Factors Associated with Increased Alpha-Tocopherol Content in Milk in Response to Maternal Supplementation with 800 IU of Vitamin E
Abstract
1. Introduction
2. Materials and Methods
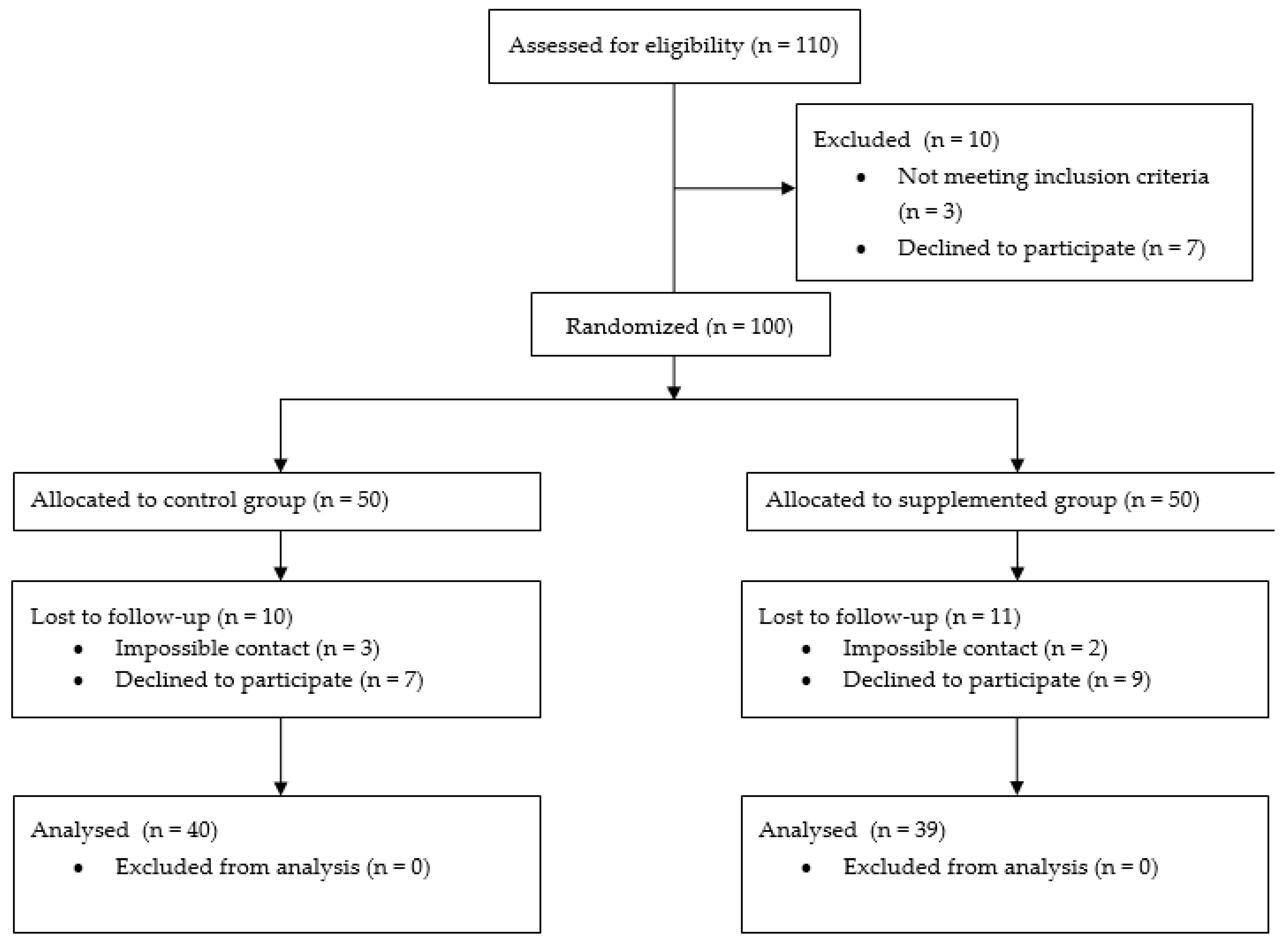
2.1. Participants and Intervention
2.2. Data Collection
2.3. Determination of Alpha-Tocopherol and Lipid Profile in Biological Samples
2.4. Dietary Intake of Vitamin E
2.5. Statistical Analysis
3. Results
3.1. General Characteristics of the Population
3.2. Effect of Vitamin E Supplementation on Serum and Breast Milk
