium adherence | 602 (36.7) | 686 (41.7) | 717 (43.5) | 684 (41.6) | |
| High adherence | 188 (11.4) | 323 (19.6) | 423 (25.7) | 590 (35.8) | |
| Mean ± SD | 7.4 ± 2.5 | 8.3 ± 2.6 | 8.8 ± 2.5 | 9.5 ± 2.6 | <0.001 |
| Nutrient Intake, Mean ± SD | |||||
| Total energy (Kcal/day) | 2382.3 ± 612.8 | 2345.0 ± 557.9 | 2340.7 ± 527.0 | 2397.1 ± 502.6 | 0.006 |
| Total fat intake (%) | 39.9 ± 7.1 | 39.9 ± 6.6 | 39.5 ± 6.3 | 38.9 ± 5.9 | <0.001 |
| Monounsaturated fat (%) | 20.7 ± 4.9 | 20.8 ± 4.7 | 20.5 ± 4.6 | 20.1 ± 4.3 | 0.002 |
| Polyunsaturated fat (%) | 6.2 ± 1.9 | 6.3 ± 1.9 | 6.4 ± 1.8 | 6.6 ± 1.8 | <0.001 |
| Saturated fat (%) | 10.1 ± 2.2 | 10.1 ± 2.1 | 9.9 ± 1.9 | 9.7 ± 1.8 | <0.001 |
| Carbohydrate intake (%) | 40.1 ± 7.5 | 40.2 ± 7.0 | 40.7 ± 6.5 | 41.3 ± 6.1 | <0.001 |
| Protein intake (%) | 15.4 ± 2.6 | 16.6 ± 2.7 | 17.2 ± 2.8 | 17.9 ± 2.6 | <0.001 |
| Fiber intake (g/day) | 21.4 ± 7.8 | 24.8 ± 7.7 | 27.1 ± 7.8 | 31.3 ± 8.8 | <0.001 |
| Vitamin A (µg/day) | 909.3 ± 624.6 | 1075.1 ± 648.9 | 1133.2 ± 587.7 | 1302.9 ± 650.3 | <0.001 |
| Vitamin B1 (mg/day) | 1.5 ± 0.4 | 1.6 ± 0.4 | 1.7 ± 0.4 | 1.8 ± 0.4 | <0.001 |
| Vitamin B6 (mg/day) | 2.0 ± 0.5 | 2.2 ± 0.5 | 2.4 ± 0.5 | 2.7 ± 0.6 | <0.001 |
| Vitamin B9 (µg/day) | 290.7 ± 82.3 | 335.6 ± 88.9 | 363.6 ± 90.9 | 416.1 ± 102.0 | <0.001 |
| Vitamin B12 (µg/day) | 9.0 ± 4.5 | 9.7 ± 4.5 | 10.1 ± 4.3 | 10.9 ± 4.5 | <0.001 |
| Vitamin C (mg/day) | 147.5 ± 66.4 | 189.0 ± 74.3 | 216.5 ± 78.3 | 255.7 ± 83.2 | <0.001 |
| Vitamin D (µg/day) | 5.3 ± 3.2 | 5.9 ± 3.3 | 6.4 ± 3.4 | 7.1 ± 3.6 | <0.001 |
| Vitamin E (mg/day) | 9.6 ± 4.1 | 10.4 ± 3.9 | 10.7 ± 3.7 | 11.7 ± 3.8 | <0.001 |
| Calcium (mg/day) | 876.6 ± 325.3 | 987.2 ± 325.1 | 1071.8 ± 320.3 | 1201.5 ± 326.2 | <0.001 |
| Phosphorus (mg/day) | 1556.4 ± 389.7 | 1699.4 ± 388.3 | 1796.7 ± 389.7 | 1985.6 ± 397.1 | <0.001 |
| Magnesium (mg/day) | 371.0 ± 99.3 | 403.7 ± 98.4 | 428.2 ± 98.8 | 479.4 ± 108.5 | <0.001 |
| Iron (mg/day) | 15.2 ± 3.9 | 16.0 ± 3.8 | 16.6 ± 3.7 | 18.1 ± 3.9 | <0.001 |
| Iodine (µg/day) | 242.9 ± 163.6 | 274.7 ± 153.1 | 297.0 ± 155.3 | 327.2 ± 151.1 | <0.001 |
| Potassium (mg/day) | 3767.9 ± 858.1 | 4262.1 ± 880.0 | 4619.6 ± 929.3 | 5256.5 ± 1053.4 | <0.001 |
| Selenium (µg/day) | 113.6 ± 36.8 | 115.9 ± 32.9 | 116.4 ± 31.3 | 122.5 ± 30.9 | <0.001 |
