Enzymatic Hydrolysis of a Collagen Hydrolysate Enhances Postprandial Absorption Rate—A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
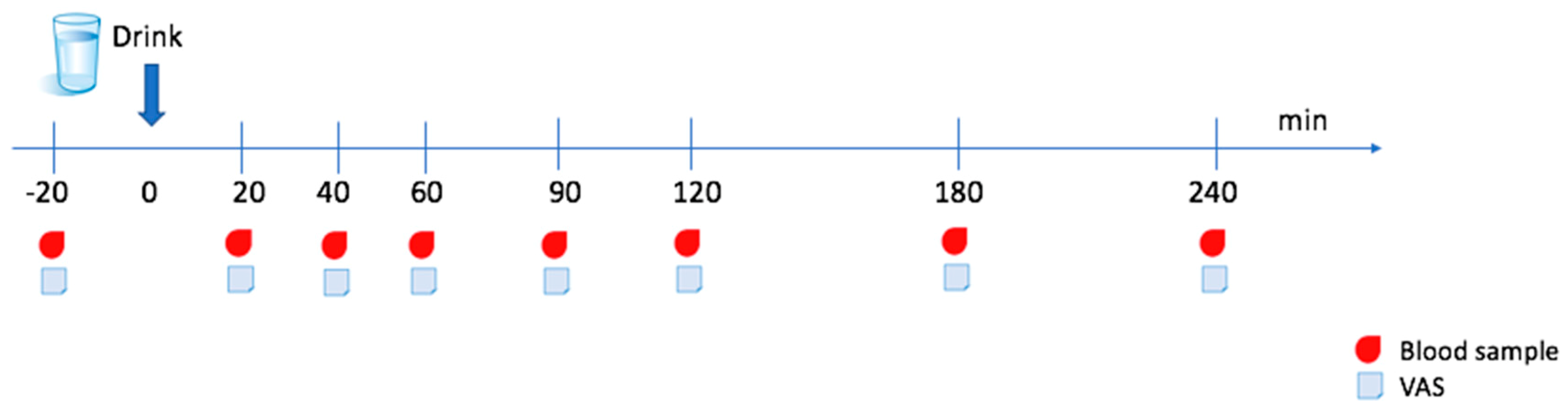
2.2. Design and Experimental Protocol
2.3. Collagen Products
2.4. Blood Samples
2.5. Visual Analogue Scale (VAS)
2.6. H NMR Spectroscopy
2.7. Statistical Analyses
3. Results
3.1. H NMR Spectroscopic Analysis
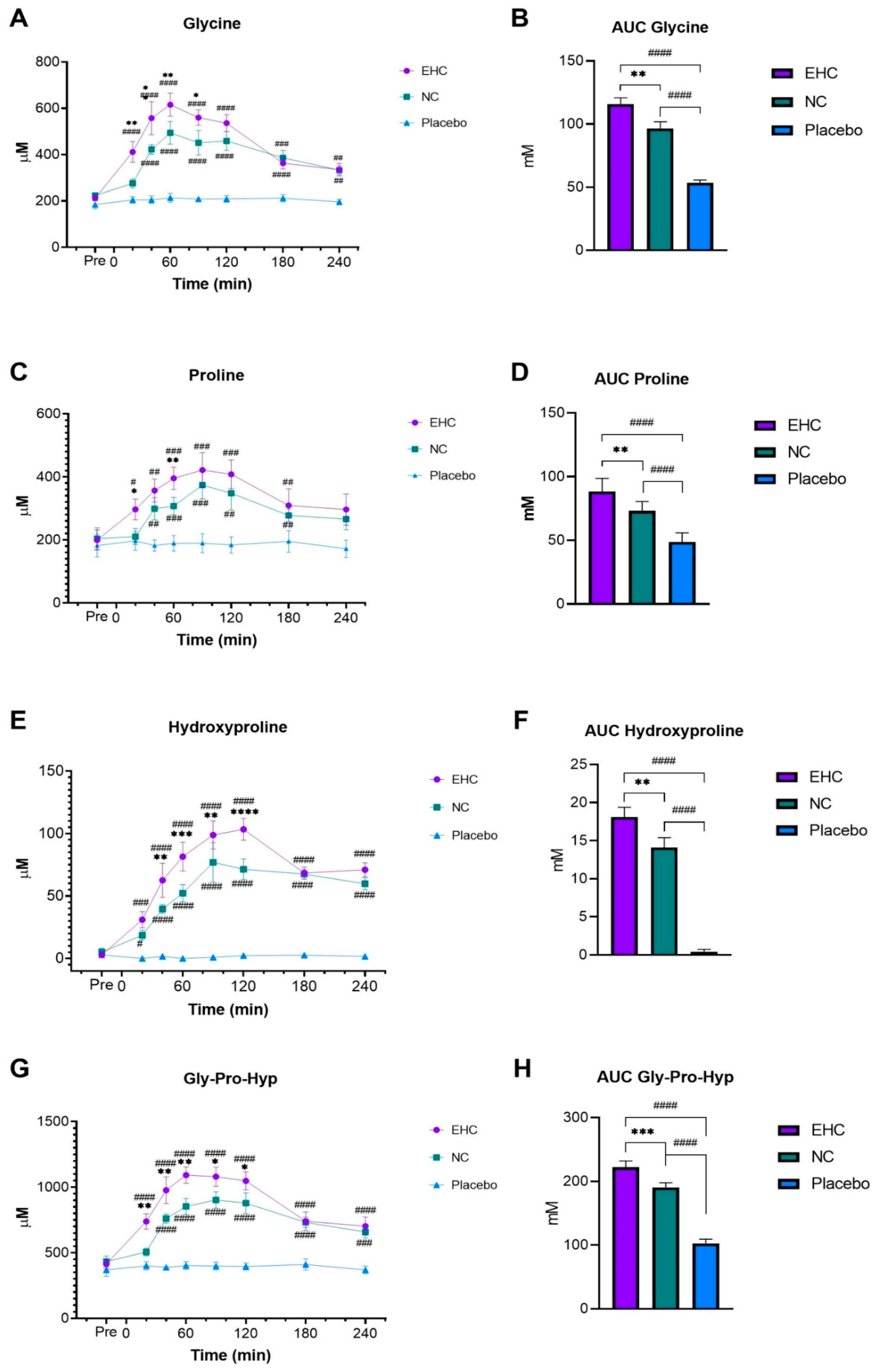
3.2. General AAs Absorption
3.3. Specific Collagen AAs
3.4. Glucose
3.5. VAS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van der Plas, A.; de Jonge, S.; de Vos, R.J.; van der Heide, H.J.; Verhaar, J.A.; Weir, A.; Tol, J.L. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br. J. Sports Med. 2012, 46, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, F.; Sánchez, L.; Amy, E.; Micheo, W. Anterior cruciate ligament injury: Return to play, function and long-term considerations. Curr. Sports Med. Rep. 2017, 16, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Dar, Q.-A.; Schott, E.M.; Catheline, S.E.; Maynard, R.D.; Liu, Z.; Kamal, F.; Farnsworth, C.W.; Ketz, J.P.; Mooney, R.A.; Hilton, M.J.; et al. Daily oral consumption of hydrolyzed type 1 collagen is chrondoprotective and anti-inflammatory in murine posttraumatic osteoarthritis. PLoS ONE 2017, 12, e0174705. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef]
- Shaw, G.; Lee-Barthel, A.; Ross, M.L.R.; Wang, B.; Baar, K. Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am. J. Clin. Nutr. 2017, 105, 136–143. [Google Scholar] [CrossRef]
- Baar, K. Stress relaxation and targeted nutrition to treat patellar tendinopathy. Int. J. Sport Nutr. Exerc. Metab. 2018, 1–18. [Google Scholar] [CrossRef]
- Praet, S.; Purdam, C.R.; Welvaert, M.; Vlahovich, N.; Lovell, G.; Burke, L.M.; Gaida, J.E.; Manzanero, S.; Hughes, D.; Waddington, G. Oral supplementation of specific collagen peptides combined with calf-strengthening exercises enhances function and reduces pain in achilles tendinopathy patients. Nutrients 2019, 11, 76. [Google Scholar] [CrossRef]
- Dressler, P.; Gehring, D.; Zdzieblik, D.; Oesser, S.; Gollhofer, A.; Koenig, D. Improvement of functional ankle properties following supplementation with specific collagen peptides in athletes with chronic ankle instability. J. Sport Sci. Med. 2018, 17, 298–304. [Google Scholar] [CrossRef]