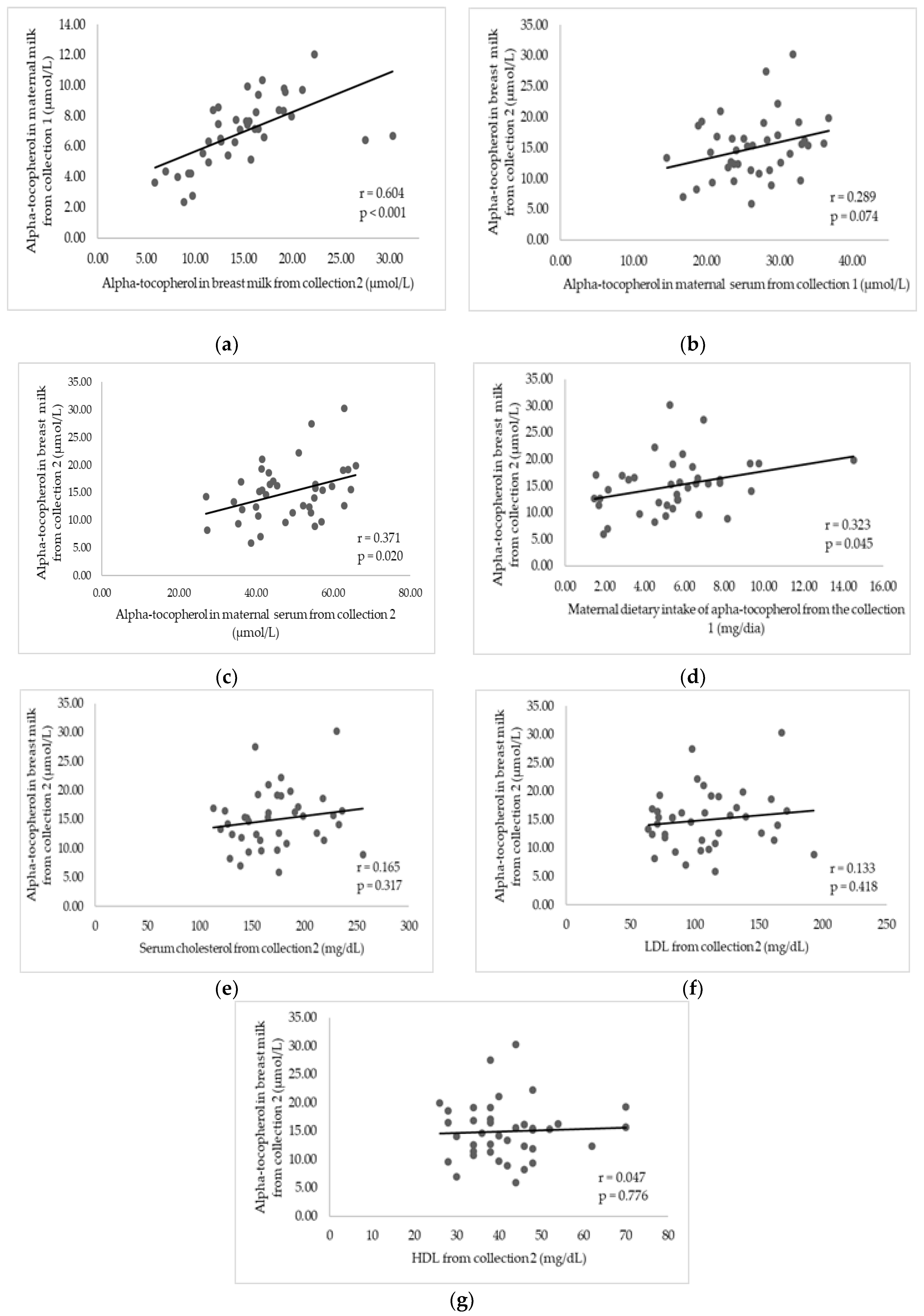
3.3. Factors Associated with Alpha-Tocopherol in Breast Milk after Supplementation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Debier, C. Vitamin E during pre- and postnatal periods. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2007; Volume 76, pp. 357–373. ISBN 9780123735928. [Google Scholar]
- Brasil. Saúde da Criança: Aleitamento Materno e Alimentação Complementar, 2rd ed.; Ministério da Saúde: Brasília, Brazil, 2015; ISBN 9788533422902.
