Can Serum Iron Concentrations in Early Healthy Pregnancy Be Risk Marker of Pregnancy-Induced Hypertension?
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Participants
2.3. Method and Data Collection
2.4. Serum Iron Determination
2.5. Statistical Analyses
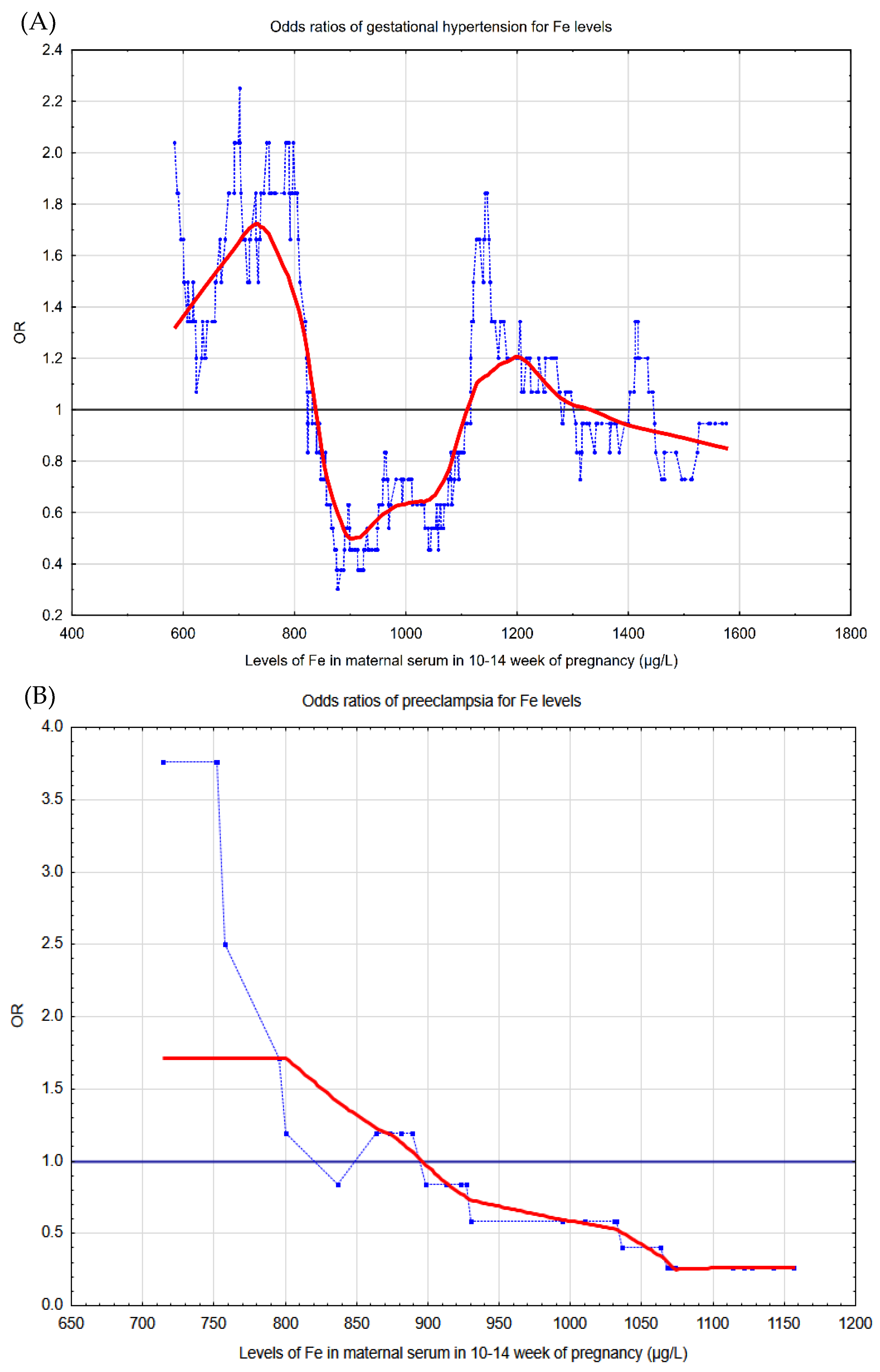
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koenig, M.D.; Tussing-Humphreys, L.; Day, J.; Cadwell, B.; Nemeth, E. Hepcidin and iron homeostasis during pregnancy. Nutrients 2014, 6, 3062–3083. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, S.U.; Pipper, C.B.; Ellervik, C.; Pociot, F.; Kyvsgaard, J.N.; Svensson, J. Association between Neonatal Whole Blood Iron Content and Cytokines, Adipokines, and Other Immune Response Proteins. Nutrients 2019, 11, 543. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef]
- Imam, M.U.; Zhang, S.; Ma, J.; Wang, H.; Wang, F. Antioxidants Mediate Both Iron Homeostasis and Oxidative Stress. Nutrients 2017, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Armon-Omer, A.; Waldman, C.; Simaan, N.; Neuman, H.; Tamir, S.; Shahien, R. New Insights on the Nutrition Status and Antioxidant Capacity in Multiple Sclerosis Patients. Nutrients 2019, 11, 427. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Sukiennicki, G.; Muszyńska, M.; Gackowski, D.; Kąklewski, K.; Durda, K.; Jaworska, K.; Huzarski, T.; Gronwald, J.; Byrski, T.; et al. Plasma micronutrients, trace elements, and breast cancer in BRCA1 mutation carriers: An exploratory study. Cancer Causes Control 2012, 23, 1065–1074. [Google Scholar] [CrossRef]
- Johnstone, D.M.; Graham, R.M.; Trinder, D.; Riveros, C.; Olynyk, J.K.; Scott, R.J.; Moscato, P.; Milward, E.A. Changes in brain transcripts related to Alzheimer’s disease in a model of HFE hemochromatosis are not consistent with increased Alzheimer’s disease risk. J. Alzheimers Dis. 2012, 30, 791–803. [Google Scholar] [CrossRef]
- Fu, S.; Li, F.; Zhou, J.; Liu, Z. The Relationship Between Body Iron Status, Iron Intake and Gestational Diabetes: A Systematic Review and Meta-Analysis. Medicine 2016, 95, e2383. [Google Scholar] [CrossRef]
- Duhig, K.; Vandermolen, B.; Shennan, A. Recent advances in the diagnosis and management of pre-eclampsia. F1000Research 2018, 7, 242. [Google Scholar] [CrossRef]
