Uncontrolled Eating during Pregnancy Predicts Fetal Growth: The Healthy Mom Zone Trial
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Intervention
2.3. Measures
2.4. Analysis
3. Results
3.1. Descriptive Characteristics
3.2. Unconditional Polynomial Models for Fetal Weight Change
3.3. Conditional Polynomial Models for Fetal Weight Change
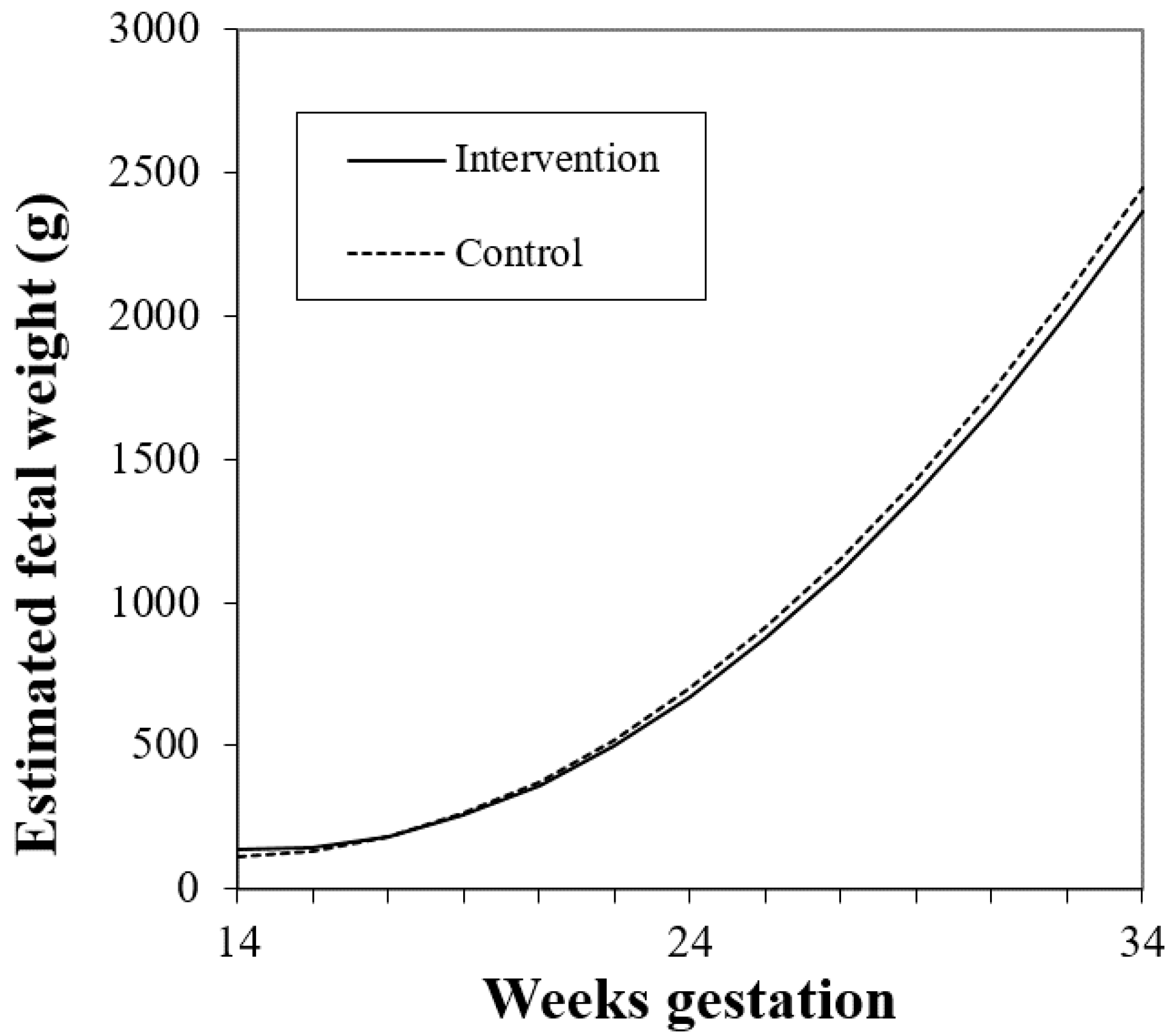
3.3.1. Treatment Effect of Intervention and Fetal Growth
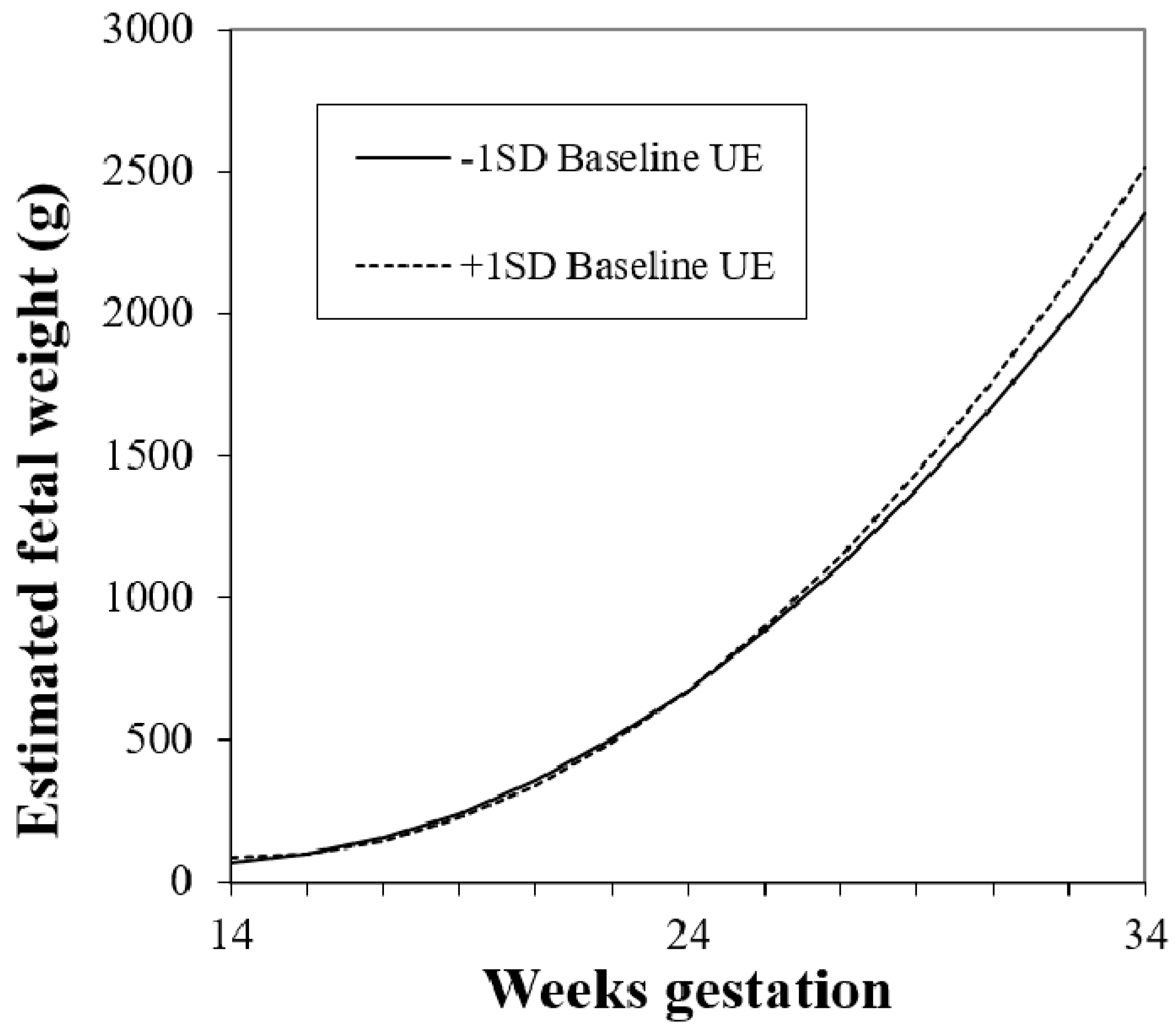
3.3.2. Individual Eating Behaviors and Fetal Growth
3.3.3. Cognitive Restraint × Disinhibited Eating and Fetal Growth
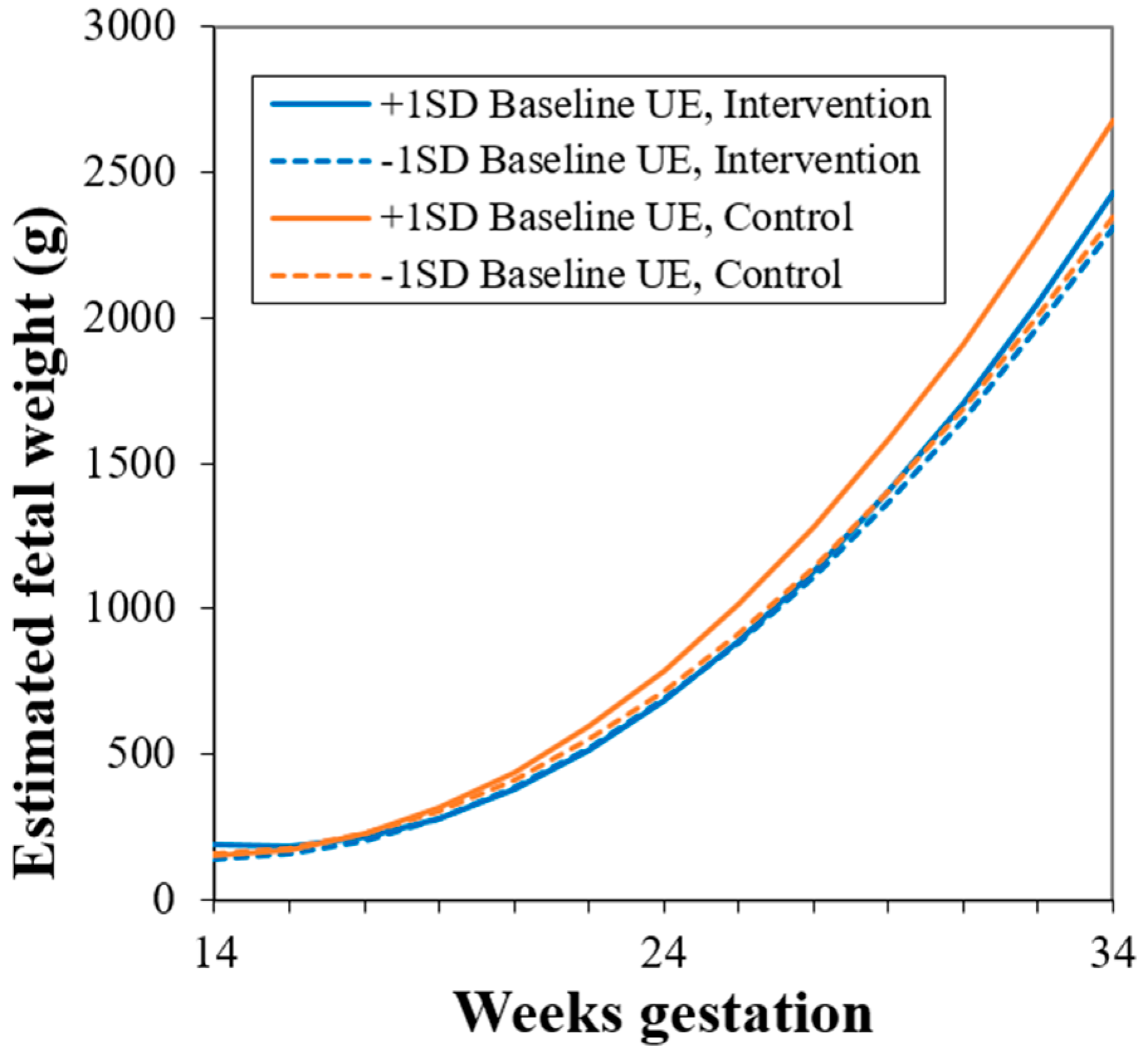
3.3.4. Post-hoc Analysis: Study Group × Uncontrolled Eating Interaction
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press (US): Washington, DC, USA, 2009. [Google Scholar]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Lawman, H.G.; Fryar, C.D.; Kruszon-Moran, D.; Kit, B.K.; Flegal, K.M. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 through 2013–2014. JAMA 2016, 315, 2292–2299. [Google Scholar] [PubMed]
- Hales, C.M.; Fryar, C.D.; Carroll, M.D.; Freedman, D.S.; Ogden, C.L. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. JAMA 2018, 319, 1723–1725. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E. Intergenerational programming of metabolic disease: Evidence from human populations and experimental animal models. Cell. Mol. Life Sci. 2013, 70, 1597–1608. [Google Scholar] [CrossRef]
- Adamo, K.B.; Ferraro, Z.M.; Brett, K.E. Can we modify the intrauterine environment to halt the intergenerational cycle of obesity? Int. J. Environ. Res. Public Health 2012, 9, 1263–1307. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Rivera, D.E.; Thomas, D.M.; Navarro-Barrientos, J.E.; Downs, D.S.; Savage, J.S.; Collins, L.M. A Dynamical Systems Model for Improving Gestational Weight Gain Behavioral Interventions. In Proceedings of the American Control Conference, Montreal, QC, Canada, 27–29 June 2012; pp. 4059–4064. [Google Scholar]