- Traber, M.G. Vitamin E. In Present Knowledge in Nutrition, 10th ed.; Erdman, J.W., Jr., Macdonald, I.A., Zeisel, S.H., Eds.; ILSI Press: Washington, DC, USA, 2012; pp. 214–229. ISBN 978-0-470-95917-6. [Google Scholar]
- Schulpis, K.H.; Michalakakou, K.; Gavrili, S.; Karikas, G.A.; Lazaropoulou, C.; Vlachos, G.; Bakoula, C.; Papassotiriou, I. Maternal-neonatal retinol and alpha-tocopherol serum concentrations in Greeks and Albanians. Acta Paediatr. 2004, 93, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Fares, S.; Feki, M.; Khouaja-Mokrani, C.; Sethom, M.M.; Jebnoun, S.; Kaabachi, N. Nutritional practice effectiveness to achieve adequate plasma vitamin A, E and D during the early postnatal life in Tunisian very low birth weight infants. J. Matern.-Fetal Neonatal Med. 2015, 28, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Kositamongkol, S.; Suthutvoravut, U.; Chongviriyaphan, N.; Feungpean, B.; Nuntnarumit, P. Vitamin A and E status in very low birth weight infants. J. Perinatol. 2011, 31, 471–476. [Google Scholar] [CrossRef]
- Rodrigues, K.D.S.R. Estado Nutricional em Vitamina E de Mães e Crianças Pré-Termo e Termo do Nascimento aos 3 Meses Pós-Parto. Ph.D. Thesis, Departamento de Bioquímica, Universidade Federal do Rio Grande do Norte, Natal, Brazil, June 2016; 148p. [Google Scholar]
- Lima, M.S.R.; Dimenstein, R.; Ribeiro, K.D.S. Vitamin E concentration in human milk and associated factors: A literature review. J. Pediatria 2014, 90, 440–448. [Google Scholar] [CrossRef]
- Hampel, D.; Shahab-Ferdows, S.; Islam, M.M.; Peerson, J.M.; Allen, L.H. Vitamin Concentrations in Human Milk Vary with Time within Feed, Circadian Rhythm, and Single-Dose Supplementation. J. Nutr. 2017, 147, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Debier, C.; Pottier, J.; Goffe, C.; Larondelle, Y. Present knowledge and unexpected behaviours of vitamins A and E in colostrum and milk. Livest. Prod. Sci. 2005, 98, 135–147. [Google Scholar] [CrossRef]
- Tijerina-Sáenz, A.; Innis, S.; Kitts, D. Antioxidant capacity of human milk and its association with vitamins A and E and fatty acid composition. Acta Paediatr. 2009, 98, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Stuetz, W.; Carrara, V.; Mc Gready, R.; Lee, S.; Sriprawat, K.; Po, B.; Hanboonkunupakarn, B.; Grune, T.; Biesalski, H.; Nosten, F. Impact of Food Rations and Supplements on Micronutrient Status by Trimester of Pregnancy: Cross-Sectional Studies in the Maela Refugee Camp in Thailand. Nutrients 2016, 8, 66. [Google Scholar] [CrossRef]
- Szlagatys-Sidorkiewicz, A.; Zagierski, M.; Jankowska, A.; Łuczak, G.; Macur, K.; Bączek, T.; Korzon, M.; Krzykowski, G.; Martysiak-Żurowska, D.; Kamińska, B. Longitudinal study of vitamins A, E and lipid oxidative damage in human milk throughout lactation. Early Hum. Dev. 2012, 88, 421–424. [Google Scholar] [CrossRef]
