Effects of Polyunsaturated Fatty Acids on Nonspecific Typical Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials
Abstract
1. Introduction
2. Methods
2.1. Study Selection Criteria
2.2. Search Strategy and Study Selection
2.3. Quality Assessment and Data Extraction
2.4. Evidence Synthesis and Statistical Analysis
3. Results
3.1. Characteristics and Quality of Included Studies
3.2. TBUT Improvement
3.3. Schirmer Test Score Improvement
3.4. Osmolarity Improvement
3.5. OSDI Score Improvement
4. Discussion
4.1. Key Findings
4.2. Comparison with the Largest Trial
4.3. Comparison with Previous Synthesized Evidence
4.4. Limitations and Future Direction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALA | α-linolenic acid |
| CI | confidence interval |
| DED | dry eye disease |
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| GLA | gamma-Linolenic acid |
| LA | linoleic acid |
| MD | mean difference |
| OSDI | ocular surface disease index |
| PUFA | polyunsaturated fatty acid |
| RCT | randomized clinical trial |
| SD | standard deviation |
| SE | standard error |
| TBUT | tear breakup time |
References
- Pflugfelder, S.C.; de Paiva, C.S. The pathophysiology of dry eye disease: What we know and future directions for research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Vanrell, R.; Peral, A. International dry eye workshop (dews). Update of the disease. Arch. Soc. Esp. Oftalmol. 2007, 82, 733–734. [Google Scholar]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. Tfos dews ii report executive summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. Tfos dews ii definition and classification report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. Tfos dews ii management and therapy report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef]
- Messmer, E.M. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Arztebl Int. 2015, 112, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Endres, S.; Ghorbani, R.; Kelley, V.E.; Georgilis, K.; Lonnemann, G.; van der Meer, J.W.; Cannon, J.G.; Rogers, T.S.; Klempner, M.S.; Weber, P.C.; et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N. Engl. J. Med. 1989, 320, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Zurier, R.B.; Rossetti, R.G.; Seiler, C.M.; Laposata, M. Human peripheral blood t lymphocyte proliferation after activation of the t cell receptor: Effects of unsaturated fatty acids. Prostaglandins Leukot. Essent. Fatty Acids 1999, 60, 371–375. [Google Scholar] [CrossRef]
