Frequency and Quantity of Egg Intake Is Not Associated with Dyslipidemia: The Hellenic National Nutrition and Health Survey (HNNHS)
Abstract
1. Introduction
2. Methods
2.1. Study Design and Subjects
2.2. Clinical, Dietary and Anthropometric Data
2.3. Egg Quantification
2.4. Egg Frequency
2.5. Egg frequency X Quantity
2.6. Clinical and Anthropometrical Data
2.7. Data Management
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: A comparative risk assessment. Lancet Diabetes Endocrinol. 2014, 2, 634–647. [Google Scholar] [CrossRef]
- European Heart Network. European Cardiovascular Disease Statistics. 2017. Available online: http://www.ehnheart.org/ (accessed on 15 March 2019).
- Liu, K.; Daviglus, M.L.; Loria, C.M.; Colangelo, L.A.; Spring, B.; Moller, A.C.; Lloyd-Jones, D.M. Healthy Lifestyle Through Young Adulthood and the Presence of Low Cardiovascular Disease Risk Profile in Middle Age: The Coronary Artery Risk Development in (Young) Adults (CARDIA) Study. Circulation 2012, 125, 996–1004. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. J. Prev. Cardiol. 2016, 23, NP1–NP96. [Google Scholar]
- Stamler, J.; Stamler, R.; Neaton, J.D.; Wentworth, D.; Daviglus, M.L.; Garside, D.; Dyer, A.R.; Liu, K.; Greenland, P. Low Risk-Factor Profile and Long-term Cardiovascular and Noncardiovascular Mortality and Life Expectancy. JAMA 1999, 282, 2012–2018. [Google Scholar] [CrossRef]
- Stamler, J.; Wentworth, D.; Neaton, J.D. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA 1986, 256, 2823–2828. [Google Scholar] [CrossRef]
- Stamler, J.; Daviglus, M.L.; Garside, D.B.; Dyer, A.R.; Greenland, P.; Neaton, J.D. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 2000, 284, 311–318. [Google Scholar] [CrossRef]
- USDA Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Available online: https://health.gov/dietaryguidelines/2015-scientific-report. (accessed on 10 March 2019).
- Rong, Y.; Chen, L.; Zhu, T.; Song, Y.; Yu, M.; Shan, Z.; Sands, A.; Hu, F.B.; Liu, L. Egg consumption and risk of coronary heart disease and stroke: Dose-response meta-analysis of prospective cohort studies. BMJ 2013, 346, e8539. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Cayssials, V.; Cleries, R.; Redondo, M.L.; Sánchez, M.J.; Rodríguez-Barranco, M.; Sánchez-Cruz, J.J.; Mokoroa, O.; Gil, L.; Amiano, P.; et al. Moderate egg consumption and all-cause and specific-cause mortality in the Spanish European Prospective into Cancer and Nutrition (EPIC-Spain) study. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef]
- Kanter, M.M.; Kris-Etherton, P.M.; Fernandez, M.L.; Vickers, K.C.; Katz, D.L. Exploring the Factors That Affect Blood Cholesterol and Heart Disease Risk: Is Dietary Cholesterol as Bad for You as History Leads Us to Believe? Adv. Nutr. 2012, 3, 711–717. [Google Scholar] [CrossRef]
- Díez-Espino, J.; Basterra-Gortari, F.J.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Schröder, H.; Estruch, R.; Ros, E.; Gómez-Gracia, E.; Arós, F.; et al. Egg consumption and cardiovascular disease according to diabetic status: The PREDIMED study. Clin. Nutr. 2017, 36, 1015–1021. [Google Scholar] [CrossRef]
- Clayton, Z.S.; Fusco, E.; Kern, M. Egg consumption and heart health: A review. Nutrition 2017, 37, 79–85. [Google Scholar] [CrossRef]
- Spence, J.D.; Jenkins, D.J.; Davignon, J. Dietary cholesterol and egg yolks: Not for patients at risk of vascular disease. Can. J. Cardiol. 2010, 26, e336–e339. [Google Scholar] [CrossRef]
- Alexander, D.D.; Miller, P.E.; Vargas, A.J.; Weed, D.L.; Cohen, S.S. Meta-analysis of Egg Consumption and Risk of Coronary Heart Disease and Stroke. J. Am. Coll. Nutr. 2016, 35, 704–716. [Google Scholar] [CrossRef]
- Subramanian, S.; Han, C.Y.; Chiba, T.; McMillen, T.S.; Wang, S.A.; Haw, I.I.I.; Kirk, E.A.; O’Brien, K.D.; Chait, A. Dietary Cholesterol Worsens Adipose Tissue Macrophage Accumulation and Atherosclerosis in Obese LDL Receptor-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 685–691. [Google Scholar] [CrossRef]
- Djousse, L.; Gaziano, J.M. Egg consumption in relation to cardiovascular disease and mortality: The Physicians’ Health Study. Am. J. Clin. Nutr. 2008, 87, 964–969. [Google Scholar] [CrossRef]
- Magriplis, E.; Dimakopoulos, I.; Karageorgou, D.; Mitsopoulou, A.V.; Bakogianni, I.; Micha, R.; Michas, G.; Ntouroupi, T.; Tsaniklidou, S.M.; Argyri, K.; et al. Aims, design and preliminary findings of the Hellenic National Nutrition and Health Survey (HNNHS). BMC Med. Res. Methodol. 2019, 19, 37. [Google Scholar] [CrossRef]
- US Department of Agriculture. AMPM. USDA Automated Multiple-Pass Method. Available online: https://www.ars.usda.gov/northeast-area/beltsville457md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research458group/docs/ampm-usda-automated-multiple-pass-method/ (accessed on 20 April 2019).