- Olafsdottir, A.S.; Wagner, K.-H.; Thorsdottir, I.; Elmadfa, I. Fat-Soluble Vitamins in the Maternal Diet, Influence of Cod Liver Oil Supplementation and Impact of the Maternal Diet on Human Milk Composition. Ann. Nutr. Metab. 2001, 45, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.R.S.; Ribeiro, K.D.d.S.; de Araújo, K.F.; Azevedo, G.M.M.; Pires, J.F.; Batista, S.D.; Dimenstein, R. Níveis de alfa-tocoferol no soro e leite materno de puérperas atendidas em maternidade pública de Natal, Rio Grande do Norte. Revista Brasileira de Saúde Materno Infantil 2009, 9, 423–428. [Google Scholar] [CrossRef]
- Clemente, H.A.; Ramalho, H.M.M.; Lima, M.S.R.; Grilo, E.C.; Dimenstein, R. Maternal Supplementation with Natural or Synthetic Vitamin E and Its Levels in Human Colostrum. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Kuchan, M.J.; Lai, C.-S.; Jensen, S.K.; Sherry, C.L. Supplementation with RRR- or all-rac -α-Tocopherol Differentially Affects the α-Tocopherol Stereoisomer Profile in the Milk and Plasma of Lactating Women. J. Nutr. 2017, 147, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G. Vitamin E Inadequacy in Humans: Causes and Consequences. Adv. Nutr. 2014, 5, 503–514. [Google Scholar] [CrossRef]
- Medeiros, J.F.P.; da Silva Ribeiro, K.D.; Lima, M.S.R.; das Neves, R.A.M.; Lima, A.C.P.; Dantas, R.C.S.; da Silva, A.B.; Dimenstein, R. α-Tocopherol in breast milk of women with preterm delivery after a single postpartum oral dose of vitamin E. Br. J. Nutr. 2016, 115, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Lira, L.Q. Efeito de Dois Protocolos de Suplementação Materna com Alfa-Tocoferol Sobre o Soro e o Leite de Lactantes até 60 Dias Pós-Parto. Ph.D. Thesis, Departamento de Bioquímica, Universidade Federal do Rio Grande do Norte, Natal, Brazil, December 2017; 127p. [Google Scholar]
- de Melo, L.R.M.; Clemente, H.A.; Bezerra, D.F.; Dantas, R.C.S.; Ramalho, H.M.M.; Dimenstein, R. Effect of maternal supplementation with vitamin E on the concentration of α-tocopherol in colostrum. J. Pediatria 2017, 93, 40–46. [Google Scholar] [CrossRef]
- Cortês da Silva, A.L.; da Silva Ribeiro, K.D.; Miranda de Melo, L.R.; Fernandes Bezerra, D.; Carvalho de Queiroz, J.L.; Santa Rosa Lima, M.; Franco Pires, J.; Soares Bezerra, D.; Osório, M.M.; Dimenstein, R. Vitamina e no leite humano e sua relação com o requerimento nutricional do recém-nascido a termo. Revista Paulista de Pediatria 2017, 35, 158–164. [Google Scholar] [CrossRef]
- Ma, D.; Ning, Y.; Gao, H.; Li, W.; Wang, J.; Zheng, Y.; Zhang, Y.; Wang, P. Nutritional Status of Breast-Fed and Non-Exclusively Breast-Fed Infants from Birth to Age 5 Months in 8 Chinese Cities. Asia Pac. J. Clin. Nutr. 2014, 23, 282–292. [Google Scholar] [CrossRef]
- Antonakou, A.; Chiou, A.; Andrikopoulos, N.K.; Bakoula, C.; Matalas, A.-L. Breast milk tocopherol content during the first six months in exclusively breastfeeding Greek women. Eur. J. Nutr. 2011, 50, 195–202. [Google Scholar] [CrossRef]
- GPower Software. Available online: http://www.gpower.hhu.de (accessed on 19 June 2017).
