Vitamin D and the Risk of Depression: A Causal Relationship? Findings from a Mendelian Randomization Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources for MR Analyses
2.2. Testing Mendelian Randomization Assumptions
2.3. Statistical Analysis
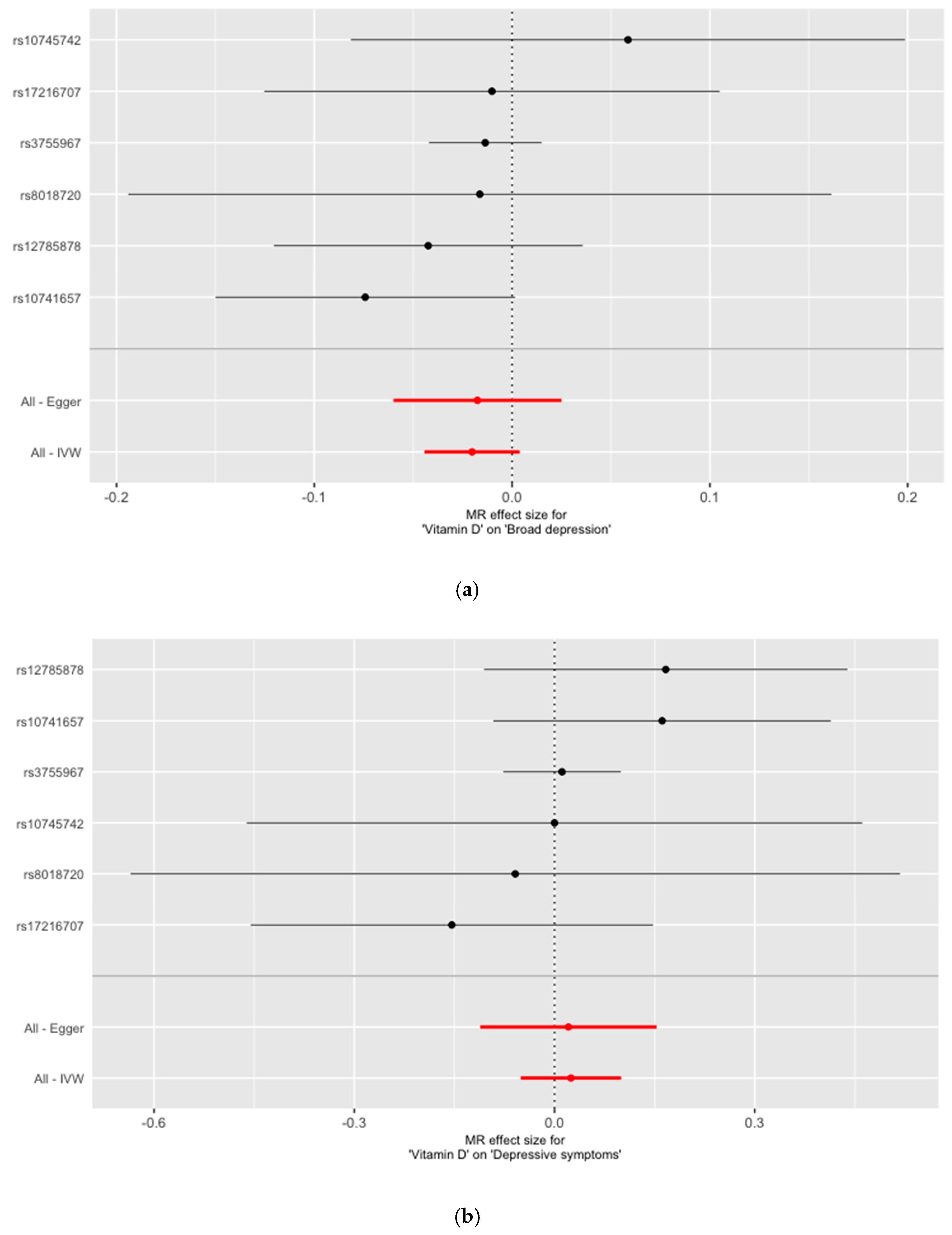
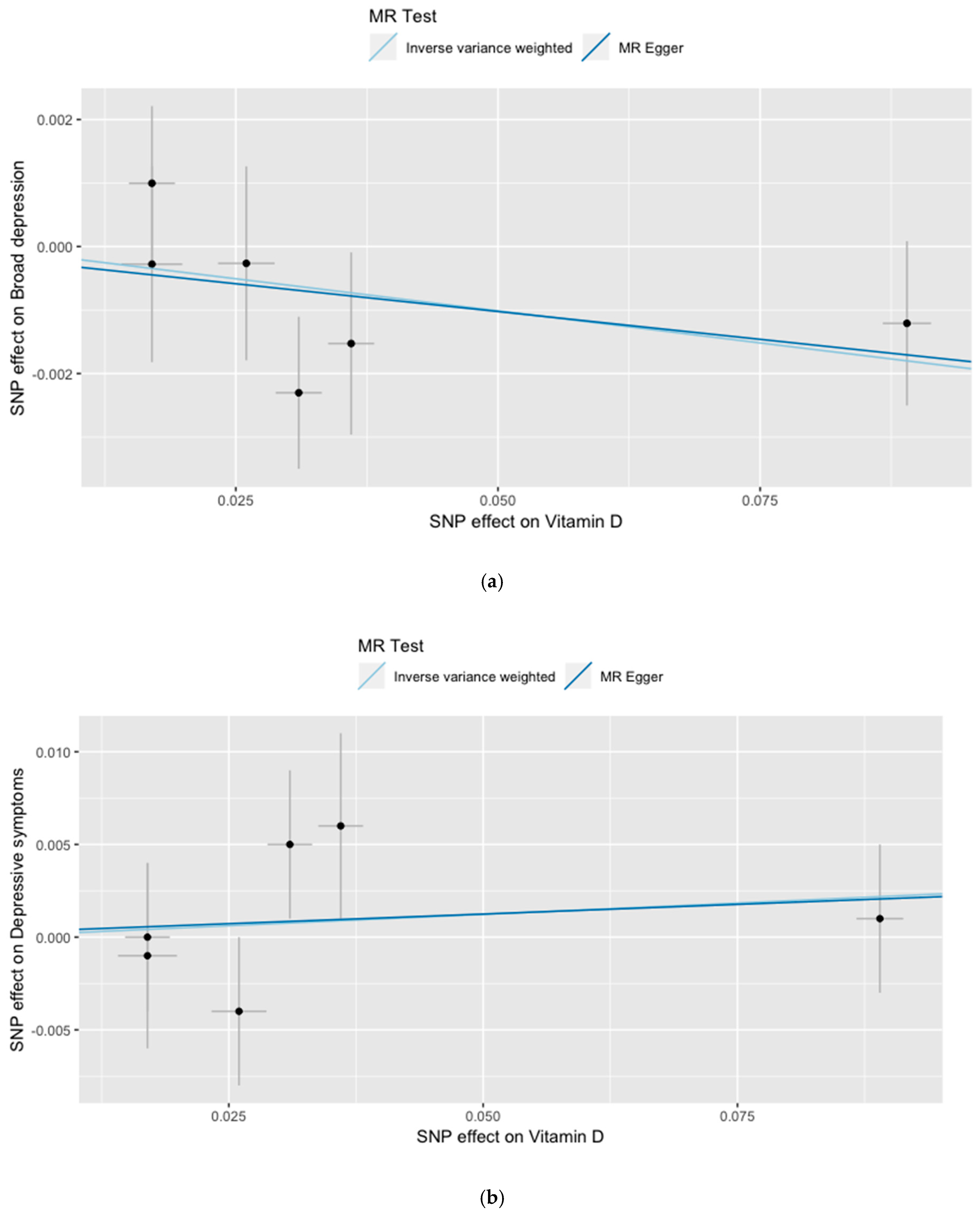
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanza-Martinez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef]
- Föcker, M.; Antel, J.; Ring, S.; Hahn, D.; Kanal, O.; Ozturk, D.; Hebebrand, J.; Libuda, L. Vitamin D and mental health in children and adolescents. Eur. Child Adolesc. Psychiatry 2017, 26, 1043–1066. [Google Scholar] [CrossRef] [PubMed]
- Husmann, C.; Frank, M.; Schmidt, B.; Jöckel, K.H.; Antel, J.; Reissner, V.; Libuda, L.; Hebebrand, J.; Föcker, M. Low 25(OH)-vitamin D concentrations are associated with emotional and behavioral problems in German children and adolescents. PLoS ONE 2017, 12, e0183091. [Google Scholar] [CrossRef]
- Schäfer, T.K.; Herrmann-Lingen, C.; Meyer, T. Association of circulating 25-hydroxyvitamin D with mental well-being in a population-based, nationally representative sample of German adolescents. Qual. Life Res. 2016, 25, 3077–3086. [Google Scholar] [CrossRef] [PubMed]
- Tolppanen, A.M.; Sayers, A.; Fraser, W.D.; Lewis, G.; Zammit, S.; Lawlor, D.A. The association of serum 25-hydroxyvitamin D3 and D2 with depressive symptoms in childhood—A prospective cohort study. J. Child Psychol. Psychiatry 2012, 53, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Föcker, M.; Antel, J.; Grasemann, C.; Fuhrer, D.; Timmesfeld, N.; Ozturk, D.; Peters, T.; Hinney, A.; Hebebrand, J.; Libuda, L. Effect of an vitamin D deficiency on depressive symptoms in child and adolescent psychiatric patients—A randomized controlled trial: Study protocol. BMC Psychiatry 2018, 18, 57. [Google Scholar] [CrossRef]
- Shaffer, J.A.; Edmondson, D.; Wasson, L.T.; Falzon, L.; Homma, K.; Ezeokoli, N.; Li, P.; Davidson, K.W. Vitamin D supplementation for depressive symptoms: A systematic review and meta-analysis of randomized controlled trials. Psychosom. Med. 2014, 76, 190–196. [Google Scholar] [CrossRef]
- Spedding, S. Vitamin D and depression: A systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients 2014, 6, 1501–1518. [Google Scholar] [CrossRef]