- Bello, A.E.; Oesser, S. Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: A review of the literature. Curr. Med. Res. Opin. 2006, 22, 2221–2232. [Google Scholar] [CrossRef]
- García-Coranado, J.M.; Martínez-Olvera, L.; Elizondo-Omana, R.E.; Acosta-Olivo, C.A.; Cavazos, F.V.; Simental-Mendía, L.E.; Simental-Mendía, M. Effect of collagen supplementation on osteoarthritis symptoms: A meta-analysis of randomized placebo-controlled trials. Int. Orthop. 2018, 43, 531–538. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Nuite, M.; Krishnan, N.; Ruthazer, R.; Price, L.L.; Burstein, D.; Griffith, J.; Flechsenhar, K. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: A pilot randomized controlled trial. Osteoarthr. Cartil. 2011, 19, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Machado, G.C.; Eyles, J.P.; Ravi, V.; Hunter, D.J. Dietary supplements for treating osteoarthritis: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.L.; Sebastianelli, W.; Fleschenhar, K.R.; Aukermann, D.F.; Meza, F.; Millard, R.L.; Sherbondy, P.S.; Albert, A. 24-Week study on the use of collagen hydrolysate as a dietary supplement in athletes with activity-related joint pain. Curr. Med. Res. Opin. 2008, 24, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Zdzieblik, D.; Oesser, S.; Gollhofer, A.; Konig, D. Improvement of activity-related knee joint discomfort following supplementation of specific collagen peptides. Appl. Physiol. Nutr. Metab. 2017, 42, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Sancturay, M.R.; Qu, Y.; Khajavi, S.H.; Van Zandt, A.E.; Dyandra, M.; Frese, S.A.; Barile, D.; German, J.B. Personalizing protein nourishment. Crit. Rew. Food Sci. Nutr. 2017, 57, 3313–3331. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Crombach, N.; Gijsen, A.P.; Walrand, S.; Fauquant, J.; Kies, A.K.; Lemosquet, S.; Saris, W.H.M.; Boirie, Y.; van Loon, L.J.C. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with is intact protein. Am. J. Clin. Nutr. 2009, 90, 106–115. [Google Scholar] [CrossRef]
- Bertram, H.C.; Jakobsen, L.M.A. Nutrimetabolomics: Integrating metabolomics in nutrition to disentangle intake of animal-based foods. Metabolomics 2018, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Vangsoe, M.T.; Thogersen, R.; Bertram, H.C.; Heckmann, L.-H.L.; Hansen, M. Ingestion of insect protein isolate enhances blood amino acid concentrations similar to soy protein in a human trial. Nutrients 2018, 10, 1357. [Google Scholar] [CrossRef]
