Plasma Metabolites Associated with Coffee Consumption: A Metabolomic Approach within the PREDIMED Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects Selection
2.3. Metabolomics
2.4. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
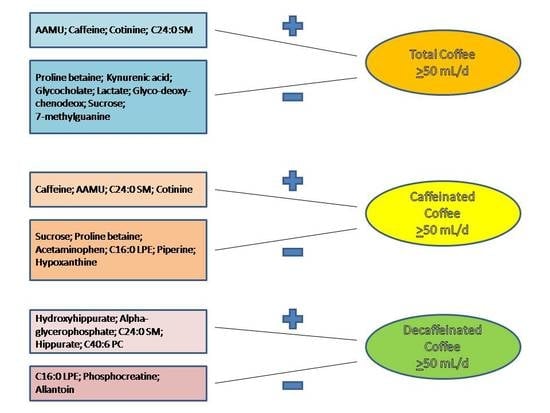
3.2. Associations between Plasma Metabolites and Total Coffee Consumption
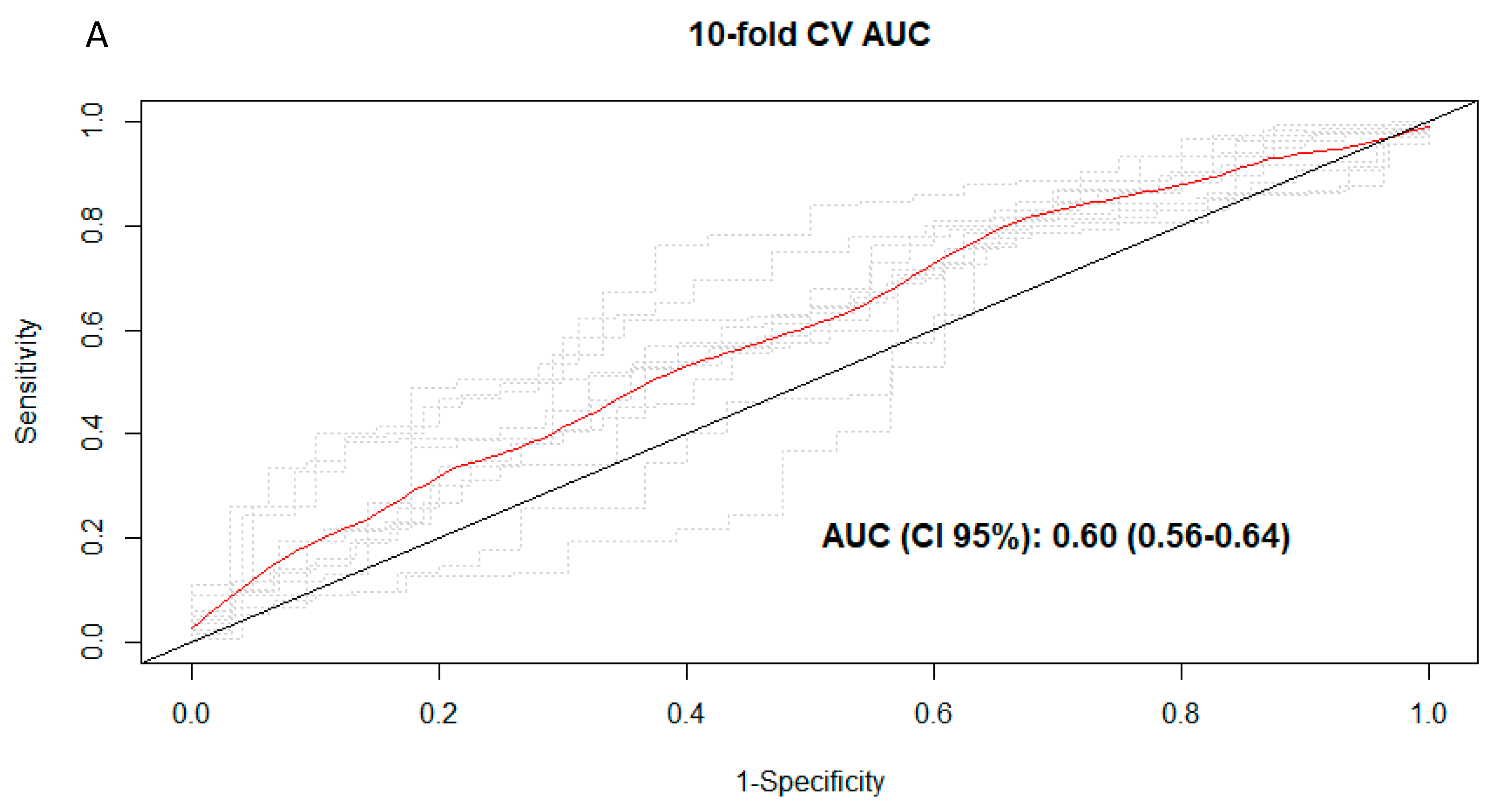
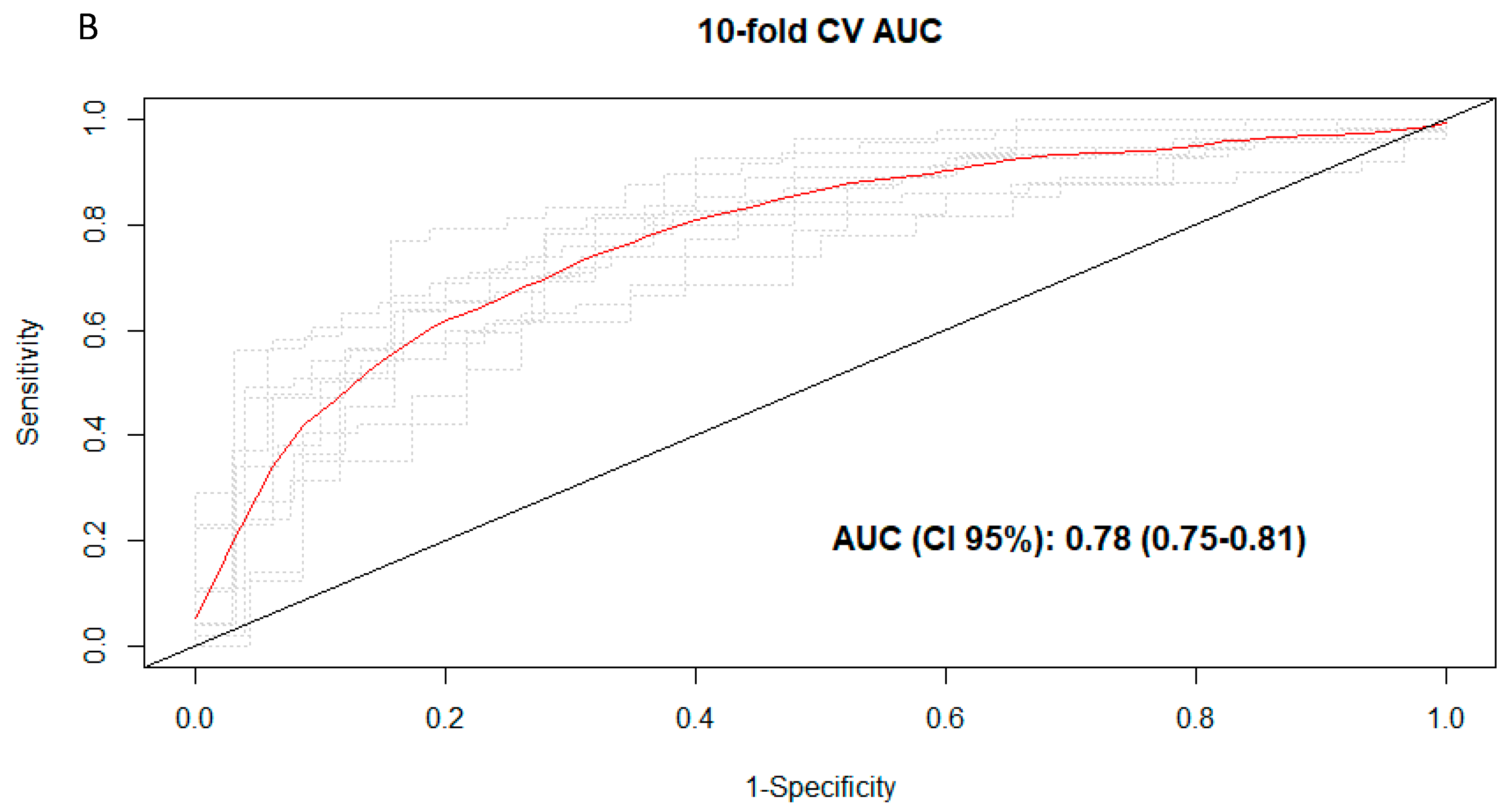
3.3. Discrimination of Total and Types of Coffee Consumption
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAMU | 5-acetylamino-6-amino-3-methyluracil |
| alpha-GP | alpha-glycerophosphate |
| AUC | area under the curve |
| IFN-γ | interferon-γ |
| LC–MS | liquid chromatography tandem mass spectrometry |
| LC-CoA | long-chain coenzyme A |
| LPE | lyso-phosphatidylethanolamine |
| SM | sphingomyelin |
| TAG | triacylglycerol |
References
- International Coffee Organization. Annual Review. 2015/2016; International Coffee Organization: London, UK, 2017. [Google Scholar]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Bhupathiraju, S.N.; Chen, M.; van Dam, R.M.; Hu, F.B. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: A systematic review and a dose-response meta-analysis. Diabetes Care 2014, 37, 569–586. [Google Scholar] [CrossRef]
- Satija, A.; Yu, E.; Willett, W.C.; Hu, F.B. Understanding nutritional epidemiology and its role in policy. Adv. Nutr. 2015, 6, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Guertin, K.A.; Moore, S.C.; Sampson, J.N.; Huang, W.Y.; Xiao, Q.; Stolzenberg-Solomon, R.Z.; Sinha, R.; Cross, A.J. Metabolomics in nutritional epidemiology: Identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am. J. Clin. Nutr. 2014, 100, 208–217. [Google Scholar] [CrossRef]