- Fallucca, S.; Vasta, M.; Sciullo, E.; Balducci, S.; Fallucca, F. Birth weight: Genetic and intrauterine environment in normal pregnancy. Diabetes Care 2009, 32, e149. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; Armstrong, J.; Dorosty, A.R.; Emmett, P.M.; Ness, A.; Rogers, I.; Steer, C.; Sherriff, A. Early life risk factors for obesity in childhood: Cohort study. BMJ 2005, 330, 1357. [Google Scholar] [CrossRef]
- Martin, J.A.; Hamilton, B.E.; Ventura, S.J.; Osterman, M.J.; Kirmeyer, S.; Mathews, T.J.; Wilson, E.C. Births: Final data for 2009. Natl. Vital Stat. Rep. 2011, 60, 1–70. [Google Scholar]
- Nohr, E.A.; Vaeth, M.; Baker, J.L.; Sorensen, T.I.; Olsen, J.; Rasmussen, K.M. Pregnancy outcomes related to gestational weight gain in women defined by their body mass index, parity, height, and smoking status. Am. J. Clin. Nutr. 2009, 90, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-N.; Sung, F.-C.; Li, C.-Y.; Chang, C.-H.; Lin, R.-S.; Lin, C.-C.; Chiang, C.-C.; Chuang, L.-M. Low birth weight and high birth weight infants are both at an increased risk to have type 2 diabetes among schoolchildren in Taiwan. Diabetes Care 2003, 26, 343–348. [Google Scholar] [CrossRef]
- Muktabhant, B.; Lawrie, T.A.; Lumbiganon, P.; Laopaiboon, M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Pauley, A.M.; Hohman, E.; Savage, J.S.; Rivera, D.E.; Guo, P.; Leonard, K.S.; Downs, D.S. Gestational Weight Gain Intervention Impacts Determinants of Healthy Eating and Exercise in Overweight/Obese Pregnant Women. J. Obes. 2018, 2018, 6469170. [Google Scholar] [CrossRef]
- Downs, D.S. Obesity in Special Populations: Pregnancy. Prim. Care 2016, 43, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Downs, D.S.; Savage, J.S.; Rauff, E.L. Falling Short of Guidelines? Nutrition and Weight Gain Knowledge in Pregnancy. J. Women’s Heal. Care 2014, 3, 1–6. [Google Scholar]
- Dong, Y.; Rivera, D.E.; Downs, D.S.; Savage, J.S.; Thomas, D.M.; Collins, L.M. Hybrid Model Predictive Control for Optimizing Gestational Weight Gain Behavioral Interventions. In Proceedings of the American Control Conference, Washington, DC, USA, 17–19 June 2013; pp. 1970–1975. [Google Scholar]