- Vellekkatt, F.; Menon, V. Efficacy of vitamin D supplementation in major depression: A meta-analysis of randomized controlled trials. J. Postgrad. Med. 2018. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R.; Powers, S.I.; Spangler, L.; Larson, J.; Michael, Y.L.; Millen, A.E.; Bueche, M.N.; Salmoirago-Blotcher, E.; Wassertheil-Smoller, S.; Brunner, R.L.; et al. Vitamin D supplementation and depression in the women’s health initiative calcium and vitamin D trial. Am. J. Epidemiol. 2012, 176, 1–13. [Google Scholar] [CrossRef]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Jacka, F.N.; Dodd, S.; Nicholson, G.; Berk, M. Annual high-dose vitamin D3 and mental well-being: Randomised controlled trial. Br. J. Psychiatry 2011, 198, 357–364. [Google Scholar] [CrossRef]
- Zheng, J.; Baird, D.; Borges, M.C.; Bowden, J.; Hemani, G.; Haycock, P.; Evans, D.M.; Smith, G.D. Recent Developments in Mendelian Randomization Studies. Curr. Epidemiol. Rep. 2017, 4, 330–345. [Google Scholar] [CrossRef]
- König, I.R.; Greco, F.M.D. Mendelian randomization: Progressing towards understanding causality. Ann. Neurol. 2018, 84, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Michaelsson, K.; Melhus, H.; Larsson, S.C. Serum 25-Hydroxyvitamin D Concentrations and Major Depression: A Mendelian Randomization Study. Nutrients 2018, 10, 1987. [Google Scholar] [CrossRef] [PubMed]
- Stapelberg, N.J.C.; Neumann, D.L.; Shum, D.; Headrick, J.P. Health, pre-disease and critical transition to disease in the psycho-immune-neuroendocrine network: Are there distinct states in the progression from health to major depressive disorder? Physiol. Behav. 2019, 198, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; O’Reilly, P.F.; Aschard, H.; Hsu, Y.H.; Richards, J.B.; Dupuis, J.; Ingelsson, E.; Karasik, D.; Pilz, S.; Berry, D.; et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat. Commun. 2018, 9, 260. [Google Scholar] [CrossRef]
- Okbay, A.; Baselmans, B.M.; De Neve, J.E.; Turley, P.; Nivard, M.G.; Fontana, M.A.; Meddens, S.F.; Linner, R.K.; Rietveld, C.A.; Derringer, J.; et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat. Genet. 2016, 48, 624–633. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Shirali, M.; Clarke, T.K.; Marioni, R.E.; Davies, G.; Coleman, J.R.I.; Alloza, C.; Shen, X.; Barbu, M.C.; et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 2018, 9, 1470. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Shirali, M.; Clarke, T.K.; Marioni, R.E.; Davies, G.; Coleman, J.R.I.; Alloza, C.; Shen, X.; Barbu, M.C.; et al. Addendum: Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 2018, 9, 3578. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Kvale, M.N.; Hesselson, S.; Hoffmann, T.J.; Cao, Y.; Chan, D.; Connell, S.; Croen, L.A.; Dispensa, B.P.; Eshragh, J.; Finn, A.; et al. Genotyping Informatics and Quality Control for 100,000 Subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) Cohort. Genetics 2015, 200, 1051–1060. [Google Scholar] [CrossRef]
- Major Depressive Disorder Working Group of the Psychiatric; Ripke, S.; Wray, N.R.; Lewis, C.M.; Hamilton, S.P.; Weissman, M.M.; Breen, G.; Byrne, E.M.; Blackwood, D.H.; Boomsma, D.I.; et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol. Psychiatry 2013, 18, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Haycock, P.C.; Burgess, S.; Wade, K.H.; Bowden, J.; Relton, C.; Davey Smith, G. Best (but oft-forgotten) practices: The design, analysis, and interpretation of Mendelian randomization studies. Am. J. Clin. Nutr. 2016, 103, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Brion, M.J.; Shakhbazov, K.; Visscher, P.M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 2013, 42, 1497–1501. [Google Scholar] [CrossRef]
- Anglin, R.E.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
| Association with Natural Log-Transformed 25(OH)vitamin D | Association with Broad Depression | Association with Depressive Symptoms | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Chromosome | Gene | Effect/Reference Allele | AF * | Effect Estimate (Beta) # | SE | p | Effect Estimate (Beta) | SE | p | Effect Estimate (Beta) | SE | p |
| rs3755967 | 4 | GC | T/C | 0.28 | −0.089 | 0.0023 | 4.74E–343 | 0.0012 | 0.0013 | 0.350 | −0.001 | 0.004 | 0.731 |
| rs10741657 | 11 | CYP2R1 | A/G | 0.4 | 0.031 | 0.0022 | 2.05E–46 | 0.002 | 0.001 | 0.055 | 0.005 | 0.004 | 0.309 |
| rs12785878 | 11 | NADSYN1_DHCR7 | T/G | 0.75 | 0.036 | 0.0022 | 3.80E–62 | −0.002 | 0.001 | 0.287 | 0.006 | 0.005 | 0.215 |
| rs10745742 | 12 | AMDHD1 | T/C | 0.4 | 0.017 | 0.0022 | 1.88E–14 | 0.001 | 0.001 | 0.412 | 0.000 | 0.004 | 0.976 |