| Zinc (mg/day) | 12.5 ± 3.5 | 13.0 ± 3.2 | 13.2 ± 3.1 | 14.1 ± 3.1 | <0.001 |
| Alcohol intake (g/day), Mean ± SD | 16.5 ± 19.4 | 11.7 ± 15.0 | 9.1 ± 12.4 | 6.8 ± 9.8 | <0.001 |
| MEN: ≤70 years | |||||
| Q1 (n = 787) | Q2 (n = 763) | Q3 (n = 973) | Q4 (n = 489) | p Value | |
| Inadequacies, mean ± SD | 3.0 ± 1.1 | 2.3 ± 1.1 | 2.0 ± 0.1 | 1.7 ± 0.7 | <0.001 1 |
| Participants, n (%) | 468 (46.8) | 156 (19.3) | 78 (11.7) | 12 (2.4) | <0.001 2 |
| WOMEN: ≤70 years | |||||
| Q1 (n = 630) | Q2 (n = 610) | Q3 (n = 884) | Q4 (n = 529) | p Value | |
| Inadequacies, mean (SD) | 2.9 ± 1.0 | 2.5 ± 1.1 | 2.1 ± 1.0 | 1.7 ± 0.8 | <0.001 1 |
| Participants, n (%) | 169 (38.2) | 145 (23.8) | 81 (11.0) | 23 (2.7) | <0.001 2 |
| MEN: >70 years | |||||
| Q1 (n = 124) | Q2 (n = 136) | Q3 (n = 111) | Q1 (n = 48) | p Value | |
| Inadequacies, mean (SD) | 2.9 ± 1.1 | 2.6 ± 1.2 | 2.0 ± 1.0 | 1.6 ± 0.9 | <0.001 1 |
| Participants, n (%) | 51 (44.0) | 37 (33.9) | 11 (10.9) | 5 (5.5) | <0.001 2 |
| WOMEN: >70 years | |||||
| Q1 (n = 138) | Q2 (n = 137) | Q1 (n = 177) | Q1 (n = 103) | p Value | |
| Inadequacies, mean (SD) | 3.0 ± 1.1 | 2.5 ± 1.1 | 2.0 ± 0.9 | 1.7 ± 0.8 | <0.001 1 |
| Participants, n (%) | 38 (42.7) | 30 (24.6) | 14 (9.8) | 6 (3.0) | <0.001 2 |
| Q1 (n = 1647) | Q2 (n = 1647) | Q3 (n = 1647) | Q4 (n = 1646) | |
| Total DDS | ||||
| Model 1 | 27.42 (20.13−37.34) | 10.00 (7.30−13.72) | 4.37 (3.14−6.09) | 1 (Ref.) |
| Model 2 | 28.56 (20.80−39.21) | 9.97 (7.25−13.70) | 4.33 (3.11−6.04) | 1 (Ref.) |
| C1 (n = 550) | C2 (n = 1315) | C3 (n = 2482) | C4 (n = 2240) | |
| Vegetable Group | ||||
| Model 1 | 19.82 (15.19−25.85) | 7.28 (5.85−9.10) | 2.74 (2.22−3.38) | 1 (Ref.) |
| Model 2 | 14.03 (10.55−18.65) | 6.21 (4.92−7.83) | 2.52 (2.02−3.14) | 1 (Ref.) |
| C1 (n = 845) | C2 (n = 4497) | C3 (n = 779) | C4 (v466) | |
| Fruit Group | ||||
| Model 1 | 19.75 (11.87−32.86) | 3.76 (2.30−6.15) | 2.23 (1.29−3.84) | 1 (Ref.) |
| Model 2 | 11.62 (6.81−19.81) | 2.71 (1.62−4.53) | 2.02 (1.15−3.57) | 1 (Ref.) |
| C1 (n = 350) | C2 (n = 4767) | C3 (n = 1390) | C4 (n = 80) | |
| Cereal Group | ||||
| Model 1 | 1.33 (0.54−3.31) | 1.13 (0.47−2.71) | 0.90 (0.37−2.19) | 1 (Ref.) |
| Model 2 | 0.83 (0.32−2.19) | 0.84 (0.33−2.14) | 0.71 (0.28−1.82) | 1 (Ref.) |