- Symons Downs, D.; Savage, J.S.; Rivera, D.E.; Smyth, J.M.; Rolls, B.J.; Hohman, E.E.; McNitt, K.M.; Kunselman, A.R.; Stetter, C.; Pauley, A.M.; et al. Individually Tailored, Adaptive Intervention to Manage Gestational Weight Gain: Protocol for a Randomized Controlled Trial in Women with Overweight and Obesity. JMIR Res. Protoc. 2018, 7, e150. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.M.; Murphy, S.A.; Strecher, V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): New methods for more potent eHealth interventions. Am. J. Prevent. Med. 2007, 32 (Suppl. 5), S112–S118. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Mullany, L.C.; Hurley, K.M.; Katz, J.; Black, R.E. Nutrition and maternal, neonatal, and child health. Semin. Perinatol. 2015, 39, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.B.; Black, A.E. Markers of the validity of reported energy intake. J. Nutr. 2003, 133 (Suppl. 3), 895S–920S. [Google Scholar] [CrossRef]
- Sui, Z.; Cramp, C.; Moran, L.J.; McNaughton, S.A.; Deussen, A.R.; Grivell, R.M.; Dodd, J.M. The characterisation of overweight and obese women who are under reporting energy intake during pregnancy. BMC Pregnancy Childbirth 2018, 18, 204. [Google Scholar]
- Guo, P.; Rivera, D.E.; Savage, J.S.; Hohman, E.E.; Pauley, A.M.; Leonard, K.S.; Symons Downs, D. System identification approaches for energy intake estimation: Enhancing interventions for managing gestational weight gain. IEEE Trans. Control Syst. Technol. 2018. [Google Scholar] [CrossRef]
- Cappelleri, J.C.; Bushmakin, A.G.; Gerber, R.; Leidy, N.K.; Sexton, C.C.; Lowe, M.R.; Karlsson, J. Psychometric analysis of the Three-Factor Eating Questionnaire-R21: Results from a large diverse sample of obese and non-obese participants. Int. J. Obes. 2009, 33, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Messick, S. Eating Inventory Manual; The Psychological Corporation: San Antonio, TX, USA, 1988. [Google Scholar]