- Altmaier, E.; Kastenmüller, G.; Römisch-Margl, W.; Thorand, B.; Weinberger, K.M.; Adamski, J.; Illig, T.; Döring, A.; Suhre, K. Variation in the human lipidome associated with coffee consumption as revealed by quantitative targeted metabolomics. Mol. Nutr. Food Res. 2009, 53, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.; Kröger, J.; Floegel, A.; Boeing, H.; Drogan, D.; Pischon, T.; Fritsche, A.; Prehn, C.; Adamski, J.; Isermann, B.; et al. Evaluation of various biomarkers as potential mediators of the association between coffee consumption and incident type 2 diabetes in the EPIC-Potsdam Study. Am. J. Clin. Nutr. 2014, 100, 891–900. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: Identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Redeuil, K.; Smarrito-Menozzi, C.; Guy, P.; Rezzi, S.; Dionisi, F.; Williamson, G.; Nagy, K.; Renouf, M. Identification of novel circulating coffee metabolites in human plasma by liquid chromatography-mass spectrometry. J. Chromatogr. A 2011, 1218, 4678–4688. [Google Scholar] [CrossRef]
- Nagy, K.; Redeuil, K.; Williamson, G.; Rezzi, S.; Dionisi, F.; Longet, K.; Destaillats, F.; Renouf, M. First identification of dimethoxycinnamic acids in human plasma after coffee intake by liquid chromatography-mass spectrometry. J. Chromatogr. A 2011, 1218, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, M.C.; Erlund, I.; Michelotti, G.A.; Herder, C.; Westerhuis, J.A.; Tuomilehto, J. Metabolomic response to coffee consumption: Application to a three-stage clinical trial. J. Intern. Med. 2018, 283, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Kuang, A.; Erlund, I.; Herder, C.; Westerhuis, J.A.; Tuomilehto, J.; Cornelis, M.C. Lipidomic Response to Coffee Consumption. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Midttun, Ø.; Ulvik, A.; Nygård, O.; Ueland, P.M. Performance of plasma trigonelline as a marker of coffee consumption in an epidemiologic setting. Am. J. Clin. Nutr. 2018, 107, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, D.; Estoppey Younes, S.; Pruijm, M.; Ponte, B.; Ackermann, D.; Ehret, G.; Ansermot, N.; Mohaupt, M.; Paccaud, F.; Vogt, B.; et al. Relation of 24-hour urinary caffeine and caffeine metabolite excretions