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- de Lira, L.Q.; Lima, M.S.R.; de Medeiros, J.M.S.; da Silva, I.F.; Dimenstein, R. Correlation of vitamin A nutritional status on alpha-tocopherol in the colostrum of lactating women: Relationship of serum retinol and alpha-tocopherol in colostrum. Matern. Child Nutr. 2013, 9, 31–40. [Google Scholar] [CrossRef]
- Nierenberg, D.W.; Nann, S.L. A method for determining concentrations of retinol, tocopherol, and five carotenoids in human plasma and tissue samples. Am. J. Clin. Nutr. 1992, 56, 417–426. [Google Scholar] [CrossRef]
- Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000; ISBN 9780309069359.
- Martin, S.S.; Blaha, M.J.; Elshazly, M.B. Comparison of a novel method vs. the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA 2013, 310, 2061–2068. [Google Scholar] [CrossRef]
- Araújo, M.O.D.; Guerra, T.M. Alimentos per Capita, 3rd ed.; Editora Universitária—UFRN: João Pessoa, Brazil, 2007; p. 323. ISBN 9788572733. [Google Scholar]
- Tomita, L.Y.; Cardoso, M.A. Relação de Medidas Caseiras, Composição Química e Receitas de Alimentos Nipo-Brasileiros; Metha: São Paulo, Brazil, 2002; p. 85. ISBN 9788588888012. [Google Scholar]
- Virtual Nutri Plus. Available online: http:/www.virtualnutriplus.com.br/ (accessed on 10 January 2018).
- Schweigert, F.J.; Bathe, K.; Chen, F.; Boscher, U.; Dudenhausen, J.W. Effect of the stage of lactation in humans on carotenoid levels in milk, blood plasma and plasma lipoprotein fractions. Eur. J. Nutr. 2004, 43, 39–44. [Google Scholar] [CrossRef]
- Didenco, S.; Gillingham, M.B.; Go, M.D.; Leonard, S.W.; Traber, M.G.; McEvoy, C.T. Increased vitamin E intake is associated with higher α-tocopherol concentration in the maternal circulation but higher α-carboxyethyl hydroxychroman concentration in the fetal circulation. Am. J. Clin. Nutr. 2011, 93, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Campos-Gimenez, E.; Redeuil, K.M.; Leveques, A.; Actis-Goretta, L.; Vinyes-Pares, G.; Zhang, Y.; Wang, P.; Thakkar, S.K. Concentrations of Carotenoids and Tocopherols in Breast Milk from Urban Chinese Mothers and Their Associations with Maternal Characteristics: A Cross-Sectional Study. Nutrients 2017, 9, 1229. [Google Scholar] [CrossRef] [PubMed]
- Dimenstein, R.; Medeiros, A.C.P.; Cunha, L.R.F.; Araújo, K.F.; Dantas, J.C.O.; Macedo, T.M.S.; Stamford, T.L.M. Vitamin E in human serum and colostrum under fasting and postprandial conditions. J. Pediatria 2010, 86, 345–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, J.; Xiao, H.; Wu, K.; Yu, Z.; Ren, Y.; Zhao, Y.; Li, K.; Li, J.; Li, D. Retinol and α-tocopherol in human milk and their relationship with dietary intake during lactation. Food Funct. 2016, 7, 1985–1991. [Google Scholar] [CrossRef]