- Lowe, M.R.; Kral, T.V. Stress-induced eating in restrained eaters may not be caused by stress or restraint. Appetite 2006, 46, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Lindroos, A.-K.; Mathiassen, M.E.; Bengtsson, C.; Lindroos, A.; Lissner, L.; Karlsson, J.; Sullivan, M.; Sjöström, L. Dietary intake in relation to restrained eating, disinhibition, and hunger in obese and nonobese Swedish women. Obes. Res. 1997, 5, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Hainer, V.; Kunešová, M.; Bellisle, F.; Parizkova, J.; Braunerova, R.; Wagenknecht, M.; Lajka, J.; Hill, M.; Stunkard, A. The eating inventory, body adiposity and prevalence of disease in a quota sample of Czech adults. Int. J. Obesity (Lond.) 2006, 30, 830–836. [Google Scholar] [CrossRef]
- Williamson, D.A.; Lawson, O.J.; Brooks, E.R.; Wozniak, P.J.; Ryan, D.H.; Bray, G.A.; Duchmann, E.G. Association of body mass with dietary restraint and disinhibition. Appetite 1995, 25, 31–41. [Google Scholar] [CrossRef]
- Hays, N.P.; Bathalon, G.P.; McCrory, M.A.; Roubenoff, R.; Lipman, R.; Roberts, S.B. Eating behavior correlates of adult weight gain and obesity in healthy women aged 55–65 y. Am. J. Clin. Nutr. 2002, 75, 476–483. [Google Scholar] [CrossRef]
- Smith, C.F.; Geiselman, P.J.; Williamson, D.A.; Champagne, C.M.; Bray, G.A.; Ryan, D.H. Association of dietary restraint and disinhibition with eating behavior, body mass, and hunger. Eat. Weight Disord. Ewd 1998, 3, 7–15. [Google Scholar] [CrossRef]
- Foster, G.D.; Wadden, T.A.; Swain, R.M.; Stunkard, A.J.; Platte, P.; Vogt, R.A. The eating inventory in obese women: Clinical correlates and relationship to weight loss. Int. J. Obes. 1998, 22, 778–785. [Google Scholar] [CrossRef]
- Lauzon-Guillain, B.; Basdevant, A.; Romon, M.; Karlsson, J.; Borys, J.M.; Charles, M.A. Is restraint eating a risk factor for weight gain in a general population? Am. J. Clin. Nutr. 2006, 83, 132–138. [Google Scholar] [CrossRef]
- Lowe, M.R.; Annunziato, R.A.; Markowitz, J.T.; Didie, E.; Bellace, D.L.; Riddell, L.; Maille, C.; McKinney, S.; Stice, E. Multiple types of dieting prospectively predict weight gain during the freshman year of college. Appetite 2006, 47, 83–90. [Google Scholar] [CrossRef]
- Olea Lopez, A.L.; Johnson, L. Associations between Restrained Eating and the Size and Frequency of Overall Intake, Meal, Snack and Drink Occasions in the UK Adult National Diet and Nutrition Survey. PLoS ONE 2016, 11, e0156320. [Google Scholar] [CrossRef] [PubMed]