- Nielsen, L.; Nyby, S.; Klingenberg, L.; Ritz, C.; Sundekilde, U.K.; Bertram, H.C.; Westerterp-Plantenga, M.S.; Liaset, B.; Kristiansen, K.; Madsen, L.; et al. Salmon in combination with high glycemic index carbohydrates increases diet-induced thermogenesis compared with salmon with low glycemic index carbohydrates–An acute randomized cross-over meal test study. Nutrients 2019, 11, 365. [Google Scholar] [CrossRef]
- López-Morales, C.A.; Vãzquez-Leyva, S.; Vallejo-Castillo, L.; Carballo-Uicab, G.; Muñoz-García, L.; Herbert-Pucheta, J.E.; Zepeda-Vallejo, L.G.; Velasco-Velázquez, M.; Pavóon, L.; Pérez-Tapia, S.M.; et al. Determination of peptide profile consistency and safety of collagen hydrolysates as quality attributes. J. Food Sci. 2019, 84, 430–438. [Google Scholar] [CrossRef]
- Czajka, A.; Kania, E.; Genovese, L.; Corbo, A.; Merone, G.; Luci, C.; Sibilla, S. Daily oral supplementation with collagen peptides combined with vitamins and other bioactive compounds improves skin elasticity and has a beneficial effect on joint and general wellbeing. Nutr. Res. 2018, 57, 97–108. [Google Scholar] [CrossRef]
- de Paz-Lugo, P.; Lupiánez, J.A.; Meléndez-Hevia, E. High glycine concentration increases collagen synthesis by articular chondrocytes in vitro: Acute glycine deficiency could be an important cause of osteoarthritis. Amino Acids 2018, 50, 1357–1365. [Google Scholar] [CrossRef]
- Baar, K. Minimizing injury and maximizing return to play: Lessons from engineered ligaments. Sports Med. 2007, 41, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Oesser, S.; Adam, M.; Babel, W.; Seifert, J. Oral administration of 14C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). J. Nutr. 1999, 129, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.L.; Holst, J.J. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysate in humans. Eur. J. Nutr. 2004, 43, 127–139. [Google Scholar] [CrossRef]
- Schmedes, M.; Bendtsen, L.; Gomes, S.; Liaset, B.; Holst, J.J.; Ritz, C.; Reitelseder, S.; Sjödin, A.; Astrup, A.; Young, J.F. The effect of casein, hydrolyzed casein, and whey proteins on urinary and postprandial plasma metabolites in overweight and moderately obese human subjects. J. Sci. Food Agric. 2018, 98, 5598–5605. