Rice Hull Extract (RHE) Suppresses Adiposity in High-Fat Diet-Induced Obese Mice and Inhibits Differentiation of 3T3-L1 Preadipocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of RHE
2.3. Animal Experiments
2.4. Serum Analysis
2.5. Histological Analysis
2.6. Western Blot Analysis
2.7. Real-Time PCR Analysis
2.8. Cell Culture and Adipocyte Differentiation
2.9. Oil Red O Staining
2.10. Statistical Analysis
3. Results
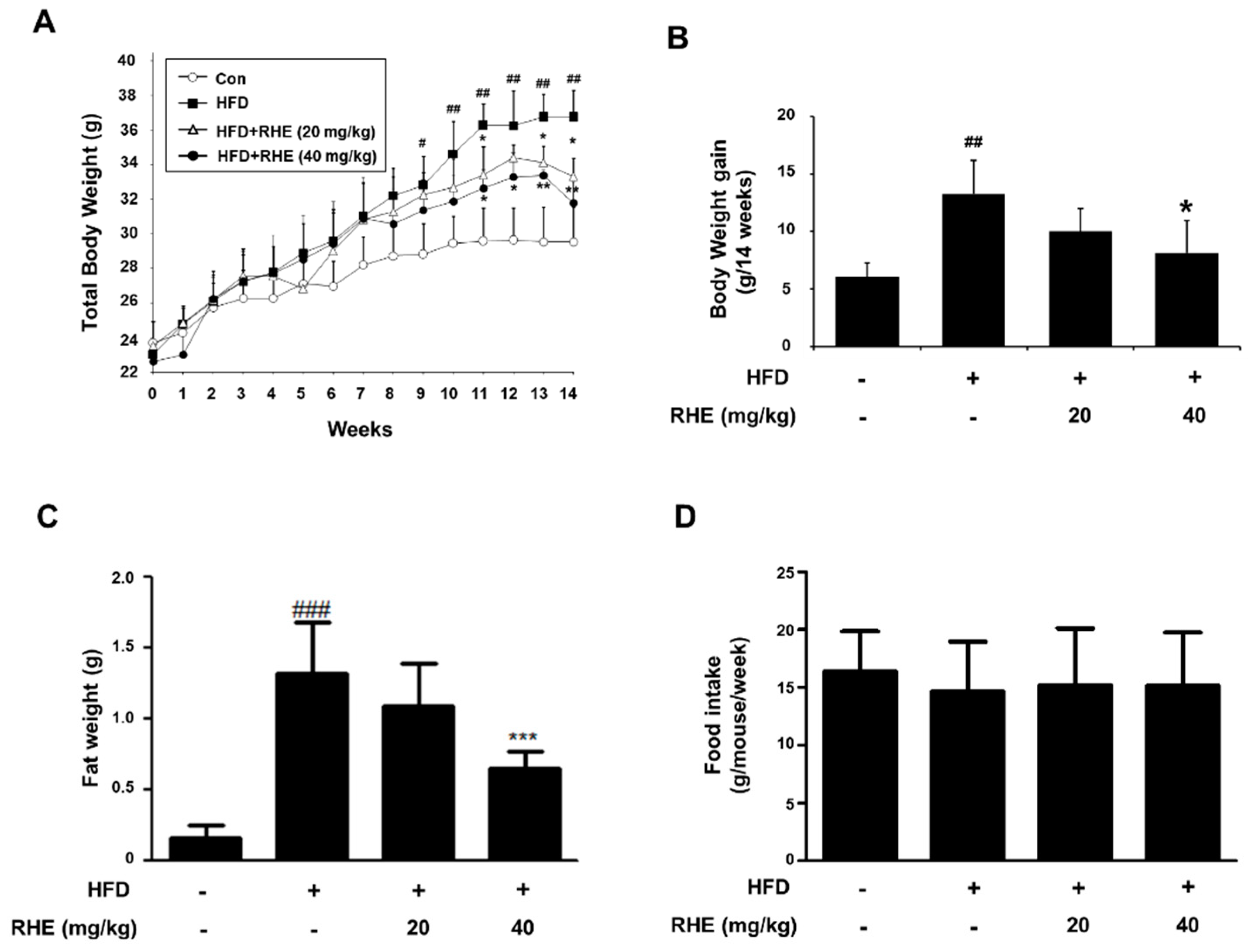
3.1. RHE Reduced Body Weight Gain and Suppressed Lipid Droplet Accumulation in Liver and Epididymis Adipose Tissue
3.2. RHE Ameliorated Adiposity and Fat Accumulation in HFD-Fed Obese Mice
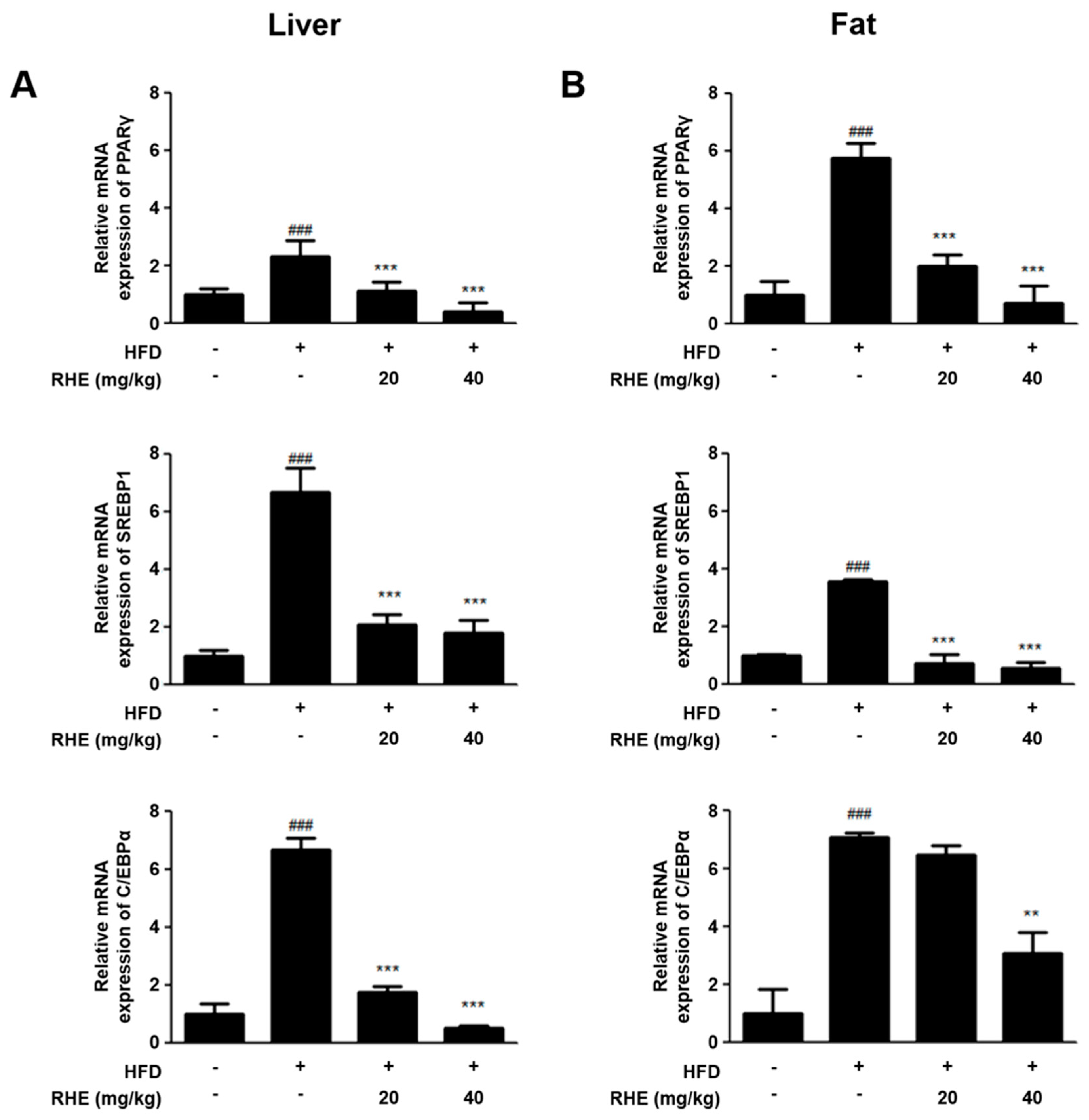
3.3. RHE Suppressed mRNA Expression of Adipogenic Genes in HFD-Fed Obese Mice
3.4. RHE Activated AMPK in HFD-Fed Obese Mice
3.5. Effects of RHE on the MAPK Pathway in HFD-Fed Obese Mice
3.6. RHE Inhibited Adipogenesis and the Expression of Adipogenic Factors in 3T3-L1 Preadipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Ethics Approval and Consent to Participate
Abbreviations
References
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Barness, L.A.; Opitz, J.M.; Gilbert-Barness, E. Obesity: Genetic, molecular, and environmental aspects. Am. J. Med. Genet. A 2007, 143, 3016–3034. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Raun, K.; Yan, F.F.; Larsen, M.O.; Tang-Christensen, M. Laboratory animals as surrogate models of human obesity. Acta Pharmacol. Sin. 2012, 33, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Carling, D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem. Sci. 2004, 29, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Kim, M.; Park, H.S.; Kim, H.S.; Jeon, M.J.; Oh, K.S.; Koh, E.H.; Won, J.C.; Kim, M.S.; Oh, G.T.; et al. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem. Biophys. Res. Commun. 2006, 340, 291–295. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef]