- Viau, S.; Maire, M.A.; Pasquis, B.; Gregoire, S.; Acar, N.; Bron, A.M.; Bretillon, L.; Creuzot-Garcher, C.P.; Joffre, C. Efficacy of a 2-month dietary supplementation with polyunsaturated fatty acids in dry eye induced by scopolamine in a rat model. Graefes. Arch. Clin. Exp. Ophthalmol. 2009, 247, 1039–1050. [Google Scholar] [CrossRef]
- Andrade, A.S.; Salomon, T.B.; Behling, C.S.; Mahl, C.D.; Hackenhaar, F.S.; Putti, J.; Benfato, M.S. Alpha-lipoic acid restores tear production in an animal model of dry eye. Exp. Eye Res. 2014, 120, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miljanovic, B.; Trivedi, K.A.; Dana, M.R.; Gilbard, J.P.; Buring, J.E.; Schaumberg, D.A. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am. J. Clin. Nutr. 2005, 82, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Oleñik, A. Effectiveness and tolerability of dietary supplementation with a combination of omega-3 polyunsaturated fatty acids and antioxidants in the treatment of dry eye symptoms: Results of a prospective study. Clin. Ophthalmol. (Auckland, N.Z.) 2014, 8, 169–176. [Google Scholar]
- Barabino, S.; Rolando, M.; Camicione, P.; Ravera, G.; Zanardi, S.; Giuffrida, S.; Calabria, G. Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea 2003, 22, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Kumar, P.; Kumar, M.; Mehra, N.; Mishra, A. A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. Int. J Ophthalmol. 2013, 6, 811–816. [Google Scholar]
- Chinnery, H.R.; Golborne, C.N.; Downie, L.E. Omega-3 supplementation is neuroprotective to corneal nerves in dry eye disease: A pilot study. Ophthalmic Physiol. Opt. 2017, 37, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Creuzot-Garcher, C.; Baudouin, C.; Labetoulle, M.; Pisella, P.J.; Mouriaux, F.; Meddeb-Ouertani, A.; El Matri, L.; Khairallah, M.; Brignole-Baudouin, F. Efficacy assessment of nutrilarm(r), a per os omega-3 and omega-6 polyunsaturated essential fatty acid dietary formulation versus placebo in patients with bilateral treated moderate dry eye syndrome. J. Fr. Ophtalmol. 