- Anton, L.; Olarerin-George, A.O.; Hogenesch, J.B.; Elovitz, M.A. Placental expression of miR-517a/b and miR-517c contributes to trophoblast dysfunction and preeclampsia. PLoS ONE 2015, 10, e0122707. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Webster, R.P. Vascular biology of preeclampsia. J. Thromb. Haemost. 2009, 7, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Ruszczynski, M.; Chmielewska, A. Effects of iron supplementation in nonanemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: A systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2010, 91, 1684–1690. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef]
- Burton, G.J.; Watson, A.L.; Hempstock, J.; Skepper, J.N.; Jauniaux, E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J. Clin. Endocrinol. Metab. 2002, 87, 2954–2959. [Google Scholar] [CrossRef]
- Mannaerts, D.; Faes, E.; Cos, P.; Briedé, J.J.; Gyselaers, W.; Cornette, J.; Gorbanev, Y.; Bogaerts, A.; Spaanderman, M.; Van Craenenbroeck, E.; et al. Oxidative stress in healthy pregnancy and preeclampsia is linked to chronic inflammation, iron status and vascular function. PLoS ONE 2018, 13, e0202919. [Google Scholar] [CrossRef]
- Liu, J.-X.; Chen, D.; Li, M.-X.; Hua, Y. Increased serum iron levels in pregnant women with preeclampsia: A meta-analysis of observational studies. J. Obstet. Gynaecol. 2019, 39, 11–16. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Ahmed, S.; Ullah, M.S.; Kabir, H.; Rahman, G.K.M.M.; Hasnat, A.; Islam, M.S. Comparative study of serum zinc, copper, manganese, and iron in preeclamptic pregnant women. Biol. Trace Elem. Res. 2013, 154, 14–20. [Google Scholar] [CrossRef]
- Basu, A.; Yu, J.Y.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Henriksen, T.; Lorentzen, B.; Garg, S.K.; Menard, M.K.; Hammad, S.M.; et al. Trace elements as predictors of preeclampsia in type 1 diabetic pregnancy. Nutr. Res. 2015, 35, 421–430. [Google Scholar] [CrossRef]
- Tande, D.L.; Ralph, J.L.; Johnson, L.K.; Scheett, A.J.; Hoverson, B.S.; Anderson, C.M. First trimester dietary intake, biochemical measures, and subsequent gestational hypertension among nulliparous women. J. Midwifery Womens Health 2013, 58, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.M.; Fernandes, V.; Matta-Coelho, C.; Paredes, S.; Pereira, M.L.; Marques, O.; Alves, M. Iron Deficiency and Obesity–Are we Diagnosing with Appropriate Indicators? Acta Med. Port. 2018, 31, 478–482. [Google Scholar] [CrossRef]
- Chełchowska, M.; Ambroszkiewicz, J.; Gajewska, J.; Jabłońska-Głąb, E.; Maciejewski, T.M.; Ołtarzewski, M. Hepcidin and Iron Metabolism in Pregnancy: Correlation with Smoking and Birth Weight and Length. Biol. Trace Elem. Res. 2016, 173, 14–20. [Google Scholar] [CrossRef]