- Martysiak-Żurowska, D.; Szlagatys-Sidorkiewicz, A.; Zagierski, M. Concentrations of alpha- and gamma-tocopherols in human breast milk during the first months of lactation and in infant formulas: Tocopherols in human milk and infant formulas. Matern. Child Nutr. 2013, 9, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.W.; Good, C.K.; Gugger, E.; Traber, M.G. Vitamin E bioavailability from fortified breakfast cereal is greater than that from encapsulated supplements. Am. J. Clin. Nutr. 2004, 79, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.S.; Leonard, S.W.; Park, S.I.; Zhao, Y.; Traber, M.G. Human vitamin E requirements assessed with the use of apples fortified with deuteriumlabeled alpha-tocopheryl acetate. Am. J. Clin. Nutr. 2006, 83, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Leonard, S.W.; Bobe, G.; Fu, X.; Saltzman, E.; Grusak, M.A.; Estande, S.L. α-Tocopherol Disappearance Rates from Plasma Depend on Lipid Concentrations: Studies Using Deuterium-Labeled Collard Greens in Younger and Older Adults. Am. J. Clin. Nutr. 2015, 101, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.K.; Johannsen, A.K.B.; Hermansen, J.E. Quantitative secretion and maximal secretion capacity of retinol, β-carotene and α-tocopherol into cows’ milk. J. Dairy Res. 1999, 66, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Weiss, W.P.; Wyatt, D.J. Effect of Dietary Fat and Vitamin E on α-Tocopherol in Milk from Dairy Cows. J. Dairy Sci. 2003, 86, 3582–3591. [Google Scholar] [CrossRef]
- Lauridsen, C.; Engel, H.; Jensen, S.K.; Craig, A.M.; Traber, M.G. Lactating Sows and Suckling Piglets Preferentially Incorporate RRR- over All-rac-alpha-Tocopherol into Milk Plasma and Tissues. J. Nutr. 2002, 132, 1258–1264. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, J.; Li, S.; Zhang, R.; Chen, L.; Wang, Y.; Zheng, M.; Wang, M.; Liu, G.; Dai, Y.; et al. Over-Expression of Human Lipoprotein Lipase in Mouse Mammary Glands Leads to Reduction of Milk Triglyceride and Delayed Growth of Suckling Pups. PLoS ONE 2011, 6, 208–295. [Google Scholar] [CrossRef]
- Monks, J.; Huey, P.U.; Hanson, L.; Eckel, R.H.; Neville, M.C.; Gavigan, S.A. lipoprotein-containing particle is transferred from the serum across the mammary epithelium into the milk of lactating mice. J. Lipid Res. 2001, 42, 686–696. [Google Scholar]
- Mardones, P.; Rigotti, A. Cellular mechanisms of vitamin e uptake: Relevance in α-tocopherol metabolism and potential implications for disease. J. Nutr. Biochem. 2004, 15, 252–260. [Google Scholar] [CrossRef]
| Characteristics | Control Group n = 40 | Supplemented Group n = 39 | p-Value |
|---|---|---|---|
| Maternal age (years), mean (SD) | 27 (6.8) | 27 (6.8) | 0.833 * |
| Postpartum age (days), mean (SD) | 57 (25.8) | 56 (23.7) | 0.833 * |
| Education level n, (%) | |||
| Incomplete primary education | 4 (10.0) | 5 (12.8) | 0.149 |
| Complete primary education | 3 (7.5) | 2 (5.1) | |
| Incomplete secondary education | 14 (35.0) | 6 (15.4) | |
| Complete secondary education | 16 (40.0) | 22 (56.4) | |
| Complete higher education | 3 (7.5) | 4 (10.3) | |
| Family income level n, (%) a | |||