- Bongers, P.; Jansen, A. Emotional Eating Is Not What You Think It Is and Emotional Eating Scales Do Not Measure What You Think They Measure. Front. Psychol. 2016, 7, 1932. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Wyatt, H.R.; Reed, G.W.; Peters, J.C. Obesity and the environment: Where do we go from here? Science 2003, 299, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Keränen, A.-M.; Savolainen, M.J.; Reponen, A.H.; Kujari, M.-L.; Lindeman, S.M.; Bloigu, R.S.; Laitinen, J.H.; Teeriniemi, A.-M. The effect of eating behavior on weight loss and maintenance during a lifestyle intervention. Prev. Med. 2009, 49, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Mumford, S.L.; Siega-Riz, A.M.; Herring, A.; Evenson, K.R. Dietary restraint and gestational weight gain. J. Am. Diet. Assoc. 2008, 108, 1646–1653. [Google Scholar] [CrossRef]
- Heery, E.; Wall, P.G.; Kelleher, C.C.; McAuliffe, F.M. Effects of dietary restraint and weight gain attitudes on gestational weight gain. Appetite 2016, 107, 501–510. [Google Scholar] [CrossRef]
- Blau, L.E.; Orloff, N.C.; Flammer, A.; Slatch, C.; Hormes, J.M. Food craving frequency mediates the relationship between emotional eating and excess weight gain in pregnancy. Eat. Behav. 2018, 31, 120–124. [Google Scholar] [CrossRef]
- Hutchinson, A.D.; Charters, M.; Prichard, I.; Fletcher, C.; Wilson, C. Understanding maternal dietary choices during pregnancy: The role of social norms and mindful eating. Appetite 2017, 112, 227–234. [Google Scholar] [CrossRef]
- Lawson, O.J.; Champagne, C.M.; Brooks, E.R.; Howat, P.M.; Wozniak, P.J.; Williamson, D.A.; Delany, J.P.; Bray, G.A.; Ryan, D.H. The association of body weight, dietary intake, and energy expenditure with dietary restraint and disinhibition. Obes. Res. 1995, 3, 153–161. [Google Scholar] [CrossRef]
- Savage, J.S.; Hoffman, L.; Birch, L.L. Dieting, restraint, and disinhibition predict women’s weight change over 6 y. Am. J. Clin. Nutr. 2009, 90, 33–40. [Google Scholar] [CrossRef]