- Tan, M.Y.; Wright, D.; Syngelaki, A.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; Greco, E.; Wright, A.; Maclagan, K.; et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: Results of SPREE. Ultrasound Obstet. Gynecol. 2018, 51, 743–750. [Google Scholar] [CrossRef]
- Rayman, M.P.; Barlis, J.; Evans, R.W.; Redman, C.W.G.; King, L.J. Abnormal iron parameters in the pregnancy syndrome preeclampsia. Am. J. Obstet. Gynecol. 2002, 187, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.-Y.; Luo, W.-P.; Zhang, C.-X. High serum iron level is associated with an increased risk of hypertensive disorders during pregnancy: A meta-analysis of observational studies. Nutr. Res. 2015, 35, 1060–1069. [Google Scholar] [CrossRef]
- Fenzl, V.; Flegar-Meštrić, Z.; Perkov, S.; Andrišić, L.; Tatzber, F.; Žarković, N.; Duić, Ž. Trace elements and oxidative stress in hypertensive disorders of pregnancy. Arch. Gynecol. Obstet. 2013, 287, 19–24. [Google Scholar] [CrossRef]
- Das, S.S.; Dhall, G.I.; Dhall, K.; Dash, S. Significance of serum iron levels as a biochemical marker in pregnancy-induced hypertension. Int. J. Gynaecol. Obstet. 1994, 45, 3–9. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; De-Regil, L.M.; Dowswell, T.; Viteri, F.E. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2012, 12, CD004736. [Google Scholar] [PubMed]
- Słomka, A.; Zekanowska, E.; Piotrowska, K.; Kwapisz, J. [Iron metabolism and maternal-fetal iron circulation]. Postepy Hi.g Med. Dosw. 2012, 66, 876–887. [Google Scholar] [CrossRef]
- Sukiennicki, G.M.; Marciniak, W.; Muszyńska, M.; Baszuk, P.; Gupta, S.; Białkowska, K.; Jaworska-Bieniek, K.; Durda, K.; Lener, M.; Pietrzak, S.; et al. Iron levels, genes involved in iron metabolism and antioxidative processes and lung cancer incidence. PLoS ONE 2019, 14, e0208610. [Google Scholar] [CrossRef] [PubMed]
- Brunacci, F.; Rocha, V.S.; De Carli, E.; Espósito, B.P.; Ruano, R.; Colli, C. Increased serum iron in preeclamptic women is likely due to low hepcidin levels. Nutr. Res. 2018, 53, 32–39. [Google Scholar] [CrossRef]
- Tabrizi, F.M.; Pakdel, F.G. Serum Level of Some Minerals during Three Trimesters of Pregnancy in Iranian Women and Their Newborns: A Longitudinal Study. Indian J. Clin. Biochem. 2014, 29, 174–180. [Google Scholar] [CrossRef]
