Dietary Patterns Are Related to Clinical Characteristics in Memory Clinic Patients with Subjective Cognitive Decline: The SCIENCe Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Dietary Assessment
2.3. Outcome Measures (Clinical Characteristics)
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, M.; International, A.s.D. World Alzheimer Report 2015. The Global Impact of Dementia: An Analysis of Prevalence, Incid Ence, Cost And Trends; Alzheimer’s Disease International (ADI): London, UK, 2015. [Google Scholar]
- Prince, M.; Ali, G.C.; Guerchet, M.; Prina, A.M.; Albanese, E.; Wu, Y.T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res. Ther. 2016, 8, 23. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chetelat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical alzheimer’s disease. Alzheimers Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef] [PubMed]
- van Harten, A.C.; Mielke, M.M.; Swenson-Dravis, D.M.; Hagen, C.E.; Edwards, K.K.; Roberts, R.O.; Geda, Y.E.; Knopman, D.S.; Petersen, R.C. Subjective cognitive decline and risk of mci: The mayo clinic study of aging. Neurology 2018, 91, e300–e312. [Google Scholar] [CrossRef] [PubMed]
- Slot, R.E.R.; Sikkes, S.A.M.; Berkhof, J.; Brodaty, H.; Buckley, R.; Cavedo, E.; Dardiotis, E.; Guillo-Benarous, F.; Hampel, H.; Kochan, N.A.; et al. Subjective cognitive decline and rates of incident alzheimer’s disease and non-alzheimer’s disease dementia. Alzheimer’s Dement. 2018, 15, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- Morris, M.C.; Wang, Y.; Barnes, L.L.; Bennett, D.A.; Dawson-Hughes, B.; Booth, S.L. Nutrients and bioactives in green leafy vegetables and cognitive decline: Prospective study. Neurology 2018, 90, e214–e222. [Google Scholar] [CrossRef]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef]
- Kang, J.H.; Ascherio, A.; Grodstein, F. Fruit and vegetable consumption and cognitive decline in aging women. Ann. Neurol. 2005, 57, 713–720. [Google Scholar] [CrossRef]
- van de Rest, O.; Berendsen, A.A.; Haveman-Nies, A.; de Groot, L.C. Dietary patterns, cognitive decline, and dementia: A systematic review. Adv. Nutr. 2015, 6, 154–168. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Guyer, H.; Langa, K.M.; Yaffe, K. Neuroprotective diets are associated with better cognitive function: The health and retirement study. J. Am. Geriatr. Soc. 2017, 65, 1857–1862. [Google Scholar] [CrossRef]
- Lourida, I.; Soni, M.; Thompson-Coon, J.; Purandare, N.; Lang, I.A.; Ukoumunne, O.C.; Llewellynb, D.J. Mediterranean diet, cognitive function, and dementia: A systematic review. Epidemiology 2013, 24, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Association of mediterranean diet with mild cognitive impairment and alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimers Dis. 2014, 39, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Wengreen, H.; Munger, R.G.; Cutler, A.; Quach, A.; Bowles, A.; Corcoran, C.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A. Prospective study of dietary approaches to stop hypertension- and mediterranean-style dietary patterns and age-related cognitive change: The cache county study on memory, health and aging. Am. J. Clin. Nutr. 2013, 98, 1263–1271. [Google Scholar] [CrossRef]
