Satiating Effect of a Ketogenic Diet and Its Impact on Muscle Improvement and Oxidation State in Multiple Sclerosis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Statistical Analysis
2.3. Procedure
2.4. Intervention
2.5. Measurements
- Nutritional and dietary anamnesis: Just before starting the study, a nutritional and dietary anamnesis was carried out for each patient with the Food Frequency Questionnaire (FFQ) [44]. This tool made it possible to determine how often foods belonging to different groups were consumed: dairy products, vegetables, fruits, juices, nuts, meat, fish, seafood, eggs, tubers, rice, legumes, pasta, cold meats and sausages, snacks, pastries and biscuits, chocolate bars, soft drinks, fermented alcohol and distilled alcohol.
- Body composition: Measurements related to weight, height, skin folds and body perimeters and diameters were taken using the Faulkner method, taking into account the protocol currently established by The International Society for the Advancement of Kinanthropometry (ISAK). Furthermore, we collaborated with an ISAK level 2 certified anthropometrist [45]. A portable clinical scale, SECA model, with a 150–200 kg capacity and 100 g precision was used, a stadiometer, model SECA 220 Hamburg, Germany with 0.1 cm precision, a mechanical skinfold caliper, model Holtain LTD Crymych UK with a 0.2 mm precision and measurement range from 0 to 48 mm, a dermographic pencil, a metal, inextensible and narrow anthropometric tape, model Lufkin W606PM with 0.2 mm precision and a bicondylar pachymeter to measure the diameter of small bones, model Holtain, with 1 mm precision and measuring range from 0 to 48 mm.
- Blood test and marker analysis: A blood test was carried out at 9 a.m. on an empty stomach, then the serum was separated from the plasma after centrifuging the samples. The BHB levels were measured with a commercial kit (Randox Laboratories, Crumlin, UK) and PON1 activity by using 4-Nitrophenyl acetate [46]. In both cases, an automated clinical biochemistry analyser (Olympus A 400, Tokyo, Japan) was used. Ghrelin was quantified by means of a commercial method, ELISA kit (BioVendor, Asheville, NC, USA).