- Karageorgou, D.; Magriplis, E.; Mitsopoulou, A.V.; Dimakopoulos, I.; Bakogianni, I.; Micha, R.; Michas, G.; Chourdakis, M.; Ntouroupi, T.; Tsaniklidou, S.M.; et al. Dietary patterns and lifestyle characteristics in adults: Results from the Hellenic National Nutrition and Health Survey (HNNHS). Public Health 2019, in press. [Google Scholar]
- Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ). Available online: http://www.ipaq.ki.se/scoring.pdf. (accessed on 15 March 2019).
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Gray, J.; Griffin, B. Eggs and dietary cholesterol—Dispelling the myth. Nutr. Bull. 2009, 34, 66–70. [Google Scholar] [CrossRef]
- Soliman, G. Dietary Cholesterol and the Lack of Evidence in Cardiovascular Disease. Nutrients 2018, 10, 780. [Google Scholar] [CrossRef]
- Mutungi, G.; Ratliff, J.; Puglisi, M.; Torres-Gonzalez, M.; Vaishnav, U.; Leite, J.O.; Quann, E.; Volek, J.S.; Fernandez, M.L. Dietary Cholesterol from Eggs Increases Plasma HDL Cholesterol in Overweight Men Consuming a Carbohydrate-Restricted Diet. J. Nutr. 2008, 138, 272–276. [Google Scholar] [CrossRef]
- Dawber, T.R.; Nickerson, R.J.; Brand, F.N.; Pool, J. Eggs, serum cholesterol, and coronary heart disease. Am. J. Clin. Nutr. 1982, 36, 617–625. [Google Scholar] [CrossRef]
- Goldberg, S.; Gardener, H.; Tiozzo, E.; Kuen, C.Y.; Elkind, M.S.; Sacco, R.L.; Rundek, T. Egg consumption and carotid atherosclerosis in the Northern Manhattan study. Atherosclerosis 2014, 235, 273–280. [Google Scholar] [CrossRef]
- Larsson, S.C.; Åkesson, A.; Wolk, A. Egg consumption and risk of heart failure, myocardial infarction, and stroke: Results from 2 prospective cohorts. Am. J. Clin. Nutr. 2015, 102, 1007–1013. [Google Scholar] [CrossRef]