- Berendsen, A.A.M.; Kang, J.H.; van de Rest, O.; Feskens, E.J.M.; de Groot, L.; Grodstein, F. The dietary approaches to stop hypertension diet, cognitive function, and cognitive decline in american older women. J. Am. Med. Dir. Assoc. 2017, 18, 427–432. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. Mind diet slows cognitive decline with aging. Alzheimers Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, A.M.; Kang, J.H.; Feskens, E.J.M.; de Groot, C.; Grodstein, F.; van de Rest, O. Association of long-term adherence to the mind diet with cognitive function and cognitive decline in american women. J. Nutr. Health Aging 2018, 22, 222–229. [Google Scholar] [CrossRef]
- Abbatecola, A.M.; Russo, M.; Barbieri, M. Dietary patterns and cognition in older persons. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 10–13. [Google Scholar] [CrossRef]
- Vauzour, D.; Camprubi-Robles, M.; Miquel-Kergoat, S.; Andres-Lacueva, C.; Banati, D.; Barberger-Gateau, P.; Bowman, G.L.; Caberlotto, L.; Clarke, R.; Hogervorst, E.; et al. Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res. Rev. 2017, 35, 222–240. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (finger): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.-N.; Dantoine, T.; Dartigues, J.-F.; et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (mapt): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef]
- Wesselman, L.M.P.; Schild, A.-K.; Coll-Padros, N.; van der Borg, W.E.; Meurs, J.H.P.; Hooghiemstra, A.M.; Slot, R.E.R.; Sannemann, L.; Rami, L.; Molinuevo, J.L.; et al. Wishes and preferences for an online lifestyle program for brain health—A mixed methods study. Alzheimer’s Dement. Translational Res. Clin. Interv. 2018, 4, 141–149. [Google Scholar] [CrossRef]
- Smart, C.M.; Karr, J.E.; Areshenkoff, C.N.; Rabin, L.A.; Hudon, C.; Gates, N.; Ali, J.I.; Arenaza-Urquijo, E.M.; Buckley, R.F.; Chetelat, G.; et al. Non-pharmacologic interventions for older adults with subjective cognitive decline: Systematic review, meta-analysis, and preliminary recommendations. Neuropsychol. Rev. 2017, 27, 245–257. [Google Scholar] [CrossRef]
- Slot, R.E.R.; Verfaillie, S.C.J.; Overbeek, J.M.; Timmers, T.; Wesselman, L.M.P.; Teunissen, C.E.; Dols, A.; Bouwman, F.H.; Prins, N.D.; Barkhof, F.; et al. Subjective cognitive impairment cohort (science): Study design and first results. Alzheimers Res. Ther. 2018, 10, 76. [Google Scholar] [CrossRef]