- Appetite Assessment: Participants completed an appetite questionnaire for one whole day, immediately before eating a meal and again after 2 h (breakfast, lunch and dinner). The appetite questionnaire used a visual analogue scale (VAS), which has been found to be a reliable and valid tool for appetite assessment [47]. This questionnaire included eight questions followed by 10 cm horizontal lines, where 0 represented “sensation not felt at all” and 10 represented “sensation strongly felt”. Subjects were asked to mark the line at the point which corresponded to how they were feeling at that particular time. The questionnaire was developed by Parker et al. [48] to assess human appetite. The distance from the beginning of the line to the participants’ mark was measured from the left-hand side.
2.6. Ethical Concerns
3. Results
3.1. Dietary Habits of the Study Population
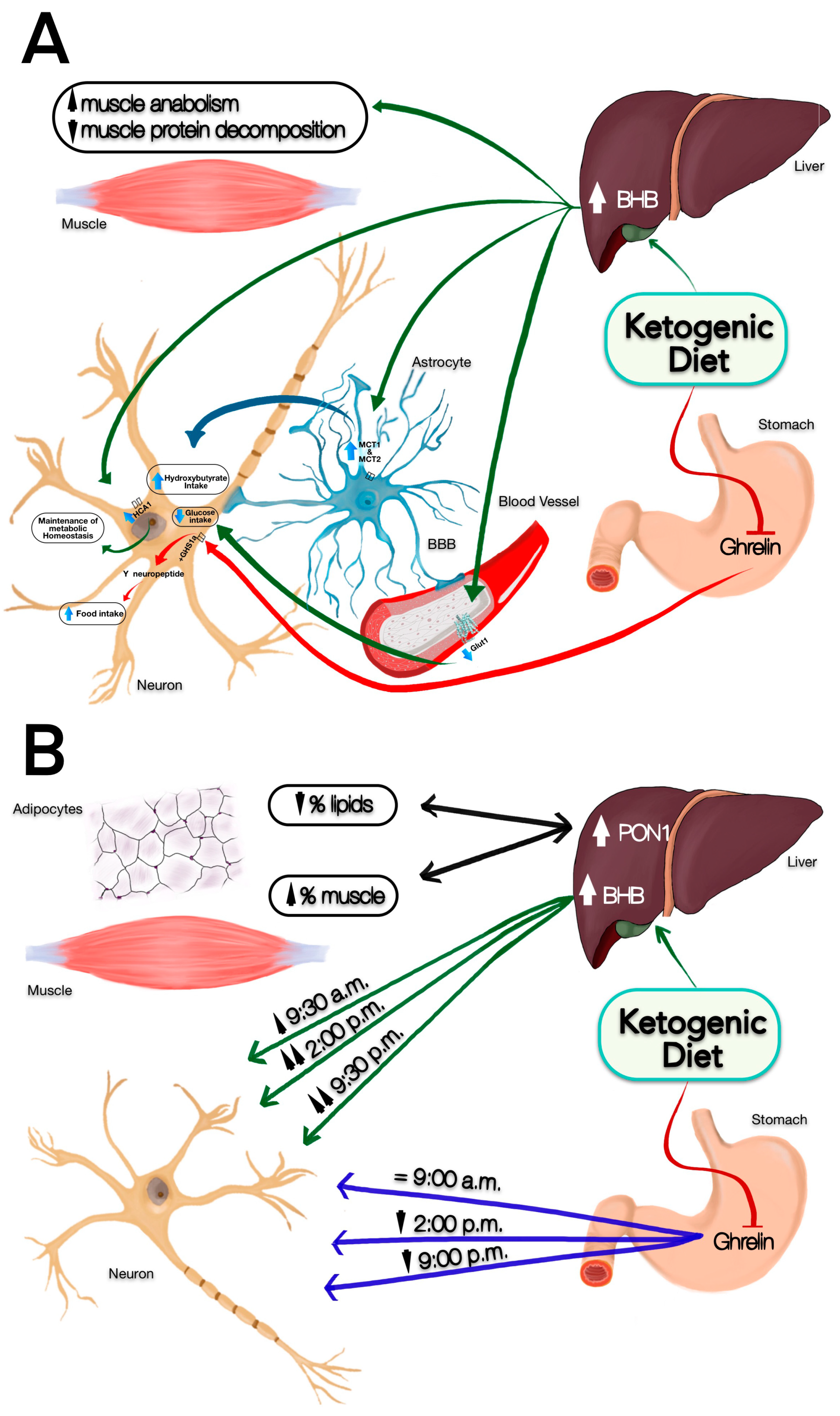
3.2. Change in Satiety Perception and BHB Production
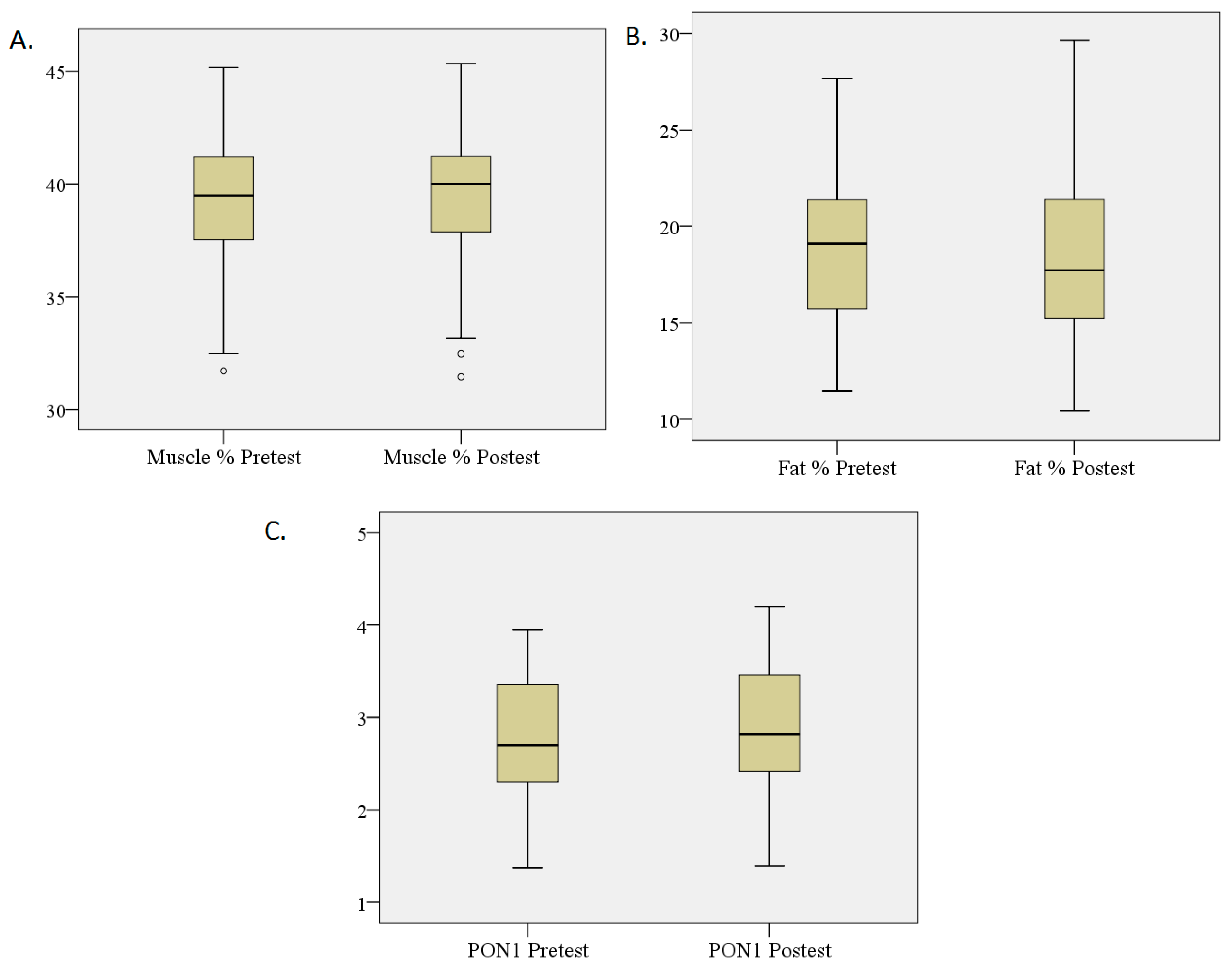
3.3. Percentage Changes in Fat and Muscle and PON1 Levels
3.4. Changes in Hunger Perception and Ghrelin Secretion
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferguson, B.; Matyszak, M.K.; Esiri, M.M.; Perry, V.H. Axonal damage in acute multiple sclerosis lesions. Brain 1997, 120, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Peterson, J.; Ransohoff, E.M.; Rudick, R.A.; Mörk, S.; Bö, L. Axonal transection in the lesions of Multiple Sclerosis. N. Engl. J. Med. 1998, 5, 278–285. [Google Scholar] [CrossRef]
- Bitsch, A.; Schuchardt, J.; Bunkowski, S.; Kuhlmann, T.; Brück, W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 2000, 6, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Kumleh, H.H.; Riazi, G.H.; Houshmand, M.; Sanati, M.H.; Gharagozli, K.; Shafa, M. Complex I deficiency in Persian multiple sclerosis patients. J. Neurol. Sci. 2006, 243, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Kent-Braun, J.A.; Ng, A.V.; Castro, M.; Weiner, M.W.; Gelinas, D.; Dudley, G.A.; Miller, R.G. Strength skeletal muscle composition and enzyme activity in multiple sclerosis. J. Appl. Physiol. 1997, 83, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Stafstrom, C.E.; Rho, J.M. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front. Pharmacol. 2012, 3, 59. [Google Scholar] [CrossRef] [PubMed]
- Deng-Bryant, Y.; Prins, M.L.; Hovda, D.A.; Harris, N.G. Ketone diet prevents alterations in brain metabolism in Young but not adult rats after traumatic brain injury. J. Neurotrauma 2011, 28, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Yang, X.; An, L.; Gao, B.; Liu, X.; Liu, S. Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson’s disease. Brain Res. 2009, 1286, 25–31. [Google Scholar] [CrossRef] [PubMed]