- Prusty, D.; Park, B.H.; Davis, K.E.; Farmer, S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jang, Y.J.; Park, B.; Yim, J.H.; Lee, H.K.; Rhee, D.K.; Pyo, S. Ramalin inhibits differentiation of 3T3-L1 preadipocytes and suppresses adiposity and body weight in a high-fat diet-fed C57BL/6J mice. Chem. Biol. Interact. 2016, 257, 71–80. [Google Scholar] [CrossRef]
- Raskin, I.; Ribnicky, D.M.; Komarnytsky, S.; Ilic, N.; Poulev, A.; Borisjuk, N.; Brinker, A.; Moreno, D.A.; Ripoll, C.; Yakoby, N.; et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002, 20, 522–531. [Google Scholar] [CrossRef]
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice bran: A novel functional ingredient. Crit. Rev. Food Sci. Nutr. 2014, 54, 807–816. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Utilization of rice husk ash as novel adsorbent: A judicious recycling of the colloidal agricultural waste. Adv. Colloid Interface Sci. 2009, 152, 39–47. [Google Scholar] [CrossRef]
- Salanti, A.; Zoia, L.; Orlandi, M.; Zanini, F.; Elegir, G. Structural characterization and antioxidant activity evaluation of lignins from rice husk. J. Agric. Food Chem. 2010, 58, 10049–10055. [Google Scholar] [CrossRef]
- Vir, O.M.; Singh, B.B.; Tomar, B.S. Specialty rice for therapeutic purposes, good health and processed food products. In Proceedings of the National Symposium on Basmati Rice Research: Current Trends and Future Prospects, SVBP University of Agriculture and Technology, Meerut, India, 6–7 September 2005. [Google Scholar]
- Butsat, S.; Weerapreeyakul, N.; Siriamornpun, S. Changes in phenolic acids and antioxidant activity in Thai rice husk at five growth stages during grain development. J. Agric. Food Chem. 2009, 57, 4566–4571. [Google Scholar] [CrossRef]
- Yang, J.Y.; Kang, M.Y.; Nam, S.H.; Friedman, M. Antidiabetic effects of rice hull smoke extract in alloxan-induced diabetic mice. J. Agric. Food Chem. 2012, 60, 87–94. [Google Scholar] [CrossRef]
- Kim, C.Y.; Chung, K.S.; Cheon, S.Y.; Lee, J.H.; Park, Y.B.; An, H.J. Rice Hull Extract Suppresses Benign Prostate Hyperplasia by Decreasing Inflammation and Regulating Cell Proliferation in Rats. J. Med. Food 2016, 19, 746–754. [Google Scholar] [CrossRef]