- Snowdon, D.A. Animal product consumption and mortality because of all causes combined, coronary heart disease, stroke, diabetes, and cancer in Seventh-day Adventists. Am. J. Clin. Nutr. 1988, 48 (Suppl. 3), 739–748. [Google Scholar] [CrossRef]
- Spence, J.D.; Jenkins, D.J.; Davignon, J. Egg yolk consumption and carotid plaque. Atherosclerosis 2012, 224, 469–473. [Google Scholar] [CrossRef]
- Nakamura, Y.; Okamura, T.; Tamaki, S.; Kadowaki, T.; Hayakawa, T.; Kita, Y.; Okayama, A.; Ueshima, H.; NIPPON DATA80 Research Group. Egg consumption, serum cholesterol, and cause-specific and all-cause mortality: The National Integrated Project for Prospective Observation of Non-communicable Disease and Its Trends in the Aged, 1980 (NIPPON DATA80). Am. J. Clin. Nutr. 2004, 80, 58–63. [Google Scholar] [CrossRef]
- Zhong, V.W.; Van Horn, L.; Cornelis, M.C.; Wilkins, J.T.; Ning, H.; Carnethon, M.R.; Greenland, P.; Mentz, R.J.; Tucker, K.L.; Zhao, L.; et al. Associations of Dietary Cholesterol or Egg Consumption with Incident Cardiovascular Disease and Mortality. JAMA 2019, 321, 1081–1095. [Google Scholar] [CrossRef]
- Shin, J.Y.; Xun, P.; Nakamura, Y.; He, K. Egg consumption in relation to risk of cardiovascular disease and diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 146–159. [Google Scholar] [CrossRef]
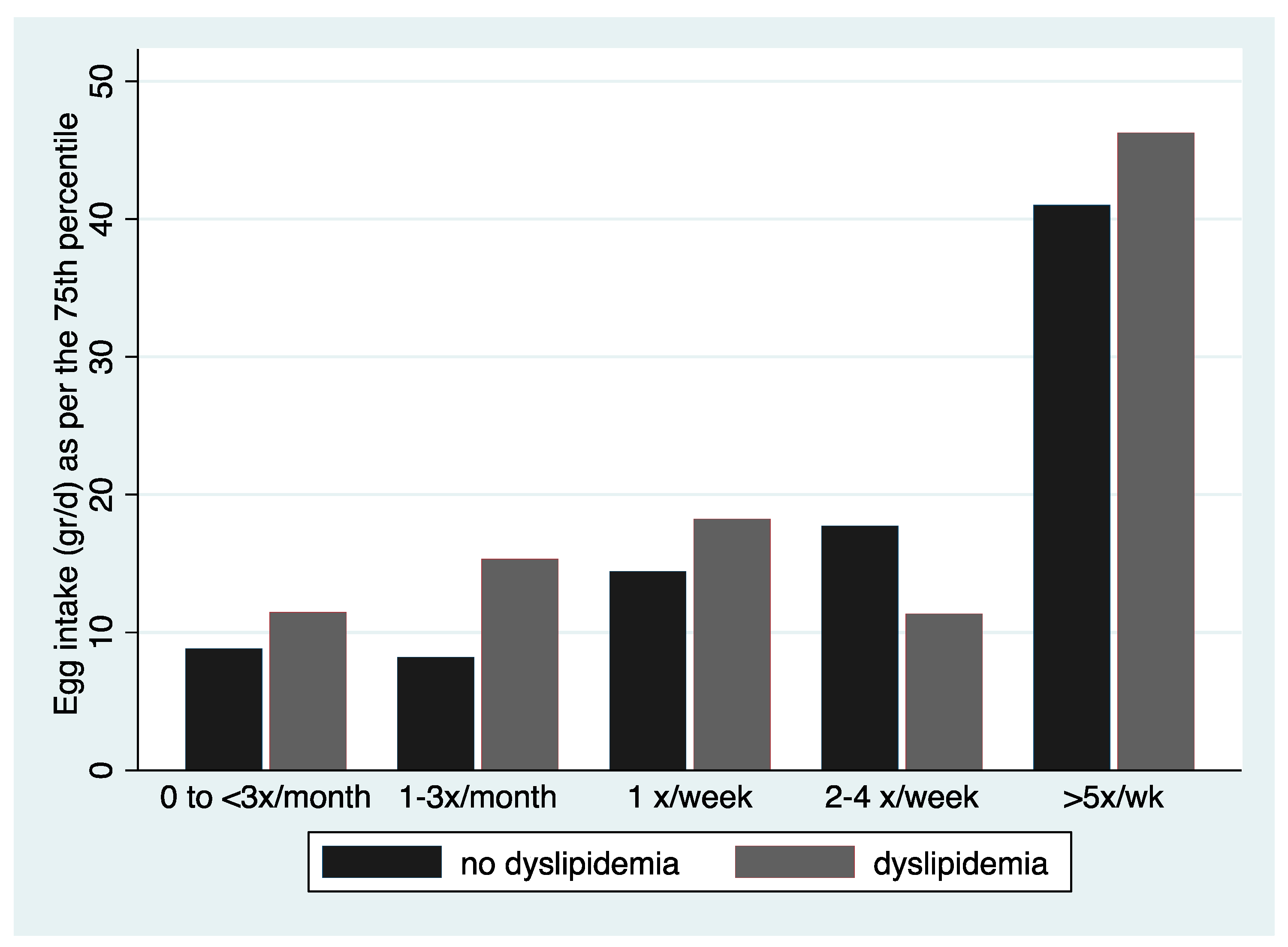
 Denotes significant difference in frequency of consumption; No overlap in CIs denotes statistically significant differences in fasted cholesterol level for those consuming eggs 1–3 times per month and 2–4 times a week or >5 times a week.