- de la Rubia Ortí, J.E.; García-Pardo, M.P.; Drehmer, E.; Sancho Cantus, D.; Julián Rochina, M.; Aguilar, M.A.; Hu Yang, I. Improvement of Main Cognitive Functions in Patients with Alzheimer’s Disease after Treatment with Coconut Oil Enriched Mediterranean Diet: A Pilot Study. J. Alzheimers Dis. 2018, 2, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.R.; Hernandez, C.M.; Campos, K.; Truckenbrod, L.; Federico, Q.; Moon, B.; McQuail, J.A.; Maurer, A.P.; Bizon, J.L.; Burke, S.N. A ketonic Diet Improves Cognition and Has Biochemical Effects in Prefrontal Cortex That Are Dissociable from Hippocampus. Front. Aging Neurosci. 2018, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.A.; Barkhof, F.; De Luca, J.; Frisén, J.; Geurts, J.J.G.; Hulst, H.E.; Sastre-Garriga, J.; Filippi, M.; MAGNIMS Study Group. The hippocampus in multiple sclerosis. Lancet Neurol. 2018, 17, 918–926. [Google Scholar] [CrossRef]
- Jaeger, S.; Paul, F.; Scheel, M.; Brandt, A.; Heine, J.; Pach, D.; Witt, C.M.; Bellmann-Strobl, J.; Finke, C. Multiple sclerosis-related fatigue: Altered resting-state functional connectivity of the ventral striatum and dorsolateral prefrontal cortex. Mult. Scler. 2019, 4, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. B-Hydroxybutyrate: A Signaling Metabolite. Anuu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef]
- Nair, K.S.; Welle, S.L.; Halliday, D.; Campbell, R.G. Effect of beta-hydroxybutyrate on whole-body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. J. Clin. Investig. 1988, 82, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J. Physiol. 2017, 595, 2857–2871. [Google Scholar] [CrossRef]
- Thompson, J.R.; Wu, G. The effect of ketone bodies on nitrogen metabolism in skeletal muscle. Comp. Biochem. Physiol. B 1991, 100, 209–216. [Google Scholar] [CrossRef]
- Thomsen, H.H.; Ritting, N.; Johannsen, M.; Moller, A.B.; Jorgensen, J.O.; Jessen, N.; Møller, N. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: Anticatabolic impact of keton bodies. Am. J. Clin. Nutr. 2018, 108, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Lange, D.J.; Voustianiouk, A.; MacGrogan, D.; Ho, L.; Suth, J. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006, 7, 29. [Google Scholar]
- Krotkiewski, M. Value of VLCD supplementation with medium chain triglycerides. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1393–1400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, H.R.; Kim, J.; Lim, H.; Park, Y.K. Two-Week Exclusive Supplementation of Modified Ketogenic Nutrition Drink Reserves Lean Body Mass and Improves Blood Lipid Profile in Obese Adults: A Randomized Clinical Trial. Nutrients 2018, 10, 1895. [Google Scholar] [CrossRef] [PubMed]