with self-reported consumption of coffee and other caffeinated beverages in the general population. Nutr. Metab. (Lond.) 2016, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Babyak, M.A. What you see may not be what you get: A brief, nontechnical introduction to overfitting in regression-type models. Psychosom. Med. 2004, 66, 411. [Google Scholar] [PubMed]
- Rostagno, M.A.; Manchón, N.; D’Arrigo, M.; Guillamón, E.; Villares, A.; García-Lafuente, A.; Ramos, A.; Martínez, J.A. Fast and simultaneous determination of phenolic compounds and caffeine in teas, mate, instant coffee, soft drink and energetic drink by high-performance liquid chromatography using a fused-core column. Anal Chim. Acta 2011, 685, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevención con DietaMediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.Á.; Corella, D.; Salas-Salvadó, J.; Ros, E.; Covas, M.I.; Fiol, M.; Wärnberg, J.; Arós, F.; Ruíz-Gutiérrez, V.; Lamuela-Raventós, R.M.; et al. Cohort profile: Design and methods of the PREDIMED study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef]
- Papandreou, C.; Bulló, M.; Zheng, Y.; Ruiz-Canela, M.; Yu, E.; Guasch-Ferré, M.; Toledo, E.; Clish, C.; Corella, D.; Estruch, R.; et al. Plasma trimethylamine-N-oxide and related metabolites are associated with type 2 diabetes risk in the Prevención con DietaMediterránea (PREDIMED) trial. Am. J. Clin. Nutr. 2018, 108, 163–173. [Google Scholar]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.A.; Salas-Salvadó, J.; Martín-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef]
- Moreiras, O.; Carvajal, A.; Cabrera, L.C.C. Tablas de Composición de Alimentos “FoodCompositionTables” Pirámide; Pirámide: Madrid, Spain, 2005. [Google Scholar]