- Giannakou, K.; Evangelou, E.; Papatheodorou, S.I. Genetic and non-genetic risk factors for pre-eclampsia: Umbrella review of systematic reviews and meta-analyses of observational studies. Ultrasound Obstet. Gynecol. 2018, 51, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, C.; Wang, Q.; Zhang, Z. Supplementation of folic acid in pregnancy and the risk of preeclampsia and gestational hypertension: A meta-analysis. Arch. Gynecol. Obstet. 2018, 298, 697–704. [Google Scholar] [CrossRef]
- Haider, B.A.; Olofin, I.; Wang, M.; Spiegelman, D.; Ezzati, M.; Fawzi, W.W.; Nutrition Impact Model Study Group (anaemia). Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2013, 346, f3443. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.C.; Hayes, D.J.; Jones, R.L. Effects of micronutrients on placental function: Evidence from clinical studies to animal models. Reproduction 2018, 156, R69–R82. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Tian, F.-J.; Lin, Y. Oxidative Stress in Placenta: Health and Diseases. Biomed. Res. Int. 2015, 2015, 293271. [Google Scholar] [CrossRef]
- Lewandowska, M.; Sajdak, S.; Lubiński, J. Serum Selenium Level in Early Healthy Pregnancy as a Risk Marker of Pregnancy Induced Hypertension. Nutrients 2019, 11, 1028. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Scherr, R.E.; Lanoue, L.; Keen, C.L. Influence of copper on early development: Prenatal and postnatal considerations. Biofactors 2010, 36, 136–152. [Google Scholar] [CrossRef]
- McArdle, H.J.; Andersen, H.S.; Jones, H.; Gambling, L. Copper and iron transport across the placenta: Regulation and interactions. J. Neuroendocrinol. 2008, 20, 427–431. [Google Scholar] [CrossRef]
- Carpenè, E.; Andreani, G.; Isani, G. Metallothionein functions and structural characteristics. J. Trace Elem. Med. Biol. 2007, 21 (Suppl. 1), 35–39. [Google Scholar] [CrossRef]
- Sharashenidze, A.; Panchulidze, L.; Sanikidze, T. Alterations in Placenta Redox-Status During Experimental Model Of Hypoxia-Induced Preeclampsia. Georgian Med. News 2017, 268, 86–90. [Google Scholar]
- Escudero, C.; Calle, A. [Iron, oxygen and placental development in the etiology of preeclampsia. Effects of high altitude in Ecuador]. Rev. Med. Chile 2006, 134, 491–498. [Google Scholar] [PubMed]
- Kadyrov, M.; Schmitz, C.; Black, S.; Kaufmann, P.; Huppertz, B. Pre-eclampsia and maternal anaemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta 2003, 24, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Cantor, A.G.; Bougatsos, C.; Dana, T.; Blazina, I.; McDonagh, M. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: A systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2015, 162, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Jirakittidul, P.; Sirichotiyakul, S.; Ruengorn, C.; Techatraisak, K.; Wiriyasirivaj, B. Effect of iron supplementation during early pregnancy on the development of gestational hypertension and pre-eclampsia. Arch. Gynecol. Obstet. 2018, 298, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Ziaei, S.; Norrozi, M.; Faghihzadeh, S.; Jafarbegloo, E. A randomised placebo-controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin > or = 13.2 g/dl. BJOG 2007, 114, 684–688. [Google Scholar] [CrossRef] [PubMed]
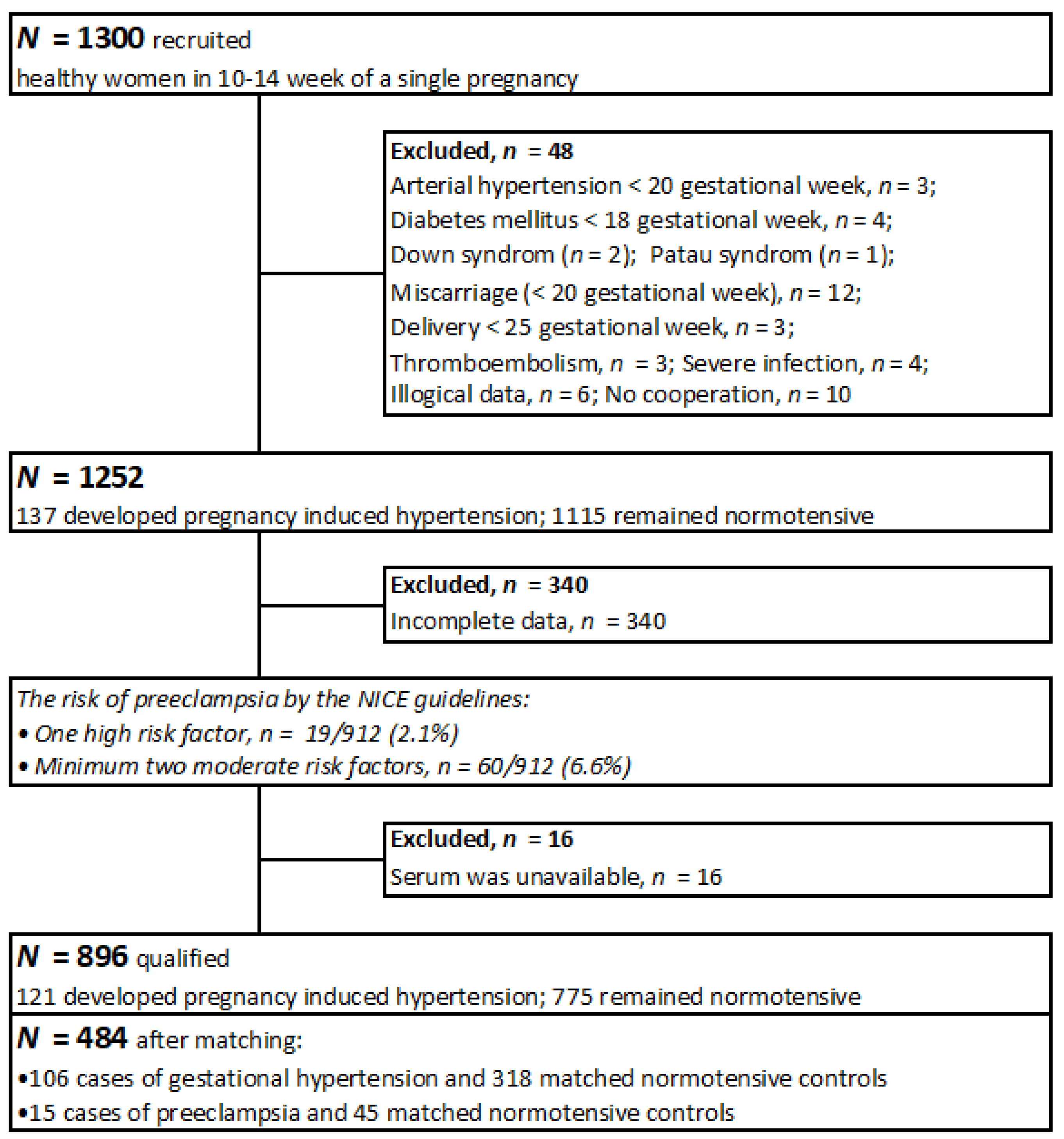
| Characteristics | Controls (n = 363) * | Cases (n = 121) * | p ** |
|---|---|---|---|
| N (%) or Mean (SD); Median | N (%) or Mean (SD); Median | ||
| Basic: | |||
| Maternal age (years) | 35.05 (3.99); 36.00 | 35.05 (4.21); 36.00 | 0.907 |
| Maternal age (range) | (22–45) | (19–45) | |
| Pre-pregnancy BMI (kg/m2) | 25.03 (4.40); 24.38 | 26.76 (5.37); 26.47 | 0.003 |
| Pre-pregnancy BMI (range) | (16.52–39.41) | (18.17–42.91) | |
| Pre-pregnancy BMI ≥30 kg/m2 | 48 (13.22%) | 34 (28.10%) | 0.0002 |
| Gestational age at recruitment (weeks) | 12.25 (0.80); 12.00 | 11.55 (0.82); 11.00 | 1.97 × 10−16 |
| Gestational age at recruitment (range) | (10–14) | (10–14) | |
| Primiparous | 141 (38.84%) | 56 (46.28%) | 0.149 |
| Prior PE *** | 2 (0.55%) | 13 (10.74%) | <0.001 |
| Pre-term delivery in history | 19 (5.23%) | 7 (5.79%) | 0.816 |
| ART | 18 (4.96%) | 11 (9.09%) | 0.097 |
| Women who had never smoked | 302 (83.20%) | 92 (76.03%) | 0.080 |
| Pack-years during recruitment | 19.25 (32.51); 6.63 | 21.21 (32.26); 8.75 | 0.748 |
| Vitamin supplementation | |||
| Folic acid supplementation in I trimester | 126 (34.71%) | 22 (18.18%) | 0.0006 |
| Multivitamin supplementation in II-III trimester | 184 (50.69%) | 50 (41.32%) | 0.074 |
| Microelement concentrations (µg/L) **** | |||
| Iron | 1043.85 (338.77); 995.66 | 948.86 (333.73); 908.27 | 0.011 |
| Selenium | 62.89 (8.70); 62.02 | 57.51 (6.54); 57.40 | <0.00001 |
| Copper | 1767.53 (338.67); 1746.67 | 1698.33 (298.37); 1671.44 | 0.059 |
| Zinc | 628.03 (174.57); 607.49 | 610.19 (87.58); 607.66 | 0.690 |
| Outcomes | |||
| PE | - | 15 | - |
| GDM | 73 (20.11%) | 23 (19.01%) | 0.792 |
| Gestational age at delivery (weeks) | 38.71 (1.77); 39.0 | 37.99 (2.62); 39.0 | 0.011 |
| Delivery <37 week | 23 (6.34%) | 16 (13.22%) | 0.016 |
| Newborn birthweight (g) | 3385.28 (546.81); 3400.00 | 3113.06 (785.36); 3150.00 | 0.0003 |
| Birthweight <10 centiles | 21 (5.79%) | 22 (18.18%) | 0.00003 |