- Le Foll, C.; Dunn-Meynell, A.A.; Miziorko, H.M.; Levin, B.E. Regulation of hypothalamic neuronal sensing and food intake by ketone bodies and fatty acids. Diabetes 2014, 63, 1259–1269. [Google Scholar] [CrossRef]
- Bueno, N.B.; de Melo, I.S.; de Oliveira, S.L.; da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomized controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef]
- Paoli, A. Ketogenic diet for obesity: Friend or foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef]
- Pérez Guisado, J. Las dietas cetogénicas: Beneficios adicionales a la pérdida de peso y efectos secundarios infundados. Archivos Latinoamericanos de Nutrición 2008, 58, 223–229. [Google Scholar]
- Johnstone, A.M.; Murison, S.D.; Duncan, J.S.; Rance, K.A.; Speakman, J.R. Factors influencing variation in basal metabolic rate include fat-freemass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am. J. Clin. Nutr. 2005, 82, 941–948. [Google Scholar] [CrossRef]
- Volek, J.S.; Phinney, S.D.; Forsythe, C.E.; Quann, E.E.; Wood, R.J.; Puglisi, M.J.; Kraemer, W.J.; Bibus, D.M.; Fernandez, M.L.; Feinman, R.D. Carbohydrate restriction hasa more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009, 44, 297–309. [Google Scholar] [CrossRef]
- Ettinger, S. Macronutrimentos: Carbohidratos, proteínas y lípidos. In Nutrición y Dietoterapia de Krause; Mahan, L.K., Escott-Stump, S., Eds.; McGraw-Hill Interamericana: México, México, 2001; pp. 46–59. [Google Scholar]
- Di Nicol Antonio, J.J.; O’Keefe, J.H. Good Fats versus Bad Fats: A Comparison of Fatty Acids in the Promotion of Insulin Resistance, Inflammation, and Obesity. Mo. Med. 2017, 114, 303–307. [Google Scholar]
- Paoli, A.; Bianco, A.; Grimaldi, K.A.; Lodi, A.; Bosco, G. Long Term Successful Weight Loss with a Combination Biphasic Ketogenic Mediterranean Diet and Mediterranean Diet Maintenance Protocol. Nutrients 2013, 5, 5205–5217. [Google Scholar] [CrossRef]
- Rebello, C.J.; O’Neil, C.E.; Greenway, F.L. Dietary fiber and satiety: The effects of oats on satiety. Nutr. Rev. 2016, 2, 131–147. [Google Scholar] [CrossRef]
- Santos-Hernández, M.; Miralles, B.; Amigo, L.; Recio, I. Intestinal Signaling of Proteins and Digestion-Derived Products Relevant to Satiety. J. Agric. Food Chem. 2018, 39, 10123–10131. [Google Scholar] [CrossRef]