- van der Flier, W.M.; Scheltens, P. Amsterdam dementia cohort: Performing research to optimize care. J. Alzheimers Dis. 2018, 62, 1091–1111. [Google Scholar] [CrossRef]
- van Lee, L.; Geelen, A.; Hooft van Huysduynen, E.J.C.; de Vries, J.H.M.; van’t Veer, P.; Feskens, E.J.M. The dutch healthy diet index (dhd-index): An instrument to measure adherence to the dutch guidelines for a healthy diet. Nutr. J. 2012, 11, 49. [Google Scholar] [CrossRef]
- Health Council of the Netherlands. Guidelines for A Healthy Diet 2006; Health Council of the Netherlands: The Hague, The Netherlands, 2006. [Google Scholar]
- Health Council of the Netherlands. Guidelines for A Healthy Diet 2006—Background Document; Health Council of the Netherlands: The Hague, The Netherlands, 2006. [Google Scholar]
- The Netherlands Nutrition Centre. Available online: http://www.Voedingscentrum.Nl (accessed on 10 May 2019).
- van Lee, L.; Feskens, E.J.; Meijboom, S.; Hooft van Huysduynen, E.J.; van’t Veer, P.; de Vries, J.H.; Geelen, A. Evaluation of a screener to assess diet quality in the netherlands. Br. J. Nutr. 2016, 115, 517–526. [Google Scholar] [CrossRef]
- Folstein, M. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Saykin, A.J.; Wishart, H.A.; Rabin, L.A.; Santulli, R.B.; Flashman, L.A.; West, J.D.; McHugh, T.L.; Mamourian, A.C. Older adults with cognitive complaints show brain atrophy similar to that of amnestic mci. Neurology 2006, 67, 834–842. [Google Scholar] [CrossRef]
- Radloff, L.S. The ces-d scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- IBM. Ibm Spss Statistics for Windows; Version 22.0; IBM Corp: New York, NY, USA, 2011. [Google Scholar]
- Bhushan, A.; Fondell, E.; Ascherio, A.; Yuan, C.; Grodstein, F.; Willett, W. Adherence to mediterranean diet and subjective cognitive function in men. Eur. J. Epidemiol. 2018, 33, 223–234. [Google Scholar] [CrossRef]
- Yuan, C.; Fondell, E.; Bhushan, A.; Ascherio, A.; Okereke, O.I.; Grodstein, F.; Willett, W.C. Long-term intake of vegetables and fruits and subjective cognitive function in us men. Neurology 2019, 92, e63–e75. [Google Scholar] [CrossRef]
- Gardener, S.; Gu, Y.; Rainey-Smith, S.R.; Keogh, J.B.; Clifton, P.M.; Mathieson, S.L.; Taddei, K.; Mondal, A.; Ward, V.K.; Scarmeas, N.; et al. Adherence to a mediterranean diet and alzheimer’s disease risk in an australian population. Transl. Psychiatry 2012, 2, e164. [Google Scholar] [CrossRef]
- Cabout, M.; Brouwer, I.A.; Visser, M. The moodfood project: Prevention of depression through nutritional strategies. Nutr. Bull. 2017, 42, 94–103. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M.; et al. Relationships of dietary patterns, foods, and micro- and macronutrients with alzheimer’s disease and late-life cognitive disorders: A systematic review. J. Alzheimers Dis. 2017, 59, 815–849. [Google Scholar] [CrossRef]