- Mascanfroni, I.D.; Takenaka, M.C.; Yeste, A.; Patel, B.; Wu, Y.; Kenison, J.E.; Siddiqui, S.; Basso, A.S.; Otterbein, L.E.; Pardoll, D.M.; et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat. Med. 2015, 21, 638–646. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.F.; Morningstar, J.E.; Yang, Q.; Zheng, B.; Gao, Y.; Jeanfavre, S.; Scott, J.; Fernandez, C.; Zheng, H.; O’Connor, S.; et al. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J. Clin. Investig. 2017, 127, 4394–4402. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.; Jiang, S.; Korem, T.; Szymanski, J.; Chang, M.L.; Szelog, J.; Cassalman, C.; Dasuri, K.; McGuire, C.; Nagai, R.; et al. Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2017, 114, 4472–4481. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Fillâtre, Y.; Martin, J.F.; Lyan, B.; Pujos-Guillot, E.; Fezeu, L.; Hercberg, S.; Comte, B.; Galan, P.; Touvier, M.; et al. New biomarkers of coffee consumption identified by the non-targeted metabolomic profiling of cohort study subjects. PLoS ONE 2014, 9, e93474. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Sabroe, M.; Poulsen, H.E. Measurement of caffeine and five of the major metabolites in urine by high-performance liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 2005, 40, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Moran, V.E. Cotinine: Beyond that Expected, More than a Biomarker of Tobacco Consumption. Front. Pharmacol. 2012, 3, 173. [Google Scholar] [CrossRef] [PubMed]
- Bjørngaard, J.H.; Nordestgaard, A.T.; Taylor, A.E.; Treur, J.L.; Gabrielsen, M.E.; Munafò, M.R.; Nordestgaard, B.G.; Åsvold, B.O.; Romundstad, P.; Davey Smith, G. Heavier smoking increases coffee consumption: Findings from a Mendelian randomization analysis. Int. J. Epidemiol. 2017, 46, 1958–1967. [Google Scholar] [CrossRef] [PubMed]
- Getty-Kaushik, L.; Richard, A.M.; Corkey, B.E. Free fatty acid regulation of glucose-dependent intrinsic oscillatory lipolysis in perifused isolated rat adipocytes. Diabetes 2005, 54, 629–637. [Google Scholar] [CrossRef][Green Version]