- Wang, X.T.; Dvorak, R.D. Sweet future fluctuating blood glucose levels affect future discounting. Psychol. Sci. 2010, 21, 183–188. [Google Scholar] [CrossRef]
- Luo, S.; Monterosso, J.R.; Sarpelleh, K.; Page, K.A. Differential effects of fructose versus glucose on brain and appetitive responses to food cues and decisions for food rewards. Proc. Natl. Acad. Sci. USA 2015, 20, 6509–6514. [Google Scholar] [CrossRef]
- Tébar Massó, F.J.; Garaulet Aza, M. Regulación del apetito: Nuevos conceptos. Rev. Esp. Obes. 2003, 1, 13–20. [Google Scholar]
- Vásquez-Machado, M.; Ulate-Montero, G. Regulación del peso corporal y del apetito. Acta Méd. Costarric. 2010, 52, 79–89. [Google Scholar]
- Date, Y.; Kojima, M.; Hosoda, H.; Sawaguchi, A.; Mondal, M.S.; Suganuma, T. Ghrelin a novel growth hormone-releasingacylatedpeptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000, 141, 4255–4261. [Google Scholar] [CrossRef]
- Banks, W.A.; Tschop, M.; Robinson, S.M.; Heiman, M.L. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J. Pharmacol. Exp. Ther. 2002, 302, 822–827. [Google Scholar] [CrossRef]
- Nakazato, N.; Murakami, D.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Cyranka, M.; Clarke, K.; de Wet, H.A. Ketone Ester Drink Lowers Human Ghrelin and Appetite. Obesity (Silver Spring) 2018, 26, 269–273. [Google Scholar] [CrossRef]
- Anuradha, C.V.; Balakrishnan, S.D. Increased lipoprotein susceptibility to oxidation following long distance running in trained subjects. Clin. Chim. Acta 1998, 271, 97–103. [Google Scholar] [CrossRef]
- Weinberg, F.; Chandel, N.S. Reactive oxygen species-dependent signaling regulates cancer. Cell. Mol. Life Sci. 2009, 66, 3663–3673. [Google Scholar] [CrossRef]
- Gershuni, V.M.; Yan, S.L.; Medici, V. Nutritional ketosis for weight management and reversal of metabolic syndrome. Curr. Nutr. Rep. 2018, 7, 97–106. [Google Scholar] [CrossRef]
- Kappelle, P.J.; de Boer, J.F.; Perton, F.G.; Annema, W.; de Vries, R.; Dullaart, R.P. Increased LCAT activity and hyperglycemia decrease the antioxidative functionality of HDL. Eur. J. Clin. Investig. 2012, 42, 487–495. [Google Scholar] [CrossRef]
- Trinidad-Rodriguez, I.; Fernández-Ballart, J.; Cucó-Pastor, G.; Biarnès-Jordà, E.; Arija-Val, V. Validación de un cuestionario de frecuencia de consumo corto: Reproducibilidad y validez. Nutr. Hosp. 2008, 3, 242–252. [Google Scholar]
- Wood, R. International Society for the Advancement of Kinanthropometry (ISAK). Topend Sports Website. 2008. Available online: https://www.topendsports.com/testing/isak.htm (accessed on 25 March 2019).