2011, 34, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Deinema, L.A.; Vingrys, A.J.; Wong, C.Y.; Jackson, D.C.; Chinnery, H.R.; Downie, L.E. A randomized, double-masked, placebo-controlled clinical trial of two forms of omega-3 supplements for treating dry eye disease. Ophthalmology 2017, 124, 43–52. [Google Scholar] [CrossRef]
- Kangari, H.; Eftekhari, M.H.; Sardari, S.; Hashemi, H.; Salamzadeh, J.; Ghassemi-Broumand, M.; Khabazkhoob, M. Short-term consumption of oral omega-3 and dry eye syndrome. Ophthalmology 2013, 120, 2191–2196. [Google Scholar] [CrossRef]
- Kawakita, T.; Kawabata, F.; Tsuji, T.; Kawashima, M.; Shimmura, S.; Tsubota, K. Effects of dietary supplementation with fish oil on dry eye syndrome subjects: Randomized controlled trial. Biomed. Res. 2013, 34, 215–220. [Google Scholar] [CrossRef]
- Pinazo-Duran, M.D.; Galbis-Estrada, C.; Pons-Vazquez, S.; Cantu-Dibildox, J.; Marco-Ramirez, C.; Benitez-del-Castillo, J. Effects of a nutraceutical formulation based on the combination of antioxidants and omega-3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disorders. Clin. Interv. Aging 2013, 8, 139–148. [Google Scholar] [CrossRef]
- Sheppard, J.D., Jr.; Singh, R.; McClellan, A.J.; Weikert, M.P.; Scoper, S.V.; Joly, T.J.; Whitley, W.O.; Kakkar, E.; Pflugfelder, S.C. Long-term supplementation with n-6 and n-3 pufas improves moderate-to-severe keratoconjunctivitis sicca: A randomized double-blind clinical trial. Cornea 2013, 32, 1297–1304. [Google Scholar] [CrossRef]
- Asbell, P.A.; Maguire, G.; Pistilli, M.; Ying, G.S.; Szczotka-Flynn, L.B.; Hardten, D.R.; Lin, M.C.; Shtein, R.M. N−3 fatty acid supplementation for the treatment of dry eye disease. N. Engl. J. Med. 2018, 378, 1681–1690. [Google Scholar] [PubMed]
- Brignole-Baudouin, F.; Baudouin, C.; Aragona, P.; Rolando, M.; Labetoulle, M.; Pisella, P.J.; Barabino, S.; Siou-Mermet, R.; Creuzot-Garcher, C. A multicentre, double-masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega-6 fatty acids on a conjunctival inflammatory marker in dry eye patients. Acta. Ophthalmol. 2011, 89, e591–e597. [Google Scholar] [CrossRef] [PubMed]