| rs8018720 | 14 | SEC23A | C/G | 0.82 | −0.017 | 0.0029 | 4.72E–09 | 0.0003 | 0.0015 | 0.857 | 0.001 | 0.005 | 0.780 |
| rs17216707 | 20 | CYP24A1 | T/C | 0.79 | 0.026 | 0.0027 | 8.14E–23 | 0.0003 | 0.0015 | 0.862 | −0.004 | 0.004 | 0.340 |
| Association of 25(OH)vitamin D with Broad Depression | Association of 25(OH)vitamin D with Depressive Symptoms | |||||
|---|---|---|---|---|---|---|
| SNP | Effect Estimate (Beta) | SE | p | Effect Estimate (Beta) | SE | p |
| rs3755967 | −0.0136 | 0.0145 | 0.350 | 0.0112 | 0.0450 | 0.803 |
| rs10741657 | −0.0743 | 0.0386 | 0.055 | 0.1613 | 0.1290 | 0.211 |
| rs12785878 | −0.0424 | 0.0398 | 0.287 | 0.1667 | 0.1389 | 0.230 |
| rs10745742 | 0.0586 | 0.0715 | 0.412 | 0.0000 | 0.2353 | 1.000 |
| rs8018720 | −0.0163 | 0.0907 | 0.857 | −0.0588 | 0.2941 | 0.841 |
| rs17216707 | −0.0102 | 0.0587 | 0.862 | −0.1538 | 0.1538 | 0.317 |
| All—Inverse variance weighted (IVW) | −0.0202 | 0.0123 | 0.099 | 0.0246 | 0.0384 | 0.521 |
| All—MR Egger | −0.0175 | 0.0217 | 0.464 | 0.0209 | 0.0674 | 0.772 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Libuda, L.; Laabs, B.-H.; Ludwig, C.; Bühlmeier, J.; Antel, J.; Hinney, A.; Naaresh, R.; Föcker, M.; Hebebrand, J.; König, I.R.; et al. Vitamin D and the Risk of Depression: A Causal Relationship? Findings from a Mendelian Randomization Study. Nutrients 2019, 11, 1085. https://doi.org/10.3390/nu11051085
Libuda L, Laabs B-H, Ludwig C, Bühlmeier J, Antel J, Hinney A, Naaresh R, Föcker M, Hebebrand J, König IR, et al. Vitamin D and the Risk of Depression: A Causal Relationship? Findings from a Mendelian Randomization Study. Nutrients. 2019; 11(5):1085. https://doi.org/10.3390/nu11051085
Chicago/Turabian StyleLibuda, Lars, Björn-Hergen Laabs, Christine Ludwig, Judith Bühlmeier, Jochen Antel, Anke Hinney, Roaa Naaresh, Manuel Föcker, Johannes Hebebrand, Inke R. König, and et al. 2019. "Vitamin D and the Risk of Depression: A Causal Relationship? Findings from a Mendelian Randomization Study" Nutrients 11, no. 5: 1085. https://doi.org/10.3390/nu11051085
APA StyleLibuda, L., Laabs, B.-H., Ludwig, C., Bühlmeier, J., Antel, J., Hinney, A., Naaresh, R., Föcker, M., Hebebrand, J., König, I. R., & Peters, T. (2019). Vitamin D and the Risk of Depression: A Causal Relationship? Findings from a Mendelian Randomization Study. Nutrients, 11(5), 1085. https://doi.org/10.3390/nu11051085