- Ceron, J.J.; Tecles, F.; Tvarijonaviciute, A. Serumparaoxonase 1 (PON1) measurement: Anupdate. BMC Vet. Res. 2014, 10, 74. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scalesin assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Parker, B.A.; Sturm, K.; MacIntosh, C.G.; Feinle, C.; Horowitz, M.; Chapman, I.M. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur. J. Clin. Nutr. 2004, 2, 212–218. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Harp, M.A.; McCully, K.K.; Moldavskiy, M.; Backus, D. Skeletal muscle mitochondrial capacity in people with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Rudick, R.A.; Cutter, G.; Reingold, S. The multiple sclerosis functional composite: A new clinical outcome measure for multiple sderosis trials. Mult. Scler. 2002, 8, 359–365. [Google Scholar] [CrossRef]
- Lou-Bonafonte, J.M.; Gabás-Rivera, C.; Navarro, M.A.; Osada, J. PON1 and Mediterranean Diet. Nutrients 2015, 7, 4068–4092. [Google Scholar] [CrossRef]
- Nazarewicz, R.R.; Ziolkowski, W.; Vaccaro, P.S.; Ghafourifar, P. Effect of short-term ketogenic diet on redox status of human blood. Rejuvenation Res. 2007, 10, 435–440. [Google Scholar] [CrossRef]
- Hyun-seung, R.; Su-Youn, C.; Hee-Tae, R. The effects of ketogenic diet on oxidative stress and antioxidative capacity markers of Taekwondo athletes. J. Exerc. Rehabil. 2014, 10, 362–366. [Google Scholar]
- Bough, K.J.; Wetherington, J.; Hassel, B.; Pare, J.F.; Gawryluk, J.W.; Greene, J.G.; Shaw, R.; Smith, Y.; Geiger, J.D.; Dingledine, R.J. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 2006, 60, 223–235. [Google Scholar] [CrossRef]
- Ahola-Erkkila, S.; Carroll, C.J.; Peltola-Mjösund, K.; Tulkki, V.; Mattilda, I.; Seppänen-Laakso, T.; Orešič, M.; Tyynismaa, H.; Suomalainen, A. Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum. Mol. Genet. 2010, 19, 1974–1984. [Google Scholar] [CrossRef]
- Davis, J. Huger, ghrelin and the gut. Brain Res. 2018, 15, 154–158. [Google Scholar] [CrossRef]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Ketosis and appetite-mediating nutrients and hormones after weight loss. Eur. J. Clin. Nutr. 2013, 67, 759–764. [Google Scholar] [CrossRef]
- Ratliff, J.; Mutungi, G.; Puglisi, M.J.; Volek, J.S.; Fernandez, M.L. Carbohydrate restriction (with or without additional dietary cholesterol provided by eggs) reduces insulin resistance and plasma leptin without modifying appetite hormones in adult men. Nutr. Res. 2009, 29, 262–268. [Google Scholar] [CrossRef]
- Mathur, D.; López-Rodas, G.; Casanova, B.; Burgal Marti, M. Perturbed Glucose Metabolism: Insights into Multiple Sclerosis Pathogenesis. Front. Neurol. 2014, 5, 250. [Google Scholar] [CrossRef]
- Keaney, J.F., Jr.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef]