- Larmo, P.S.; Jarvinen, R.L.; Setala, N.L.; Yang, B.; Viitanen, M.H.; Engblom, J.R.; Tahvonen, R.L.; Kallio, H.P. Oral sea buckthorn oil attenuates tear film osmolarity and symptoms in individuals with dry eye. J. Nutr. 2010, 140, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, J.C.; Butovich, I.; Uchiyama, E.; Aronowicz, J.; Agee, S.; McCulley, J.P. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea 2011, 30, 308–314. [Google Scholar] [CrossRef]
- Asbell, P.A.; Maguire, M.G.; Peskin, E.; Bunya, V.Y.; Kuklinski, E.J. Dry eye assessment and management (dream(c)) study: Study design and baseline characteristics. Contemp. Clin. Trials 2018, 71, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Molina-Leyva, I.; Molina-Leyva, A.; Bueno-Cavanillas, A. Efficacy of nutritional supplementation with omega-3 and omega-6 fatty acids in dry eye syndrome: A systematic review of randomized clinical trials. Acta. Ophthalmol. 2017, 95, e677–e685. [Google Scholar] [CrossRef]
- Creuzot, C.; Passemard, M.; Viau, S.; Joffre, C.; Pouliquen, P.; Elena, P.P.; Bron, A.; Brignole, F. Improvement of dry eye symptoms with polyunsaturated fatty acids. J. Fr. Ophtalmol. 2006, 29, 868–873. [Google Scholar] [CrossRef]
- King-Smith, P.E.; Begley, C.G.; Braun, R.J. Mechanisms, imaging and structure of tear film breakup. Ocul. Surf. 2018, 16, 4–30. [Google Scholar] [CrossRef]
- Millar, T.J.; Schuett, B.S. The real reason for having a meibomian lipid layer covering the outer surface of the tear film - a review. Exp. Eye Res. 2015, 137, 125–138. [Google Scholar] [CrossRef]
- Wong, H.; Fatt, I.I.; Radke, C.J. Deposition and thinning of the human tear film. J. Colloid. Interface Sci. 1996, 184, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ulven, S.M.; Holven, K.B. Comparison of bioavailability of krill oil versus fish oil and health effect. Vas. Health Risk Manag. 2015, 11, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Bustos, R.; Romo, L.; Yáñez, K.; Díaz, G.; Romo, C. Oxidative stability of carotenoid pigments and polyunsaturated fatty acids in microparticulate diets containing krill oil for nutrition of marine fish larvae. J. Food Eng. 2003, 56, 289–293. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Dohlman, T.H.; Amparo, F.; Arnoldner, M.A.; Jamali, A.; Hamrah, P.; Dana, R. Effects of corneal nerve density on the response to treatment in dry eye disease. Ophthalmology 2015, 122, 662–668. [Google Scholar] [CrossRef]