Denotes significant difference in frequency of consumption; No overlap in CIs denotes statistically significant differences in fasted cholesterol level for those consuming eggs 1–3 times per month and 2–4 times a week or >5 times a week.
 Denotes significant difference in frequency of consumption; No overlap in CIs denotes statistically significant differences in fasted cholesterol level for those consuming eggs 1–3 times per month and 2–4 times a week or >5 times a week.
Denotes significant difference in frequency of consumption; No overlap in CIs denotes statistically significant differences in fasted cholesterol level for those consuming eggs 1–3 times per month and 2–4 times a week or >5 times a week. 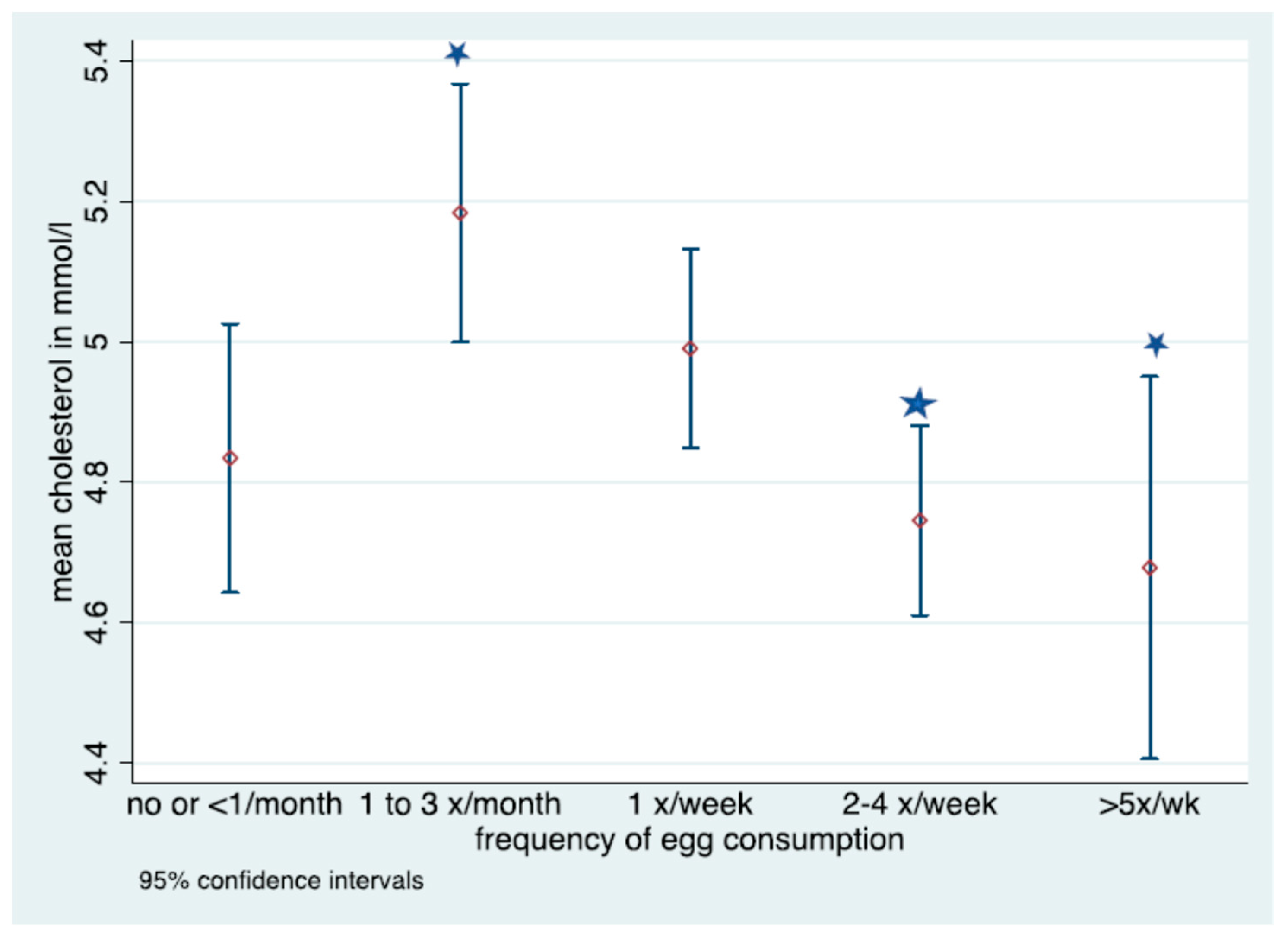
| Parameter | Component 1: Health Component | Component 2: Behavior Component |
|---|---|---|
| BMI | 0.369 | 0.325 |
| Hypertension | 0.527 | |
| Type 2 Diabetes | 0.479 | |
| Smokers | 0.513 | |
| Age | 0.513 | |
| Total Energy | 0.547 | |
| IPAQ score | −0.575 | |
| KMO = 0.776 |
| Parameter | Total (N = 3558) | No dyslipidemia (N = 2807) | Dyslipidemia (N = 751) | p–value (by lipid status) * |
| Age (years), mean (sd) | 43.9 (18.2) | 40.2 (17.1) | 57.6 (15.5) | <0.001 |
| Sex, % males | 1371 (40.3) | 1084 (40.4) | 287 (40.0) | 0.840 |
| Egg intake (grams/day), 75th–95th percentile | 13.9–55.2 | 14.4–56.2 | 12.6–54.4 | 0.289 |
| Frequency of egg consumption, n (%) | 0.001 | |||
| 0–<1 time a month** | 259 (10.7) | 192 (9.8) | 67 (14.5) | - |
| 1–3 times a month | 525 (21.6) | 411 (20.9) | 114 (24.7) | |
| 1 per week** | 789 (32.5) | 646 (32.8) | 143 (31.0) | 0.045 |
| 2–4 per week ** | 686 (28.2) | 568 (28.9) | 118 (25.5) | 0.020 |
| >5 per week ** | 172 (7.1) | 152 (7.7) | 20 (4.3) | 0.002 |
| Total Energy intake (kcal/day), mean (sd) | 1971.8 (1141.6) | 2016 (1174.4) | 1804.7 (992.7) | <0.001 |
| Total SFA (% energy), mean (sd) | 12.4 (5.1) | 12.5 (5.2) | 12.2 (4.7) | 0.216 |
| Total fiber, median (25th–75th percentile) | 17.1 (10.8–31.8) | 17.1 (10.8–33.0) | 16.6 (10.6–26.9) | 0.0296 |
| BMI (kg/m2), mean (sd) | 25.5 (4.8) | 25.0 (4.7) | 27.6 (4.7) | <0.001 |