- Hwang, C.S.; Mandrup, S.; MacDougald, O.A.; Geiman, D.E.; Lane, M.D. Transcriptional activation of the mouse obese (ob) gene by CCAAT/enhancer binding protein alpha. Proc. Natl. Acad. Sci. USA 1996, 93, 873–877. [Google Scholar] [CrossRef]
- Sakaue, H.; Ogawa, W.; Nakamura, T.; Mori, T.; Nakamura, K.; Kasuga, M. Role of MAPK phosphatase-1 (MKP-1) in adipocyte differentiation. J. Biol. Chem. 2004, 279, 39951–39957. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Kang, S. Gastrodia elata Blume water extracts improve insulin resistance by decreasing body fat in diet-induced obese rats: Vanillin and 4-hydroxybenzaldehyde are the bioactive candidates. Eur. J. Nutr. 2011, 50, 107–118. [Google Scholar] [CrossRef]
- Hokanson, J.E.; Austin, M.A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies. J. Cardiovasc. Risk 1996, 3, 213–219. [Google Scholar] [CrossRef]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPalpha induces adipogenesis through PPARgamma: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Park, J.Y.; Kang, K.S.; Park, J.H.; Hwang, G.S. Processed Panax ginseng, sun ginseng, inhibits the differentiation and proliferation of 3T3-L1 preadipocytes and fat accumulation in Caenorhabditis elegans. J. Ginseng Res. 2017, 41, 257–267. [Google Scholar] [CrossRef]
- Gwon, S.Y.; Ahn, J.Y.; Jung, C.H.; Moon, B.K.; Ha, T.Y. Shikonin suppresses ERK 1/2 phosphorylation during the early stages of adipocyte differentiation in 3T3-L1 cells. BMC Complement. Altern. Med. 2013, 13, 207. [Google Scholar] [CrossRef]
- Lee, M.; Sung, S.H. Platyphylloside isolated from betula platyphylla inhibit adipocyte differentiation and induce lipolysis via regulating adipokines including PPARgamma in 3T3-L1 cells. Pharmacogn. Mag. 2016, 12, 276–281. [Google Scholar] [CrossRef]
- Daval, M.; Foufelle, F.; Ferre, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef]