| C1 (n = 26) | C2 (n = 1254) | C3 (n = 2770) | C4 (n = 2537) | |
| Proteins group | ||||
| Model 1 | 12.33 (4.10−37.19) | 3.00 (2.48−3.62) | 2.00 (1.69−2.37) | 1 (Ref.) |
| Model 2 | 6.60 (1.96−22.24) | 2.02 (1.64−2.48) | 1.63 (1.36−1.96) | 1 (Ref.) |
| C1 (n = 686) | C2 (n = 2447) | C3 (n = 2600) | C4 (n = 854) | |
| Dairy Group | ||||
| Model 1 | 9.51 (6.88−13.14) | 3.35 (2.50−4.49) | 1.52 (1.12−2.06) | 1 (Ref.) |
| Model 2 | 6.54 (4.64−9.22) | 2.40 (1.76−3.27) | 1.24 (0.90−1.71) | 1 (Ref.) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Ibáñez, N.; Gea, A.; Martínez-González, M.A.; Salas-Salvadó, J.; Corella, D.; Zomeño, M.D.; Romaguera, D.; Vioque, J.; Aros, F.; Wärnberg, J.; et al. Dietary Diversity and Nutritional Adequacy among an Older Spanish Population with Metabolic Syndrome in the PREDIMED-Plus Study: A Cross-Sectional Analysis. Nutrients 2019, 11, 958. https://doi.org/10.3390/nu11050958
Cano-Ibáñez N, Gea A, Martínez-González MA, Salas-Salvadó J, Corella D, Zomeño MD, Romaguera D, Vioque J, Aros F, Wärnberg J, et al. Dietary Diversity and Nutritional Adequacy among an Older Spanish Population with Metabolic Syndrome in the PREDIMED-Plus Study: A Cross-Sectional Analysis. Nutrients. 2019; 11(5):958. https://doi.org/10.3390/nu11050958
Chicago/Turabian StyleCano-Ibáñez, Naomi, Alfredo Gea, Miguel A. Martínez-González, Jordi Salas-Salvadó, Dolores Corella, M. Dolors Zomeño, Dora Romaguera, Jesús Vioque, Fernando Aros, Julia Wärnberg, and et al. 2019. "Dietary Diversity and Nutritional Adequacy among an Older Spanish Population with Metabolic Syndrome in the PREDIMED-Plus Study: A Cross-Sectional Analysis" Nutrients 11, no. 5: 958. https://doi.org/10.3390/nu11050958
APA StyleCano-Ibáñez, N., Gea, A., Martínez-González, M. A., Salas-Salvadó, J., Corella, D., Zomeño, M. D., Romaguera, D., Vioque, J., Aros, F., Wärnberg, J., Martínez, J. A., Serra-Majem, L., Estruch, R., Tinahones, F. J., Lapetra, J., Pintó, X., Tur, J. A., García-Ríos, A., Riquelme-Gallego, B., ... Bueno-Cavanillas, A. (2019). Dietary Diversity and Nutritional Adequacy among an Older Spanish Population with Metabolic Syndrome in the PREDIMED-Plus Study: A Cross-Sectional Analysis. Nutrients, 11(5), 958. https://doi.org/10.3390/nu11050958
