| Socio-Demographic Characteristics | Controls (n = 363) * | Cases (n = 121) * | p ** |
|---|---|---|---|
| n (%) | n (%) | ||
| Education Levels (Available data, n) | 305 | 105 | 0.042 |
| Higher | 201 (65.90%) | 57 (54.29%) | |
| Secondary | 76 (24.92%) | 28 (26.67%) | |
| Vocational | 26 (8.53%) | 17 (16.19%) | |
| Elementary | 2 (0.66%) | 3 (2.86%) | |
| Financial status (available data, n) *** | 141 | 63 | 0.002 |
| (lower levels) | 14 (9.93%) | 5 (7.94%) | |
| (medium level) | 32 (22.70%) | 26 (41.27%) | |
| (higher levels) | 95 (67.38%) | 32 (50.79%) | |
| Place of residence (available data, n) | 362 | 120 | 0.585 |
| Country | 110 (30.39%) | 30 (25.00%) | |
| Town <50,000 of residence | 104 (28.73%) | 35 (29.17%) | |
| Big city >50,000 of residence | 148 (40.88%) | 56 (46.67%) |
| Quartile | Iron (µg/L)! | Risk of Pregnancy-Induced Hypertension | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | OR * | (CI 95%) | p *** | AOR ** | (CI 95%) | p *** | ||
| Q1 | 217.55–801.20 | 45 | 75 | 2.19 | (1.24–3.88) | 0.007 | 1.98 | (1.01–3.90) | 0.048 |
| Q2 | 801.20–982.33 | 22 | 99 | 0.83 | (0.65–2.32) | 0.519 | 0.92 | (0.53–2.23) | 0.816 |
| Q3 | 982.33–1211.75 | 27 | 93 | 1.06 | (0.58–1.95) | 0.850 | 1.43 | (0.72–2.85) | 0.310 |
| Q4 | 1211.75–2806.24 | 26 | 95 | 1 | 1 | ||||
| Disease | Prediction Indicators of Disease for Iron Concentrations * | ||||
|---|---|---|---|---|---|
| Cut-Off Point (µg/L) | AUC | p ** | Sensitivity | Specificity | |
| Pregnancy induced hypertension | 843.21 | 0.578 | 0.013 | 45.8% | 74.0% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowska, M.; Sajdak, S.; Lubiński, J. Can Serum Iron Concentrations in Early Healthy Pregnancy Be Risk Marker of Pregnancy-Induced Hypertension? Nutrients 2019, 11, 1086. https://doi.org/10.3390/nu11051086
Lewandowska M, Sajdak S, Lubiński J. Can Serum Iron Concentrations in Early Healthy Pregnancy Be Risk Marker of Pregnancy-Induced Hypertension? Nutrients. 2019; 11(5):1086. https://doi.org/10.3390/nu11051086
Chicago/Turabian StyleLewandowska, Małgorzata, Stefan Sajdak, and Jan Lubiński. 2019. "Can Serum Iron Concentrations in Early Healthy Pregnancy Be Risk Marker of Pregnancy-Induced Hypertension?" Nutrients 11, no. 5: 1086. https://doi.org/10.3390/nu11051086
APA StyleLewandowska, M., Sajdak, S., & Lubiński, J. (2019). Can Serum Iron Concentrations in Early Healthy Pregnancy Be Risk Marker of Pregnancy-Induced Hypertension? Nutrients, 11(5), 1086. https://doi.org/10.3390/nu11051086