- Lehtisalo, J.; Ngandu, T.; Valve, P.; Antikainen, R.; Laatikainen, T.; Strandberg, T.; Soininen, H.; Tuomilehto, J.; Kivipelto, M.; Lindstrom, J. Nutrient intake and dietary changes during a 2-year multi-domain lifestyle intervention among older adults: Secondary analysis of the finnish geriatric intervention study to prevent cognitive impairment and disability (finger) randomised controlled trial. Br. J. Nutr. 2017, 118, 291–302. [Google Scholar] [CrossRef]
- Rosenberg, A.; Ngandu, T.; Rusanen, M.; Antikainen, R.; Backman, L.; Havulinna, S.; Hanninen, T.; Laatikainen, T.; Lehtisalo, J.; Levalahti, E.; et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The finger trial. Alzheimers Dement. 2018, 14, 263–270. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, J.; Song, D.; Deng, R.; Wei, J.; Zhang, Z. Increased consumption of fruit and vegetables is related to a reduced risk of cognitive impairment and dementia: Meta-analysis. Front Aging Neurosci. 2017, 9, 18. [Google Scholar] [CrossRef]
- World Helath Organization. Healthy Diet. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 10 May 2019).
| Nutritional Item (Mean ± SD) | Total SCD Group (N = 165) | General Dutch Population [30] |
|---|---|---|
| DHD-FFQ total score | 54.3 (12.2) | 57.6 (9.6) |
| Vegetables | 6.6 (2.9) | 6.7 (2.6) |
| Fruit | 7.8 (2.8) | 8.0 (2.7) |
| Fibers | 7.5 (1.9) | 7.8 (1.9) |
| Fish | 6.2 (3.4) | 5.5 (3.2) |
| Saturated fat * | 4.5 (4.1) | 5.5 (4.0) |
| Trans fatty acids * | 7.3 (4.6) | 9.2 (2.7) |
| Salt * | 5.9 (3.0) | 6.3 (2.8) |
| Alcohol * | 8.7 (2.7) | 8.6 (2.7) |
| Nutritional Item | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| Saturated fat | 0.837 | 0.004 | −0.031 |
| Salt | 0.821 | −0.031 | 0.025 |
| Trans fatty acids | 0.807 | 0.094 | 0.010 |
| Vegetables | −0.042 | 0.792 | −0.137 |
| Fruit | 0.205 | 0.718 | 0.129 |
| Fibers | −0.566 | 0.649 | 0.098 |
| Alcohol | −0.017 | 0.172 | 0.868 |
| Fish | −0.003 | 0.457 | −0.581 |
| Nutritional Score | Model | MMSE | CCI | CES-D |
|---|---|---|---|---|
| DHD-FFQ Total Score | 1 | β 0.10 (−0.01–0.03) | β −0.01 (−0.21–0.18) | β −0.11 (−0.15–0.02) |
| 2 | β 0.12 (−0.01.–0.03) | β 0.03 (−0.16–0.24) | β −0.13 (−0.16–0.02) | |
| “Low-Fat-low-Salt” Component | 1 | β −0.10 (−0.36–0.08) | β −0.01 (−2.5–2.2) | β −0.17 * (−2.1–−0.10) |
| 2 | β −0.10 (−0.36–0.09) | β 0.04 (−1.8–3.0) | β −0.18 * (−2.27–−0.16) | |
| “high-Veggy” Component | 1 | β 0.27 ** (0.17–0.59) | β −0.04 (−2.9–1.8) | β 0.03 (−0.82–1.25) |
| 2 | β 0.30 ** (0.21–0.64) | β −0.02 (−2.7–2.0) | β 0.02 (−0.91–1.23) | |
| “Low-Alcohol-low-Fish” Component | 1 | β −0.09 (−0.35–0.09) | β 0.15 (−0.12–4.5) | β 0.01 (−0.99–1.08) |
| 2 | β −0.10 (−0.37–0.08) | β 0.12 (−0.60–4.1) | β 0.01 (−0.98–1.13) |
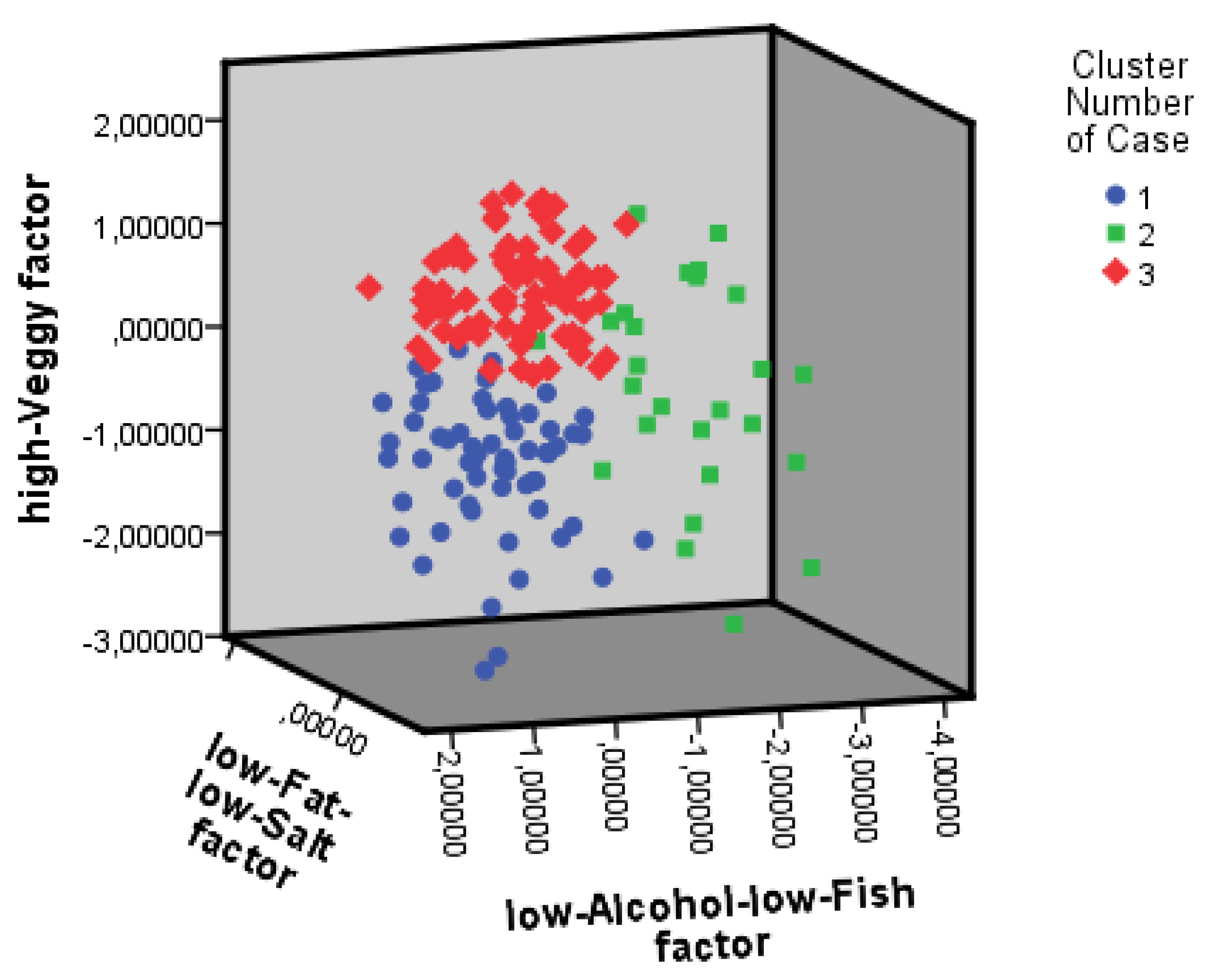
| Characteristic Mean (SD) | Cluster 1 n = 61 | Cluster 2 n = 26 | Cluster 3 n = 78 |