- Pallister, T.; Jackson, M.A.; Martin, T.C.; Zierer, J.; Jennings, A.; Mohney, R.P.; MacGregor, A.; Steves, C.J.; Cassidy, A.; Spector, T.D.; et al. Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci. Rep. 2017, 7, 13670. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Lang, T.; Bader, M.; Beusch, A.; Schlagbauer, V.; Hofmann, T. High-Throughput Quantitation of Proline Betaine in Foods and Suitability as a Valid Biomarker for Citrus Consumption. J. Agric. Food Chem. 2017, 65, 1613–1619. [Google Scholar] [CrossRef]
- Maughan, R.J.; Griffin, J. Caffeine ingestion and fluid balance: A review. J. Hum. Nutr. Diet. 2003, 16, 411–420. [Google Scholar] [CrossRef]
- Leila Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine-The Bioactive Compound of Black Pepper: From Isolation to Medicinal Formulations. Compr. Rev. Food Sci. Food Saf. 2016, 16. [Google Scholar] [CrossRef]
- Tamae, K.; Kawai, K.; Yamasaki, S.; Kawanami, K.; Ikeda, M.; Takahashi, K.; Miyamoto, T.; Kato, N.; Kasai, H. Effect of age, smoking and other lifestyle factors on urinary 7-methylguanine and 8-hydroxydeoxyguanosine. Cancer Sci. 2009, 100, 715–721. [Google Scholar] [CrossRef]
- Giofré, M.R.; Meduri, G.; Pallio, S.; Calandra, S.; Magnano, A.; Niceforo, D.; Cinquegrani, M.; di Leo, V.; Mazzon, E.; Sturniolo, G.C.; et al. Gastric permeability to sucrose is increased in portal hypertensive gastropathy. Eur. J. Gastroenterol. Hepatol. 2000, 12, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Loftfield, E.; Shiels, M.S.; Graubard, B.I.; Katki, H.A.; Chaturvedi, A.K.; Trabert, B.; Pinto, L.A.; Kemp, T.J.; Shebl, F.M.; Mayne, S.T.; et al. Associations of Coffee Drinking with Systemic Immune and Inflammatory Markers. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Gostner, J.M.; Schroecksnadel, S.; Jenny, M.; Klein, A.; Ueberall, F.; Schennach, H.; Fuchs, D. Coffee extracts suppress tryptophan breakdown in mitogen-stimulated peripheral blood mononuclear cells. J. Am. Coll. Nutr. 2015, 34, 212–223. [Google Scholar] [CrossRef]
- Woollett, L.A.; Buckley, D.D.; Yao, L.; Jones, P.J.; Granholm, N.A.; Tolley, E.A.; Tso, P.; Heubi, J.E. Cholic acid supplementation enhances cholesterol absorption in humans. Gastroenterology 2004, 126, 724–731. [Google Scholar] [CrossRef]