| <1 Minimum wage | 16 (40.0) | 23 (59.0) | 0.092 |
| >1 Minimum wage | 24 (60.0) | 16 (41.0) | |
| Type of delivery n, (%) | |||
| Vaginal | 15 (37.5) | 13 (33.3) | 0.699 |
| Caesarian | 25 (62.5) | 26 (66.7) | |
| Parity status n, (%) | |||
| Primiparous | 21 (52.5) | 17 (43.6) | 0.405 |
| Multtiparou | 19 (47.5) | 22 (56.4) | |
| BMI classification (kg/m2), (%) b | |||
| Low weight | 1 (2.5) | 0 (0) | 0.735 |
| Normal | 15 (37.5) | 18 (46.2) | |
| Overweight | 16 (40.0) | 13 (33.3) | |
| Obese | 8 (20.0) | 8 (20.5) | |
| Type of maternal breastfeeding n, (%) | |||
| Exclusive maternal breastfeeding | 35 (87.5) | 33 (84.6) | 0.711 |
| Maternal breast milk and other milks | 5 (12.5) | 6 (15.4) | |
| Calorie intake (Kcal/day), mean (SD) | 3248.4 (711.2) | 3270.7 (868.4) | 0.970 * |
| Intake of alpha-tocopherol (mg/day), mean (SD) | 8.7 (3.4) | 8.8 (3.5) | 0.901 * |
| Intake of total fat (g/dia), mean (SD) | 69.2 (23.6) | 69.9 (25.8) | 0.905 * |
| Biochemical Indicators Evaluated | Control Group | Supplemented Group | Differences between Control Group and Supplemented Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Collection 1 | Collection 2 | Change | p-Value * | Collection 1 | Collection 2 | Change | p-Value * | p-Value ** | ||
| Collection 1 CG × SG | Collection 2 CG × SG | |||||||||
| Serum alpha-tocopherol (µmol/L) | 26.37 (4.6) | 26.34 (4.92) | 0.03 (2.5) | 0.876 | 26.38 (5.4) | 48.27 (10.5) | 21.89 (7.4) | 0.001 | 0.996 | <0.001 |
| Alpha-tocopherol in breast milk (µmol/L) | 6.91 (1.8) | 6.94 (2.0) | 0.03 (1.2) | 0.935 | 6.98 (2.2) | 15.00 (5.1) | 8.02 (4.2) | <0.001 | 0.883 | <0.001 |
| Serum cholesterol (mg/dL) | 177 (41.0) | 178 (42.0) | 1.70 (33.5) | 0.750 | 179 (44.0) | 173 (36.0) | 6.28 (16.0) | 0.190 | 0.834 | 0.498 |
| Serum triglycerides (mg/dL) | 143 (99.0) | 130 (86.0) | 13.38 (57.8) | 0.151 | 129 (66.0) | 109 (51.0) | 19.23 (32.9) | 0.08 | 0.439 | 0.195 |
| HDL (mg/dL) | 40 (14.0) | 41 (15.0) | 0.25 (12.9) | 0.903 | 42 (11.0) | 42 (10.0) | 0.31 (8.0) | 0.811 | 0.574 | 0.724 |
| LDL (mg/dL) | 111 (35.0) | 114 (40.0) | 3.28 (34.4) | 0.535 | 113 (43.0) | 110 (35.0) | 3.74 (14.7) | 0.129 | 0.762 | 0.603 |
| Variables | Greater Effect of Supplementation (Quartiles 2–4) * | |
|---|---|---|
| 95% CI | p-Value | |
| Alpha-tocopherol in milk collection 1 (µmol/L) | 0.998–1.024 | 0.104 |
| Alpha-tocopherol in serum collection 1 (µmol/L) | 0.991–1.005 | 0.565 |
| Alpha-tocopherol in serum collection 2 (µmol/L) | 0.995–1.002 | 0.387 |
| Dietary intake of vitamin E collection 1 (mg/day) | 0.209–0.877 | 0.020 ** |
| Serum cholesterol collection 1 (mg/dL) | 0.937–1.163 | 0.431 |
| LDL collection 1 (mg/dL) | 0.846–1.051 | 0.289 |
| Characteristics | Quartile 1 n = 9 | Quartiles 2–4 n = 30 | p-Value |
|---|---|---|---|
| Maternal age (years), mean (SD) | 27 (6.5) | 27 (6.9) | 0.922 * |
| Postpartum age (days), mean (SD) | 58 (30.0) | 55 (22.1) | 0.749 * |
| Education level n, (%) | |||