- Rivera, D.E.; Hekler, E.; Savage, J.S.; Symons Downs, D. Intensively adaptive interventions using control systems engineering: Two illustrative examples. In Optimization of Behavioral, Biobehavioral, and Biomedical Interventions; Collins, L.M., Ed.; Springer: Cham, Switzerland, 2018; pp. 21–173. [Google Scholar]
- Ajzen, I. The theory of planned behavior. Organ. Behav. Hum. Decis. Process. 1991, 50, 179–211. [Google Scholar] [CrossRef]
- Carver, C.; Scheier, M. On the Self-Regulation of Behavior; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Carpenter, R.J.; Deter, R.L.; Park, S.K. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology 1984, 150, 535–540. [Google Scholar] [CrossRef]
- Villar, J.; Ismail, L.C.; Victora, C.G.; Ohuma, E.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Black, M.H.; Sacks, D.A.; Xiang, A.H.; Lawrence, J.M. The relative contribution of prepregnancy overweight and obesity, gestational weight gain, and IADPSG-defined gestational diabetes mellitus to fetal overgrowth. Diabetes Care 2013, 36, 56–62. [Google Scholar] [CrossRef]
- Clark, M.; Ogden, J. The impact of pregnancy on eating behaviour and aspects of weight concern. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 18–24. [Google Scholar] [CrossRef]
- Fairburn, C.G.; Stein, A.; Jones, R. Eating habits and eating disorders during pregnancy. Psychosom. Med. 1992, 54, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Shloim, N.; Hetherington, M.M.; Rudolf, M.; Feltbower, R.G. Relationship between body mass index and women’s body image, self-esteem and eating behaviours in pregnancy: A cross-cultural study. J. Health Psychol. 2015, 20, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Slane, J.D.; Levine, M.D. Association of Restraint and Disinhibition to Gestational Weight Gain among Pregnant Former Smokers. Women’s Health Issues 2015, 25, 390–395. [Google Scholar] [CrossRef][Green Version]