- Staels, B.; Fonseca, V.A. Bile acids and metabolic regulation: Mechanisms and clinical responses to bile acid sequestration. Diabetes Care 2009, 32, 237–245. [Google Scholar] [CrossRef]
- Jee, S.H.; He, J.; Appel, L.J.; Whelton, P.K.; Suh, I.; Klag, M.J. Coffee consumption and serum lipids: A meta-analysis of randomized controlled clinical trials. Am. J. Epidemiol. 2001, 153, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Ma, D.; Zhang, Y.; Liu, Z.; Wang, P. The effect of coffee consumption on serum lipids: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2012, 66, 872–877. [Google Scholar] [CrossRef]
- Arciero, P.J.; Gardner, A.W.; Calles-Escandon, J.; Benowitz, N.L.; Poehlman, E.T. Effects of caffeine ingestion on NE kinetics, fat oxidation, and energy expenditure in younger and older men. Am. J. Physiol. 1995, 268, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Engels, H.J.; Haymes, E.M. Effects of caffeine ingestion on metabolic responses to prolonged walking in sedentary males. Int. J. Sport Nutr. 1992, 2, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Il’yasova, D.; Spasojevic, I.; Base, K.; Zhang, H.; Wang, F.; Young, S.P.; Millington, D.S.; D’Agostino, R.B., Jr.; Wagenknecht, L.E. Urinary F2-isoprostanes as a biomarker of reduced risk of type 2 diabetes. Diabetes Care 2012, 35, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Kiyohara, C.; Kono, S.; Honjo, S.; Todoroki, I.; Sakurai, Y.; Nishiwaki, M.; Hamada, H.; Nishikawa, H.; Koga, H.; Ogawa, S.; et al. Inverse association between coffee drinking and serum uric acid concentrations in middle-aged Japanese males. Br. J. Nutr. 1999, 82, 125–130. [Google Scholar] [PubMed]
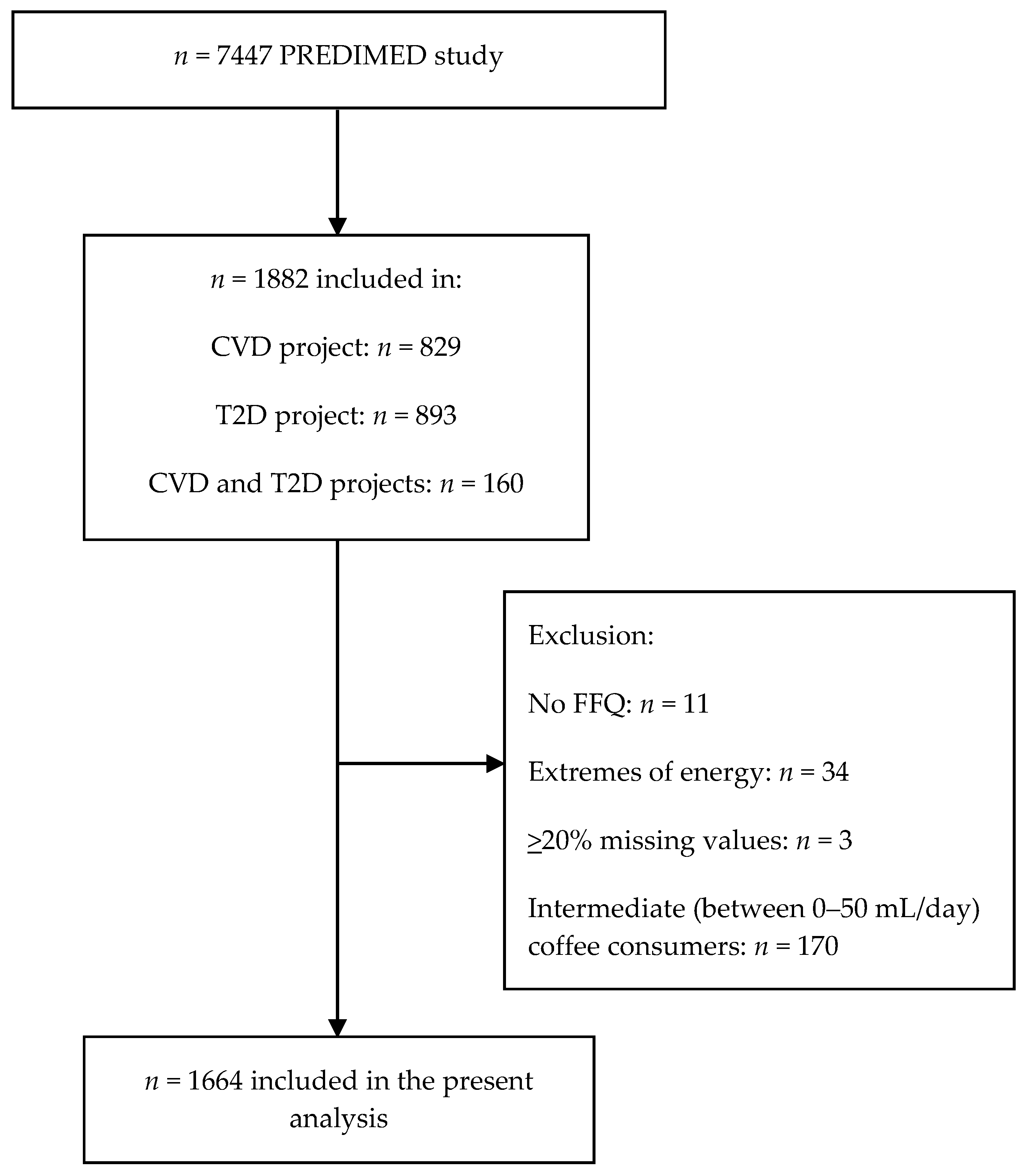
| Non-Coffee Consumers | Total Coffee Consumers | Caffeinated Coffee Consumers | Decaffeinated Coffee Consumers | Total Subjects | |
|---|---|---|---|---|---|