| Incomplete primary education | 2 (22.2) | 3 (10.0) | 0.343 |
| Complete primary education | 1 (11.1) | 1 (3.3) | |
| Incomplete secondary education | 1 (11.1) | 5 (16.7) | |
| Complete secondary education | 3 (33.3) | 19 (63.3) | |
| Complete higher education | 2 (22.2) | 2 (6.7) | |
| Family income level n, (%) a | |||
| <1 Minimum wage | 4 (44.4) | 19 (63.3) | 0.312 |
| >1 Minimum wage | 5 (55.6) | 11 (36.7) | |
| Type of delivery n, (%) | |||
| Vaginal | 2 (22.2) | 11 (36.7) | 0.420 |
| Caesarian | 7 (77.8) | 19 (63.3) | |
| Parity status n, (%) | |||
| Primiparous | 6 (66.7) | 11 (36.7) | 0.111 |
| Multtiparous | 3 (33.3) | 19 (63.3) | |
| BMI classification (kg/m2), (%) b | |||
| Low weight | 0 (0) | 0 (0) | 0.754 |
| Normal | 3 (33.3) | 14 (46.7) | |
| Overweight | 4 (44.5) | 10 (33.3) | |
| Obese | 2 (22.2) | 6 (20.0) | |
| Type of maternal breastfeeding n, (%) | |||
| Exclusive maternal breastfeeding | 8 (88.9) | 25 (83.3) | 0.685 |
| Maternal breast milk and other milks | 1 (11.1) | 5 (16.7) | |
| Calory intake (Kcal/day), mean (SD) | 2624.6 (453.7) | 3464.5 (873.4) | 0.001 * |
| Intake of alpha-tocopherol (mg/day), mean (SD) | 6.8 (2.1) | 9.3 (3.6) | 0.013 * |
| Intake of total fat (g/dia), mean (SD) | 58.5 (12.8) | 73.3 (27.8) | 0.033 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa Rebouças, A.; Costa Lemos da Silva, A.G.; Freitas de Oliveira, A.; Thalia Pereira da Silva, L.; de Freitas Felgueiras, V.; Cruz, M.S.; Silbiger, V.N.; da Silva Ribeiro, K.D.; Dimenstein, R. Factors Associated with Increased Alpha-Tocopherol Content in Milk in Response to Maternal Supplementation with 800 IU of Vitamin E. Nutrients 2019, 11, 900. https://doi.org/10.3390/nu11040900
de Sousa Rebouças A, Costa Lemos da Silva AG, Freitas de Oliveira A, Thalia Pereira da Silva L, de Freitas Felgueiras V, Cruz MS, Silbiger VN, da Silva Ribeiro KD, Dimenstein R. Factors Associated with Increased Alpha-Tocopherol Content in Milk in Response to Maternal Supplementation with 800 IU of Vitamin E. Nutrients. 2019; 11(4):900. https://doi.org/10.3390/nu11040900
Chicago/Turabian Stylede Sousa Rebouças, Amanda, Ana Gabriella Costa Lemos da Silva, Amanda Freitas de Oliveira, Lorena Thalia Pereira da Silva, Vanessa de Freitas Felgueiras, Marina Sampaio Cruz, Vivian Nogueira Silbiger, Karla Danielly da Silva Ribeiro, and Roberto Dimenstein. 2019. "Factors Associated with Increased Alpha-Tocopherol Content in Milk in Response to Maternal Supplementation with 800 IU of Vitamin E" Nutrients 11, no. 4: 900. https://doi.org/10.3390/nu11040900
APA Stylede Sousa Rebouças, A., Costa Lemos da Silva, A. G., Freitas de Oliveira, A., Thalia Pereira da Silva, L., de Freitas Felgueiras, V., Cruz, M. S., Silbiger, V. N., da Silva Ribeiro, K. D., & Dimenstein, R. (2019). Factors Associated with Increased Alpha-Tocopherol Content in Milk in Response to Maternal Supplementation with 800 IU of Vitamin E. Nutrients, 11(4), 900. https://doi.org/10.3390/nu11040900