| Educational Status (level), n (%) | <0.001 | |||
| Low | 415 (12.2) | 234 (8.7) | 181 (25.2) | |
| Medium | 1188 (35.0) | 948 (35.4) | 240 (33.4) | |
| High | 1793 (52.8) | 1496 (55.9) | 297 (41.4) | |
| Smoking status, n (%) | 0.245 | |||
| Smokers (daily or occasional) | 1147 (33.7) | 918 (34.2) | 229 (31.9) | |
| Physical activity status, n (%) | <0.001 | |||
| Sedentary | 224 (6.7) | 160 (6.1) | 64 (9.2) | |
| Low | 463 (13.9) | 338 (12.9) | 125 (17.9) | |
| Moderate | 1301 (39.1) | 1039 (39.5) | 262 (37.8) | |
| Active | 1338 (40.2) | 1092 (41.5) | 246 (35.3) | |
| Other comorbidities, n (%) | ||||
| Hypertension 1 | 561 (16.5) | 285 (10.6) | 276 (38.4) | <0.001 |
| Diabetes | 146 (4.3) | 66 (2.5) | 80 (11.2) | <0.001 |
| Population subsample | Total (N = 1051) | Males (N = 402) | Females (N = 649) | p–value for sex |
| Serum cholesterol (mmol/L), mean (sd) | 5.0 (1.1) | 4.8 (1.0) | 5.6 (1.1) | <0.001 |
| Serum HDL-c (mmol/L), mean (sd) | 1.5 (0.4) | 1.5 (0.4) | 1.4 (0.4) | 0.004 |
| Serum LDL-c (mmol/L), mean (sd) | 3.0 (0.9) | 2.8 (0.9) | 3.4 (1.0) | <0.001 |
| Fasted glucose (mmol/L), mean (sd) | 5.5 (1.0) | 5.5 (0.9) | 5.7 (1.1) | <0.001 |
| Models | Frequency of Egg Consumption | Quantity of Egg (g/day) * | Frequency × QUANTITY (g/day) * |
|---|---|---|---|
| OR (95% CI) | |||
| Presence of dyslipidemia ** | |||
| Sex adjusted | 0.83 (0.752, 0.904) | 0.91 (0.809, 1.017) | 0.87 (0.796, 0.963) |
| Multivariable 1 | 0.83 (0.755, 0.915) | 0.93 (0.831, 1.055) | 0.88 (0.801, 0.974) |
| Multivariable 2 | 0.83 (0.752, 0.911) | 0.92 (0.821, 1.041) | 0.88 (0.796, 0.968) |
| Multivariable 3 | 0.80 (0.718, 0.887) | 0.91 (0.798, 1.040) | 0.85 (0.759, 0.945) |
| Linear Regression | |||
| Models | b-coefficient (95% CI) | b-coefficient (95% CI) | b-coefficient (95% CI) |
| Cholesterol levels *** | |||
| Age & sex adjusted | −0.11 (−0.183, −0.038) | −0.10 (−0.187, −0.009) | −0.11 (−0.190, −0.044) |
| Multivariable 1 | −0.10 (−0.178, −0.031) | −0.10 (−0.190, −0.007) | −0.10 (−0.178, −0.028) |
| Multivariable 2 | −0.11 (−0.178, −0.031) | −0.10 (−0.193, −0.010) | −0.11 (−0.180, −0.031) |