| Characteristic | n = 285 | n = 1379 | n = 512 | n = 721 | n = 1664 |
| Coffee consumption (mL/day) * | 0 (0, 0) | 50 (50, 475) | 50 (50, 250) | 50 (50, 350) | 50 (0, 475) |
| Male sex, N (%) | 102 (35.8) | 591 (42.9) b | 257 (50.2) b | 265 (36.8) | 693 (41.6) |
| Age (years) | 67.64 ± 6.25 | 67.04 ± 5.94 | 66.32 ± 5.93 a | 67.71 ± 5.94 | 67.14 ± 6 |
| Body mass index (kg/m2) | 29.42 ± 3.42 | 30.03 ± 3.62 a | 29.7 ± 3.52 | 30.25 ± 3.7 a | 29.92 ± 3.59 |
| Waist circumference (cm) | 99.61 ± 9.9 | 100.29 ± 10.22 | 100.33 ± 9.67 | 100.26 ± 10.56 | 100.17 ± 10.17 |
| Smoking, N (%) | |||||
| Never | 196 (68.8) | 793 (57.5) b | 262 (51.2) b | 461 (63.9) | 989 (59.4) |
| Former | 58 (20.4) | 344 (24.9) | 131 (25.6) | 173 (24.0) | 402 (24.2) |
| Current | 31 (10.9) | 242 (17.5) | 119 (23.2) | 87 (12.1) | 273 (16.4) |
| Type 2 diabetes, N (%) | 80 (28.1) | 375 (27.2) | 150 (29.3) | 189 (26.2) | 455 (27.3) |
| Dyslipidemia, N (%) | 202 (70.9) | 1077 (78.1) b | 411 (80.3) b | 553 (76.7) | 1279 (76.9) |
| Hypertension, N (%) | 254 (89.1) | 1192 (86.4) | 426 (83.2) b | 640 (88.8) | 1446 (86.9) |
| Family history of CVD, N (%) | 79 (27.7) | 338 (24.5) | 123 (24.0) | 188 (26.1) | 417 (25.1) |
| Cardiac medication, N (%) | 25 (9) | 122 (9.1) | 42 (8.4) | 72 (10.3) | 147 (9.1) |
| Antihypertensive agents, N (%) | 211 (74.6) | 1034 (75.1) | 360 (70.5) | 566 (78.7) | 1245 (75) |
| Lipid-lowering medication, N (%) | 120 (42.3) | 653 (47.5) | 225 (44) | 358 (49.8) | 773 (46.6) |
| Insulin medication, N (%) | 8 (2.8) | 57 (4.1) | 16 (3.1) | 32 (4.5) | 65 (3.9) |
| Oral antidiabetics, N (%) | 51 (18) | 262 (19) | 112 (21.9) | 122 (17) | 313 (18.9) |
| MedDiet score | 8.73 ± 1.86 | 8.63 ± 1.86 | 8.54 ± 1.93 | 8.74 ± 1.83 | 8.65 ± 1.86 |
| Total Coffee | Caffeinated Coffee | Decaffeinated Coffee |
|---|---|---|
| AAMU 0.462 | Caffeine 0.545 | Hydroxyhippurate 0.065 |
| Caffeine 0.330 | AAMU 0.140 | Alpha-glycerophosphate 0.047 |
| Cotinine 0.022 | C24:0 SM 0.031 | C24:0 SM 0.018 |