- Bort, A.; Sanchez, B.G.; Mateos-Gomez, P.A.; Diaz-Laviada, I.; Rodriguez-Henche, N. Capsaicin targets lipogenesis in HepG2 cells through AMPK activation, AKT inhibition and PPARs regulation. Int. J. Mol. Sci. 2019, 20, 1660. [Google Scholar] [CrossRef]
- Joshi, T.; Singh, A.K.; Haratipour, P.; Sah, A.N.; Pandey, A.K.; Naseri, R.; Juyal, V.; Farzaei, M.H. Targeting AMPK signaling pathway by natural products for treatment of diabetes mellitus and its complications. J. Cell Physiol. 2019. [Google Scholar] [CrossRef]
- Shen, B.; Zhao, C.; Wang, Y.; Peng, Y.; Cheng, J.; Li, Z.; Wu, L.; Jin, M.; Feng, H. Aucubin inhibited lipid accumulation and oxidative stress via Nrf2/HO-1 and AMPK signalling pathways. J. Cell Mol. Med. 2019. [Google Scholar] [CrossRef]
- Misra, P. AMP activated protein kinase: A next generation target for total metabolic control. Expert Opin. Ther. Targets 2008, 12, 91–100. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. PPARgamma: A nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem. 2001, 276, 37731–37734. [Google Scholar] [CrossRef]
- Kohjima, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; Enjoji, M.; et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2008, 21, 507–511. [Google Scholar] [CrossRef]
- Mobbs, C.V.; Makimura, H. Block the FAS, lose the fat. Nat. Med. 2002, 8, 335–336. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Gronborg, M.; Huang, H.; Kim, J.W.; Otto, T.C.; Pandey, A.; Lane, M.D. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 9766–9771. [Google Scholar] [CrossRef]
- Camp, H.S.; Tafuri, S.R.; Leff, T. c-Jun N-Terminal Kinase Phosphorylates Peroxisome Proliferator-Activated Receptor-gamma1 and Negatively Regulates Its Transcriptional Activity. Endocrinology 1999, 140, 392–397. [Google Scholar] [CrossRef]
- Luo, Z.; Zang, M.; Guo, W. AMPK as a metabolic tumor suppressor: Control of metabolism and cell growth. Future Oncol. 2010, 6, 457–470. [Google Scholar] [CrossRef]




| Genes | Tm (°C) | Size (bp) | Sequence 5′-3′ |