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, Y.; Kubomura, D.; Sato, Y.; Sato, K. Dose-dependency changes in the levels of free and peptide forms of hydroxyproline in human plasma after collagen hydrolysate ingestion. Food Chem. 2014, 159, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Zdzieblik, D.; Oesser, S.; Baumstark, M.W.; Gollhofer, A.; König, D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomized controlled trial. Br. J. Nutr. 2015, 114, 1237–1245. [Google Scholar] [CrossRef]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious beneficial effect of nonessential amino acid, glycine: A review. Ox. Med. Cell. Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M.; Moughan, P.J. Determination of sulfur amino acids in foods as related to bioavailability. J. AOAC Int. 2008, 91, 907–913. [Google Scholar] [PubMed]
- Farup, J.; Rahbek, S.K.; Storm, A.C.; Klitgaard, S.; Jørgensen, H.; Bibby, B.M.; Serena, A.; Vissing, K. Effect of degree of hydrolysis of whey protein on in vivo plasma amino acid appearance in humans. SpringerPlus 2016, 5, 382. [Google Scholar] [CrossRef]
- Paterson, M.; Bell, K.J.; O’Connell, S.M.; Smart, C.E.; Shafat, A.S.; King, B. The role of dietary protein and fat in glycaemic control in type 1 diabetes: Implications for intensive diabetes management. Curr. Diabetes Rep. 2015, 16, 61. [Google Scholar] [CrossRef]
- Fromentin, C.; Tomé, D.; Nau, F.; Flet, L.; Luengo, C.; Azzout-Marniche, D.; Sanders, P. Fromentin, G.; Gaudichon, C. Dietary proteins contribute little to glucose production, even under optimal gluconeogenic conditions in healthy humans. Diabetes 2013, 62, 1435–1442. [Google Scholar] [CrossRef]
- Claessens, M.; Saris, W.H.M.; van Bak, M.A. Glucagon and insulin responses after ingestion of different amounts of intact and hydrolyzed proteins. Br. J. Nutr. 2008, 100, 61–69. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Branched-chain amino acids: Enzyme and substrate regulation. J. Nutr. 2006, 136, 207S–211S. [Google Scholar] [CrossRef]
- Halton, T.L.; Hu, F.B. The effects of high protein diets on thermogenesis, satiety and weight loss: A critical review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef]
- Veldhorst, M.A.B.; Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; Westerterp, K.R.; Engelen, M.P.K.J.; Brummer, R.-J.M.; Deutz, N.E.P.; Westerterp-Plantenga, M.S. A breakfast with alpha-lactalbumin, gelatin, or gelatin + TRP lowers energy intake at lunch compared with a breakfast with casein, soy, whey, or whey-GMP. Clin. Nutr. 2009, 28, 147–155. [Google Scholar] [CrossRef]