- Wellen, K.E.; Thompson, C.B. Cellular metabolic stress: Considering how cells respond to nutrient excess. Mol. Cell 2010, 40, 323–332. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Ludwig, D.S. The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002, 18, 2414–2423. [Google Scholar] [CrossRef]
- Ball, S.D.; Keller, K.R.; Moyer-Mileur, L.J.; Ding, Y.W.; Donaldson, D.; Jackson, W.D. Prolongation of satiety after low versus moderately high glycemic index meals in obese adolescents. Pediatrics 2003, 3, 488–494. [Google Scholar] [CrossRef]
- Lennerz, B.S.; Alsop, D.C.; Holsen, L.M.; Stern, E.; Rojas, R.; Ebbeling, C.B.; Goldstein, J.M.; Ludwig, D.S. Effects of dietary glycemic index on brain regions related to reward and craving in men. Am. J. Clin. Nutr. 2013, 3, 641–647. [Google Scholar] [CrossRef]
- Cecil, J.E.; Francis, J.; Read, N.W. Comparison of the effects of a high-fat and high-carbohydrate soup delivered orally and intragastrically on gastric emptying, appetite, and eating behaviour. Physiol. Behav. 1999, 2, 299–306. [Google Scholar] [CrossRef]
- Latner, J.D.; Schwartz, M. The effects of a high-carbohydrate, high-protein or balanced lunch upon later food intake and hunger ratings. Appetite 1999, 1, 119–128. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Lemmens, S.G.; Westerterp, K.R. Dietary protein—Its role in satiety, energetics, weight loss and health. Br. J. Nutr. 2012, 2, 105–112. [Google Scholar] [CrossRef]
- Nielsen, L.V.; Kristensen, M.D.; Klingenberg, L.; Ritz, C.; Belza, A.; Astrup, A.; Raben, A. Protein from Meat or Vegetable Sources in Meals Matched for Fiber Content has Similar Effects on Subjective Appetite Sensations and Energy Intake-A Randomized Acute Cross-Over Meal Test Study. Nutrients 2018, 10, 96. [Google Scholar] [CrossRef]
| Measure | Frequency | % | |
|---|---|---|---|
| MS Type | Primary progressive MS | 1 | 3.7% |
| Relapsing-remitting MS | 20 | 74.1% | |
| Secondary progressive MS | 6 | 22.2% | |
| Gender | Men | 5 | 18.5% |
| Women | 22 | 81.5% | |
| Mean | SD | ||
| Age (years) | 44.56 | 11.27 | |
| Time from MS Diagnosis (years) | 12 | 10 | |
| Measure | Mean | SD |
|---|---|---|
| No. of meals a day | 4.00 | 0.83 |
| No. of monthly intakes of main nutrients | ||
| Dairy | 16.81 | 12.89 |
| Cheeses | 11.37 | 9.86 |
| Dairy desserts | 0.78 | 2.49 |
| Vegetables | 19.26 | 8.81 |
| Fruit | 22.67 | 8.52 |
| Juice | 9.93 | 12.02 |
| Nuts | 14.89 | 10.23 |
| Meat | 12.37 | 6.97 |
| Fish | 8.07 | 4.59 |
| Seafood | 3.37 | 3.25 |
| Eggs | 10.22 | 5.24 |
| Tubers | 10.22 | 5.56 |
| Rice | 7.56 | 3.39 |
| Legumes | 6.22 | 4.59 |
| Pasta | 6.52 | 4.17 |
| Cold meats | 14.74 | 10.66 |
| Snacks | 2.89 | 3.89 |
| Pastries | 12.41 | 11.19 |
| Chocolate bars | 10.19 | 10.50 |
| Soft drinks | 5.81 | 8.87 |
| Fermented alcohol | 6.15 | 7.39 |
| Distilled alcohol | 0.15 | 0.46 |