- Pinto-Fraga, J.; Lopez-Miguel, A.; Gonzalez-Garcia, M.J.; Fernandez, I.; Lopez-de-la-Rosa, A.; Enriquez-de-Salamanca, A.; Stern, M.E.; Calonge, M. Topical fluorometholone protects the ocular surface of dry eye patients from desiccating stress: A randomized controlled clinical trial. Ophthalmology 2016, 123, 141–153. [Google Scholar] [CrossRef]
- Sheppard, J.D.; Donnenfeld, E.D.; Holland, E.J.; Slonim, C.B.; Solomon, R.; Solomon, K.D.; McDonald, M.B.; Perry, H.D.; Lane, S.S.; Pflugfelder, S.C.; et al. Effect of loteprednol etabonate 0.5% on initiation of dry eye treatment with topical cyclosporine 0.05%. Eye Contact Lens 2014, 40, 289–296. [Google Scholar] [CrossRef]
- Wan, K.H.; Chen, L.J.; Young, A.L. Efficacy and safety of topical 0.05% cyclosporine eye drops in the treatment of dry eye syndrome: A systematic review and meta-analysis. Ocul. Surf. 2015, 13, 213–225. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Y.; Li, G.; Wang, J.; Li, X. Efficacy of polyunsaturated fatty acids for dry eye syndrome: A meta-analysis of randomized controlled trials. Nutr. Rev. 2014, 72, 662–671. [Google Scholar] [CrossRef]

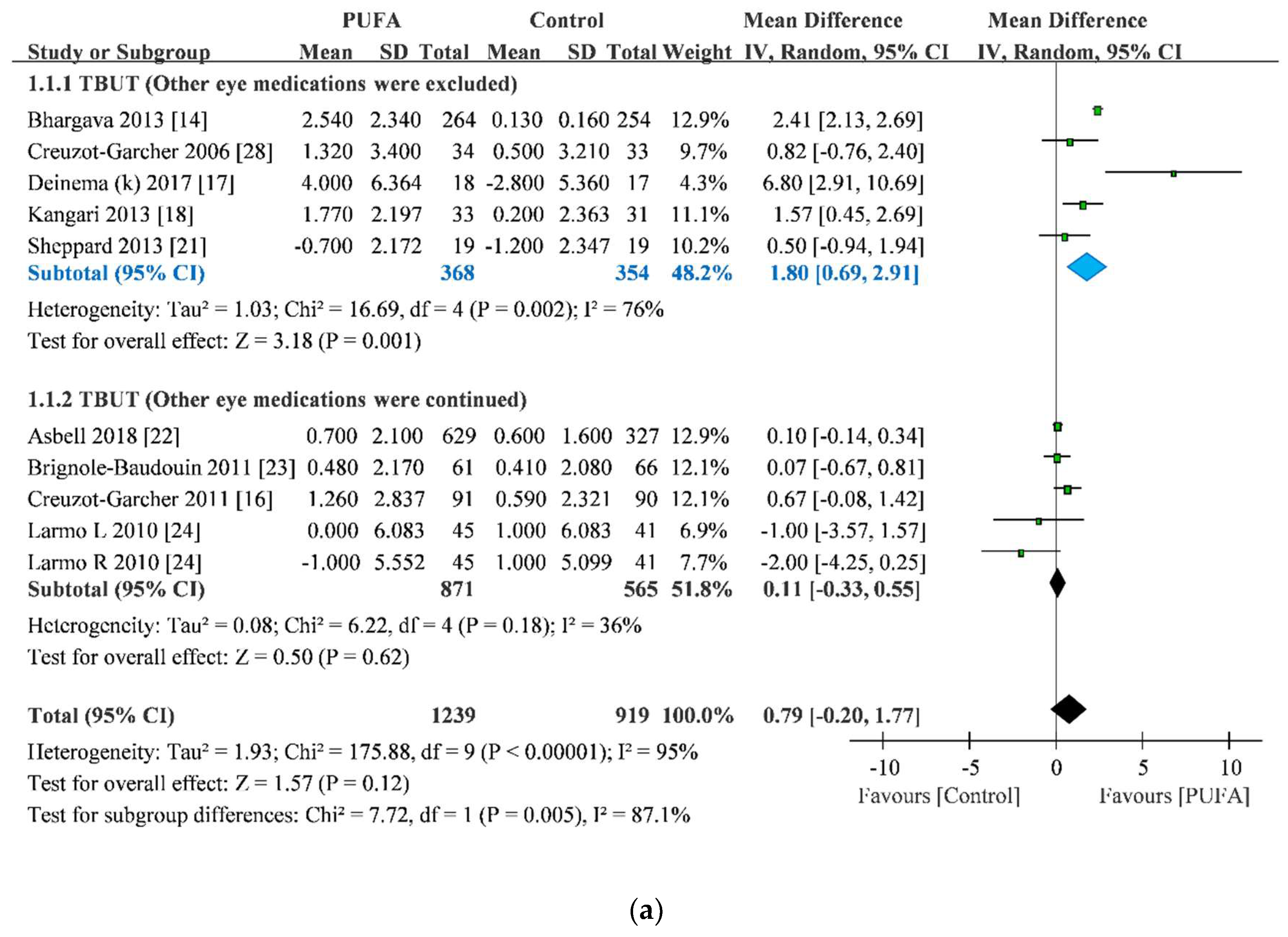
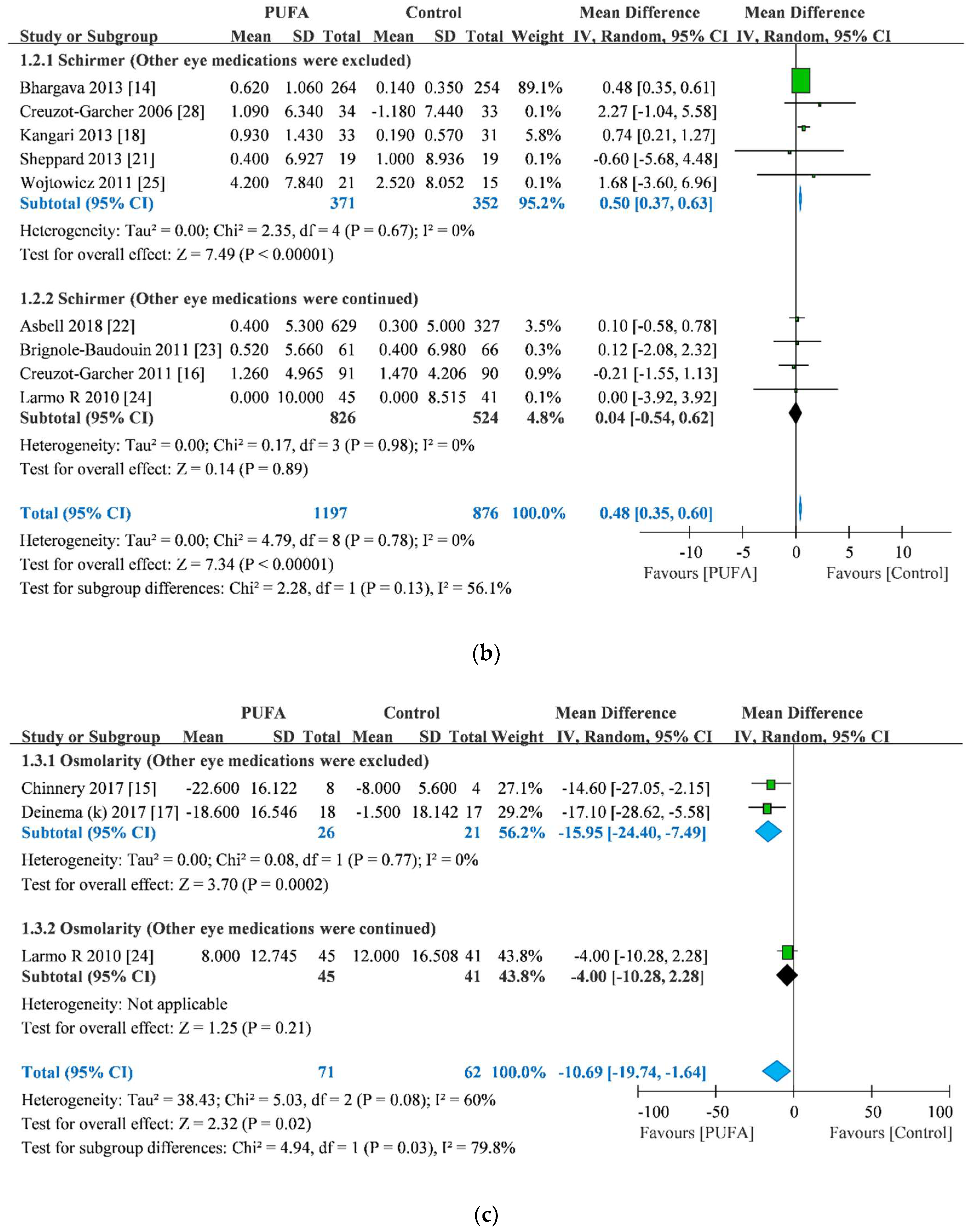

| Study | Year | Sample Size | Age | Sex (male/female) | Other Eye Medication | Treatment Duration | Anesthesia for Schirmer Test | |||
|---|---|---|---|---|---|---|---|---|---|---|
| PUFA | Control | PUFA | Control | PUFA | Control | |||||
| Asbell et al. [22] | 2018 | 349 | 186 | 58.3 ± 13.5 | 57.5 ± 12.6 | 65/284 | 36/150 | Allowed | 12 months | Yes |
| Barabino et al. [13] | 2003 | 13 | 13 | 63.4 ± 8.2 | 54.3 ± 11.3 | 4/9 | 3/10 | Limited | 1.5months | NR |
| Bhargava et al. [14] | 2013 | 264 | 254 | 38.82±4.12 | 40.06 ± 6.76 | Total: | 254/268 | Limited | 3 months | Yes |
| Brignole-Baudouin et al. [23] | 2011 | 58 | 63 | 60 ± 11.75 | 59.7 ± 11.95 | 1/57 | 3/60 | Allowed | 3 months | NR |
| Chinnery et al. [15] | 2017 | 8 | 4 | 42 ± 7 a | 46 ± 10 a | 2/6 | 1/3 | Limited | 3 months | NR |
| Creuzo-Garcher et al. [16] | 2011 | 90 | 91 | 61.28 ± 12.15 | 61.79 ± 11.64 | 8/82 | 7/84 | Allowed | 6 months | NR |