- Hochstenbach-Waelen, A.; Westerterp, K.R.; Soenen, S.; Westerterp-Plantenga, M.S. No long-term weight maintenance effects of gelatin in a supra-sustained protein diet. Physiol. Behav. 2010, 101, 237–244. [Google Scholar] [CrossRef]

| Subjects (n = 10) | |
|---|---|
| Age (y) | 26 ± 1 |
| Weight (kg) | 77 ± 6 |
| Height (cm) | 180 ± 5 |
| Activity level (h) BMI (kg/m2) | 10 ± 3 24 ± 2 |
| Metabolite | δ 1H (multiplicity) |
|---|---|
| Alanine | 1.46 (d), 3.76 (q) |
| Arginine | 1.68 (m), 1.90 (m), 3.23 (t), 3.76 (t) |
| Asparagine | 4.00 (dd), 2.94 (m), 2.84 (m) |
| Glucose | 3.233 (dd), 3.398 (m), 3.458 (m), 3.524 (dd), 3.726 (m), 3.824 (m), 3.889 (dd), 4.634 (d), 5.223 (d) |
| Glycine | 3.54 (s) |
| Histidine | 3.16 (dd), 3.23 (dd), 3.98 (dd), 7.09 (d), 7.90 (d) |
| Isoleucine | 0.926 (t), 0.997 (d), 1.246 (m), 1.475 (m), 1.968 (m), 3.661 (d) |
| Leucine | 0.948 (t), 1.700 (m), 3.722 (m) |
| Lysine | 1.46 (m), 1.71 (m), 1.89 (m), 3.02 (t), 3.74 (t) |
| Methionine | 2.157 (m), 2.631 (t), 3.851 (dd) |
| Phenylalanine | 3.19 (m), 3.98 (dd), 7.32 (d), 7.36 (m), 7.42 (m) |
| Proline | 1.99 (m), 2.06 (m), 2.34 (m), 3.33 (dt), 3.41 (dt), 4.12 (dd) |
| Serine | 3.832 (dd), 3.958 (m) |
| Threonine | 1.316 (d), 3.575 (d), 4.244 (m) |
| Tyrosine | 3.024 (dd), 3.170 (dd), 3.921 (dd), 6.877 (m), 7.170 (m) |
| Valine | 0.976 (d), 1.029 (d), 2.261 (m), 3.691 (d) |
| Hydroxyproline | 2.14 (ddd), 2.42 (M), 3.36 (ddd), 3.46 (dd), 4.33 (d), 4.35 (d) |
| Comparison | EHC vs. NC | EHC vs. Placebo | NC vs. Placebo | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AA | EHC | NC | p | EHC | Pl | p | NC | Pl | p |
| Alanine | 367.4 | 351.3 | 0.69 | 367.4 | 266.2 | 0.0002 | 351.3 | 266.2 | 0.001 |
| Arginine | 86.54 | 82.54 | 0.67 | 86.54 | 50.28 | <0.0001 | 82.54 | 50.28 | <0.0001 |
| Asparagine | 40.60 | 40.43 | 0.99 | 40.60 | 34.58 | 0.019 | 40.43 | 34.58 | 0.023 |
| Glutamate | 39.17 | 34.34 | 0.25 | 39.17 | 25.68 | 0.0006 | 34.34 | 25.68 | 0.021 |
| Glutamine | 513.5 | 521.9 | 0.87 | 513.5 | 469.2 | 0.043 | 521.9 | 469.2 | 0.015 |
| Glycine | 448.7 | 380.7 | 0.0059 | 448.7 | 204.1 | <0.0001 | 380.7 | 204.1 | <0.0001 |
| Histidine | 72.85 | 77.37 | 0.12 | 72.85 | 67.38 | 0.053 | 77.37 | 67.38 | 0.0006 |