| C24:0 SM 0.015 | Cotinine 0.015 | Hippurate 0.014 |
| C40:6 PC 0.006 |
| Total Coffee | Caffeinated Coffee | Decaffeinated Coffee |
|---|---|---|
| Proline betaine −0.031 | Sucrose −0.062 | C16:0 LPE −0.025 |
| Kynurenic acid −0.018 | Proline betaine −0.019 | Phosphocreatine −0.017 |
| Glycocholate −0.016 | Acetaminophen −0.017 | Allantoin −0.009 |
| Lactate −0.016 | C16:0 LPE −0.011 | |
| Glyco-deoxy-chenodeox −0.013 | Piperine −0.006 | |
| Sucrose −0.007 | Hypoxanthine −0.002 | |
| 7-methylguanine −0.006 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papandreou, C.; Hernández-Alonso, P.; Bulló, M.; Ruiz-Canela, M.; Yu, E.; Guasch-Ferré, M.; Toledo, E.; Dennis, C.; Deik, A.; Clish, C.; et al. Plasma Metabolites Associated with Coffee Consumption: A Metabolomic Approach within the PREDIMED Study. Nutrients 2019, 11, 1032. https://doi.org/10.3390/nu11051032
Papandreou C, Hernández-Alonso P, Bulló M, Ruiz-Canela M, Yu E, Guasch-Ferré M, Toledo E, Dennis C, Deik A, Clish C, et al. Plasma Metabolites Associated with Coffee Consumption: A Metabolomic Approach within the PREDIMED Study. Nutrients. 2019; 11(5):1032. https://doi.org/10.3390/nu11051032
Chicago/Turabian StylePapandreou, Christopher, Pablo Hernández-Alonso, Mònica Bulló, Miguel Ruiz-Canela, Edward Yu, Marta Guasch-Ferré, Estefanía Toledo, Courtney Dennis, Amy Deik, Clary Clish, and et al. 2019. "Plasma Metabolites Associated with Coffee Consumption: A Metabolomic Approach within the PREDIMED Study" Nutrients 11, no. 5: 1032. https://doi.org/10.3390/nu11051032
APA StylePapandreou, C., Hernández-Alonso, P., Bulló, M., Ruiz-Canela, M., Yu, E., Guasch-Ferré, M., Toledo, E., Dennis, C., Deik, A., Clish, C., Razquin, C., Corella, D., Estruch, R., Ros, E., Fitó, M., Arós, F., Fiol, M., Lapetra, J., Ruano, C., ... Salas-Salvadó, J. (2019). Plasma Metabolites Associated with Coffee Consumption: A Metabolomic Approach within the PREDIMED Study. Nutrients, 11(5), 1032. https://doi.org/10.3390/nu11051032