|---|---|---|---|
| Female, n (%) | 22 (36%) & | 18 (69%) # | 34 (44%) &# |
| Age, year | 62.3 (8.0) | 63.9 (8.4) | 65.1 (7.9) |
| Education, year | 9.1 (4.9) & | 11.2 (5.6) | 11.3 (4.8) & |
| BMI (kg/m2) | 26 (4) | 27 (4) | 25 (3) |
| Smoker a: n (%) | |||
| no | 23 (37%) | 13 (50%) | 40 (51%) |
| previous | 27 (44%) | 11 (42%) | 31 (40%) |
| current | 9 (15%) | 1 (4%) | 3 (4%) |
| DHD-FFQ total score | 48.0 (10.4) # | 50.5 (11.9) | 61.2 (9.6) # |
| Vegetables | 4.0 (2.1) # | 7.0 (2.5) # | 8.5 (1.8) # |
| Fruit | 6.1 (3.1) # | 7.0 (3.0) & | 9.4 (1.2) #& |
| Fibers | 6.4 (1.8) # | 7.0 (1.9) & | 8.4 (1.5) #& |
| Fish | 4.5 (3.2) # | 8.2 (2.8) #& | 7.0 (3.2) & |
| Saturated fat * | 4.1 (4.2) | 4.9 (4.2) | 4.8 (4.0) |
| Trans fatty acids * adherence yes, n (%) | 40 (66%) | 18 (69%) | 58 (74%) |
| Salt * | 5.6 (3.3) | 6.2 (2.9) | 5.9 (2.9) |
| Alcohol * | 9.7 (0.9) # | 3.4 (2.9) #& | 9.7 (1.0) & |
| MMSE | 28.3 (1.8) | 28.8 (1.4) | 28.8 (1.0) |
| CCI | 47.1 (15.9) & | 38.5 (12.7) & | 42.4 (14.9) |
| CES-D | 9.6 (6.9) | 9.7 (6.9) | 9.0 (6.5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wesselman, L.M.P.; Doorduijn, A.S.; de Leeuw, F.A.; Verfaillie, S.C.J.; van Leeuwenstijn-Koopman, M.; Slot, R.E.R.; Kester, M.I.; Prins, N.D.; van de Rest, O.; de van der Schueren, M.A.E.; et al. Dietary Patterns Are Related to Clinical Characteristics in Memory Clinic Patients with Subjective Cognitive Decline: The SCIENCe Project. Nutrients 2019, 11, 1057. https://doi.org/10.3390/nu11051057
Wesselman LMP, Doorduijn AS, de Leeuw FA, Verfaillie SCJ, van Leeuwenstijn-Koopman M, Slot RER, Kester MI, Prins ND, van de Rest O, de van der Schueren MAE, et al. Dietary Patterns Are Related to Clinical Characteristics in Memory Clinic Patients with Subjective Cognitive Decline: The SCIENCe Project. Nutrients. 2019; 11(5):1057. https://doi.org/10.3390/nu11051057
Chicago/Turabian StyleWesselman, Linda M. P., Astrid S. Doorduijn, Francisca A. de Leeuw, Sander C. J. Verfaillie, Mardou van Leeuwenstijn-Koopman, Rosalinde E. R. Slot, Maartje I. Kester, Niels D. Prins, Ondine van de Rest, Marian A. E. de van der Schueren, and et al. 2019. "Dietary Patterns Are Related to Clinical Characteristics in Memory Clinic Patients with Subjective Cognitive Decline: The SCIENCe Project" Nutrients 11, no. 5: 1057. https://doi.org/10.3390/nu11051057
APA StyleWesselman, L. M. P., Doorduijn, A. S., de Leeuw, F. A., Verfaillie, S. C. J., van Leeuwenstijn-Koopman, M., Slot, R. E. R., Kester, M. I., Prins, N. D., van de Rest, O., de van der Schueren, M. A. E., Scheltens, P., Sikkes, S. A. M., & van der Flier, W. M. (2019). Dietary Patterns Are Related to Clinical Characteristics in Memory Clinic Patients with Subjective Cognitive Decline: The SCIENCe Project. Nutrients, 11(5), 1057. https://doi.org/10.3390/nu11051057