| Hydroxyproline | 64.92 | 48.88 | 0.0087 | 64.92 | 1.484 | <0.0001 | 48.88 | 1.484 | <0.0001 |
| Isoleucine | 73.62 | 68.00 | 0.21 | 73.62 | 58.24 | 0.0003 | 68.00 | 58.24 | 0.017 |
| Leucine | 127.4 | 120.8 | 0.42 | 127.4 | 99.09 | <0.0001 | 120.8 | 99.09 | 0.0014 |
| Lysine | 122.5 | 119.1 | 0.79 | 122.5 | 99.37 | 0.0008 | 119.1 | 99.37 | 0.0034 |
| Methionine | 18.78 | 22.51 | 0.034 | 18.78 | 17.42 | 0.59 | 22.51 | 17.42 | 0.0043 |
| Proline | 335.4 | 285.8 | 0.0074 | 335.4 | 186.3 | <0.0001 | 285.8 | 186.3 | <0.0001 |
| Phenylalanine | 47.65 | 48.21 | 0.93 | 47.65 | 39.80 | 0.0003 | 48.21 | 39.80 | 0.0001 |
| Serine | 147.7 | 145.0 | 0.89 | 147.7 | 108.5 | <.0001 | 145.0 | 108.5 | <0.0001 |
| Threonine | 152.2 | 148.2 | 0.83 | 152.2 | 120.6 | 0.0006 | 148.2 | 120.6 | 0.0023 |
| Tyrosine | 56.47 | 55.91 | 0.96 | 56.47 | 48.72 | 0.011 | 55.91 | 48.72 | 0.018 |
| Valine | 278.4 | 254.9 | 0.09 | 278.4 | 213.2 | <0.0001 | 254.9 | 213.2 | 0.0027 |
| BCAA | 479.5 | 443.7 | 0.15 | 479.5 | 370.6 | <0.0001 | 443.7 | 370.6 | 0.002 |
| Total AA | 2994 | 2806 | 0.14 | 2994 | 2110 | <0.0001 | 2806 | 2110 | <0.0001 |
| EAA | 893.4 | 859.1 | 0.48 | 893.4 | 715.2 | <0.0001 | 859.1 | 715.2 | 0.0003 |
| Gly-Pro-Hp | 849.1 | 715.4 | 0.0003 | 849.1 | 391.8 | <0.0001 | 715.4 | 391.8 | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skov, K.; Oxfeldt, M.; Thøgersen, R.; Hansen, M.; Bertram, H.C. Enzymatic Hydrolysis of a Collagen Hydrolysate Enhances Postprandial Absorption Rate—A Randomized Controlled Trial. Nutrients 2019, 11, 1064. https://doi.org/10.3390/nu11051064
Skov K, Oxfeldt M, Thøgersen R, Hansen M, Bertram HC. Enzymatic Hydrolysis of a Collagen Hydrolysate Enhances Postprandial Absorption Rate—A Randomized Controlled Trial. Nutrients. 2019; 11(5):1064. https://doi.org/10.3390/nu11051064
Chicago/Turabian StyleSkov, Kathrine, Mikkel Oxfeldt, Rebekka Thøgersen, Mette Hansen, and Hanne Christine Bertram. 2019. "Enzymatic Hydrolysis of a Collagen Hydrolysate Enhances Postprandial Absorption Rate—A Randomized Controlled Trial" Nutrients 11, no. 5: 1064. https://doi.org/10.3390/nu11051064
APA StyleSkov, K., Oxfeldt, M., Thøgersen, R., Hansen, M., & Bertram, H. C. (2019). Enzymatic Hydrolysis of a Collagen Hydrolysate Enhances Postprandial Absorption Rate—A Randomized Controlled Trial. Nutrients, 11(5), 1064. https://doi.org/10.3390/nu11051064