| Creuzot-Garcher et al. [28] | 2006 | 36 | 35 | 59.7 ± 14.7 | 61.1 ± 11.1 | 2/34 | 1/34 | Limited | 6 months | No |
| Deinema et al. [17] | 2017 | 37 | 17 | Total: | 42.51 | Total: | 18/36 | Limited | 3 months | Yes |
| Kangari et al. [18] | 2013 | 33 | 31 | 60.6 ± 8.7 | 61.8 ± 8 | 15/18 | 11/20 | Limited | 1 month | No |
| Kawakita et al. [19] | 2013 | 15 | 11 | 52.5 ± 2.5 a | 51.9 ± 2.2 a | 5/10 | 1/10 | Limited | 4 months | No |
| Larmo et al. [24] | 2010 | 52 | 48 | 45 ± 18 | 46 ± 17 | 8/44 | 7/41 | Allowed | 3 months | No |
| Sheppard et al. [21] | 2013 | 19 | 19 | 62 ± 1 a | 61 ± 2 a | 0/19 | 0/19 | Limited | 6 months | NR |
| Wojtowicz et al. [25] | 2011 | 21 | 15 | Total: | 61 | Total: | 20/16 | Limited | 3 months | No |
| Outcome | Small-Study Effect | Meta-Regression by Duration | Meta-Regression by Single-eye Data | Meta-Regression by Type of Schirmer Test | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Egger test | P | Point Estimate | P | τ2 | Point Estimate | P | τ2 | Point Estimate | P | τ2 | |
| TBUT | −0.53 | 0.81 | −0.20 | <0.001 | 1.62 | 0.17 | 0.55 | 2.16 | NE | NE | NE |
| Schirmer test scores | −0.08 | 0.81 | −0.05 | 0.17 | 0.00 | −0.17 | 0.52 | 0.00 | −0.31 | 0.26 | 0.00 |
| Osmolarity | −2.83 | 0.32 | NE | NE | NE | NE | NE | NE | NE | NE | NE |
| OSDI score | −3.18 | 0.05 | 0.54 | 0.03 | 15.27 | NE | NE | NE | NE | NE | NE |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, S.-C.; Tuan, H.-I.; Kang, Y.-N. Effects of Polyunsaturated Fatty Acids on Nonspecific Typical Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2019, 11, 942. https://doi.org/10.3390/nu11050942
Chi S-C, Tuan H-I, Kang Y-N. Effects of Polyunsaturated Fatty Acids on Nonspecific Typical Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients. 2019; 11(5):942. https://doi.org/10.3390/nu11050942
Chicago/Turabian StyleChi, Sheng-Chu, Hsin-I Tuan, and Yi-No Kang. 2019. "Effects of Polyunsaturated Fatty Acids on Nonspecific Typical Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials" Nutrients 11, no. 5: 942. https://doi.org/10.3390/nu11050942
APA StyleChi, S.-C., Tuan, H.-I., & Kang, Y.-N. (2019). Effects of Polyunsaturated Fatty Acids on Nonspecific Typical Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients, 11(5), 942. https://doi.org/10.3390/nu11050942