|---|---|---|---|
| PPARγ | 55 | 148 | F: TTCGGAATCAGCTCTGTGGA |
| 55 | R: CCATTGGGTCAGCTCTTGTG | ||
| SREBP1c | 55 | 115 | F: ATCGCAAACAAGCTGACCTG |
| 55 | R: AGATCCAGGTTTGAGGTGGG | ||
| C/EBPα | 55 | 187 | F: TCGGTGCGTCTAAGATGAGG |
| 55 | R: TCAAGGCACATTTTTGCTCC |
| Indexes | ND | HFD | HT | |
|---|---|---|---|---|
| 20 mg/kg | 40 mg/kg | |||
| Triglyceride (mg/dL) | 55.00 ± 10.00 | 71.67 ± 11.55 ## | 58.13 ± 9.98 | 45.00 ± 8.86 |
| LDL-cholesterol (mg/dL) | 2.25 ± 0.87 | 11.00 ± 1.22 ### | 9.67 ± 2.50 | 9.00 ± 1.15 * |
| HDL-cholesterol (mg/dL) | 67.50 ± 2.89 | 72.50 ± 2.89 | 75.50 ± 7.07 | 78.75 ± 4.79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.-H.; Ju, J.-Y.; Chung, K.-S.; Cheon, S.-Y.; Gil, T.-Y.; Cominguez, D.C.; Cha, Y.-Y.; Lee, J.-H.; Roh, S.-S.; An, H.-J. Rice Hull Extract (RHE) Suppresses Adiposity in High-Fat Diet-Induced Obese Mice and Inhibits Differentiation of 3T3-L1 Preadipocytes. Nutrients 2019, 11, 1162. https://doi.org/10.3390/nu11051162
Kim G-H, Ju J-Y, Chung K-S, Cheon S-Y, Gil T-Y, Cominguez DC, Cha Y-Y, Lee J-H, Roh S-S, An H-J. Rice Hull Extract (RHE) Suppresses Adiposity in High-Fat Diet-Induced Obese Mice and Inhibits Differentiation of 3T3-L1 Preadipocytes. Nutrients. 2019; 11(5):1162. https://doi.org/10.3390/nu11051162
Chicago/Turabian StyleKim, Ga-Hee, Jae-Yun Ju, Kyung-Sook Chung, Se-Yun Cheon, Tae-Young Gil, Divina C. Cominguez, Yun-Yeop Cha, Jong-Hyun Lee, Seong-Soo Roh, and Hyo-Jin An. 2019. "Rice Hull Extract (RHE) Suppresses Adiposity in High-Fat Diet-Induced Obese Mice and Inhibits Differentiation of 3T3-L1 Preadipocytes" Nutrients 11, no. 5: 1162. https://doi.org/10.3390/nu11051162
APA StyleKim, G.-H., Ju, J.-Y., Chung, K.-S., Cheon, S.-Y., Gil, T.-Y., Cominguez, D. C., Cha, Y.-Y., Lee, J.-H., Roh, S.-S., & An, H.-J. (2019). Rice Hull Extract (RHE) Suppresses Adiposity in High-Fat Diet-Induced Obese Mice and Inhibits Differentiation of 3T3-L1 Preadipocytes. Nutrients, 11(5), 1162. https://doi.org/10.3390/nu11051162