| Multivariable 3 | −0.08 (−0.149, −0.009) | −0.09 (−0.175, 0.004) | −0.07 (−0.143, 0.002) |
| LDL-c levels *** | |||
| Age & sex adjusted | −0.10 (−0.163, −0.029) | −0.13 (−0.217, −0.052) | −0.12 (−0.187, −0.056) |
| Multivariable 1 | −0.09 (−0.160, −0.023) | −0.15 (−0.236, −0.067) | −0.11 (−0.182, −0.047) |
| Multivariable 2 | −0.09 (−0.160, −0.024) | −0.15 (−0.239, −0.070) | −0.12 (−0.184, −0.049) |
| Multivariable 3 | −0.07 (−0.138, −0.009) | −0.15 (−0.239, −0.070) | −0.08 (−0.147, −0.155) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magriplis, E.; Mitsopoulou, A.-V.; Karageorgou, D.; Bakogianni, I.; Dimakopoulos, I.; Micha, R.; Michas, G.; Chourdakis, M.; Chrousos, G.P.; Roma, E.; et al. Frequency and Quantity of Egg Intake Is Not Associated with Dyslipidemia: The Hellenic National Nutrition and Health Survey (HNNHS). Nutrients 2019, 11, 1105. https://doi.org/10.3390/nu11051105
Magriplis E, Mitsopoulou A-V, Karageorgou D, Bakogianni I, Dimakopoulos I, Micha R, Michas G, Chourdakis M, Chrousos GP, Roma E, et al. Frequency and Quantity of Egg Intake Is Not Associated with Dyslipidemia: The Hellenic National Nutrition and Health Survey (HNNHS). Nutrients. 2019; 11(5):1105. https://doi.org/10.3390/nu11051105
Chicago/Turabian StyleMagriplis, Emmanuella, Anastasia-Vasiliki Mitsopoulou, Dimitra Karageorgou, Ioanna Bakogianni, Ioannis Dimakopoulos, Renata Micha, George Michas, Michail Chourdakis, George P. Chrousos, Eleftheria Roma, and et al. 2019. "Frequency and Quantity of Egg Intake Is Not Associated with Dyslipidemia: The Hellenic National Nutrition and Health Survey (HNNHS)" Nutrients 11, no. 5: 1105. https://doi.org/10.3390/nu11051105
APA StyleMagriplis, E., Mitsopoulou, A.-V., Karageorgou, D., Bakogianni, I., Dimakopoulos, I., Micha, R., Michas, G., Chourdakis, M., Chrousos, G. P., Roma, E., Panagiotakos, D., Zampelas, A., HNNHS Contributors, & HNNHS Advisory Committee. (2019). Frequency and Quantity of Egg Intake Is Not Associated with Dyslipidemia: The Hellenic National Nutrition and Health Survey (HNNHS). Nutrients, 11(5), 1105. https://doi.org/10.3390/nu11051105