| Measure | Pre-Test | Post-Test | Z | p | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Before breakfast satiety | 4.44 | 3.06 | 5.15 | 2.63 | –1.143 | 0.253 |
| After breakfast satiety | 5.38 | 2.76 | 6.64 | 2.76 | –1.480 | 0.139 |
| Before lunch satiety | 3.26 | 3.16 | 6.22 | 2.58 | –3.387 | 0.001 * |
| After lunch satiety | 4.56 | 2.75 | 8.07 | 1.72 | –3.802 | 0.000 * |
| Before dinner satiety | 3.90 | 2.92 | 5.80 | 2.71 | –2.800 | 0.005 * |
| After dinner satiety | 5.46 | 2.13 | 7.89 | 2.15 | –3.876 | 0.000 * |
| BHB (Mmol/L) | 0.06 | 0.04 | 0.10 | 0.10 | –2.005 | 0.045 * |
| Fat % | 19.53 | 3.78 | 17.74 | 3.32 | –4.421 | 0.000 * |
| Muscle % | 39.39 | 2.88 | 40.22 | 2.86 | –2.955 | 0.003 * |
| PON1 (UI/L) | 2.67 | 0.62 | 2.92 | 0.68 | –3.722 | 0.000 * |
| Hunger before breakfast | 3.27 | 2.17 | 3.14 | 3.35 | –0.622 | 0.534 |
| Hunger after breakfast | 2.88 | 2.38 | 2.25 | 1.93 | –1.677 | 0.094 |
| Hunger before lunch | 6.46 | 2.13 | 2.15 | 2.37 | –4.306 | 0.000 * |
| Hunger after lunch | 5.38 | 2.27 | 1.02 | 1.80 | –4.346 | 0.000 * |
| Hunger before dinner | 5.59 | 2.24 | 2.54 | 2.99 | –4.077 | 0.000 * |
| Hunger after dinner | 3.82 | 2.66 | 0.91 | 1.71 | –3.744 | 0.000 * |
| Ghrelin (pg/mL) | 24.04 | 36.75 | 24.97 | 48.94 | –0.216 | 0.829 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benlloch, M.; López-Rodríguez, M.M.; Cuerda-Ballester, M.; Drehmer, E.; Carrera, S.; Ceron, J.J.; Tvarijonaviciute, A.; Chirivella, J.; Fernández-García, D.; de la Rubia Ortí, J.E. Satiating Effect of a Ketogenic Diet and Its Impact on Muscle Improvement and Oxidation State in Multiple Sclerosis Patients. Nutrients 2019, 11, 1156. https://doi.org/10.3390/nu11051156
Benlloch M, López-Rodríguez MM, Cuerda-Ballester M, Drehmer E, Carrera S, Ceron JJ, Tvarijonaviciute A, Chirivella J, Fernández-García D, de la Rubia Ortí JE. Satiating Effect of a Ketogenic Diet and Its Impact on Muscle Improvement and Oxidation State in Multiple Sclerosis Patients. Nutrients. 2019; 11(5):1156. https://doi.org/10.3390/nu11051156
Chicago/Turabian StyleBenlloch, María, María Mar López-Rodríguez, María Cuerda-Ballester, Eraci Drehmer, Sandra Carrera, Jose Joaquin Ceron, Asta Tvarijonaviciute, Javier Chirivella, David Fernández-García, and Jose Enrique de la Rubia Ortí. 2019. "Satiating Effect of a Ketogenic Diet and Its Impact on Muscle Improvement and Oxidation State in Multiple Sclerosis Patients" Nutrients 11, no. 5: 1156. https://doi.org/10.3390/nu11051156
APA StyleBenlloch, M., López-Rodríguez, M. M., Cuerda-Ballester, M., Drehmer, E., Carrera, S., Ceron, J. J., Tvarijonaviciute, A., Chirivella, J., Fernández-García, D., & de la Rubia Ortí, J. E. (2019). Satiating Effect of a Ketogenic Diet and Its Impact on Muscle Improvement and Oxidation State in Multiple Sclerosis Patients. Nutrients, 11(5), 1156. https://doi.org/10.3390/nu11051156