- Wright, C.M.; Parkinson, K.N.; Drewett, R.F. The influence of maternal socioeconomic and emotional factors on infant weight gain and weight faltering (failure to thrive): Data from a prospective birth cohort. Arch. Dis. Child. 2006, 91, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Arroyo, J.; Druker, S.; Sankey, H.Z.; Rosal, M.C. Knowledge, Attitudes and Provider Advice by Pre-Pregnancy Weight Status: A Qualitative Study of Pregnant Latinas with Excessive Gestational Weight Gain. Women Health 2015, 55, 805–828. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.W.; Nitzke, S.; Buist, D.; Cain, D.; Horning, S.; Eghtedary, K. I am pregnant and want to do better but I can’t: Focus groups with low-income overweight and obese pregnant women. Matern. Child Health J. 2015, 19, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.W.; Nitzke, S.; Guilford, E.; Adair, C.H.; Hazard, D.L. Motivators and barriers to healthful eating and physical activity among low-income overweight and obese mothers. J. Am. Diet Assoc. 2008, 108, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Levoy, E.; Lazaridou, A.; Brewer, J.; Fulwiler, C. An exploratory study of Mindfulness Based Stress Reduction for emotional eating. Appetite 2017, 109, 124–130. [Google Scholar] [CrossRef]
- Armitage, C.J. Randomized test of a brief psychological intervention to reduce and prevent emotional eating in a community sample. J. Public Health (Oxf.) 2015, 37, 438–444. [Google Scholar] [CrossRef]
- Katterman, S.N.; Kleinman, B.M.; Hood, M.M.; Nackers, L.M.; Corsica, J.A. Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: A systematic review. Eat. Behav. 2014, 15, 197–204. [Google Scholar] [CrossRef]
- Conway, R.; Reddy, S.; Davies, J. Dietary restraint and weight gain during pregnancy. Eur. J. Clin. Nutr. 1999, 53, 849–853. [Google Scholar] [CrossRef]
- Laraia, B.; Epel, E.; Siega-Riz, A.M. Food insecurity with past experience of restrained eating is a recipe for increased gestational weight gain. Appetite 2013, 65, 178–184. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 27) | Intervention (n = 13) | Control (n = 14) | |
|---|---|---|---|
| M (SD) Range | M (SD) Range | M (SD) Range | |
| Age (years) | 30.6 (2.9) 24–37 | 30.3 (2.8) 24–35 | 30.9 (3.2) 27–37 |
| Gestational Age at Study Start (weeks) | 10.6 (1.6) 7.3–13.0 | 10.5 (1.7) 7.3–13.0 | 10.6 (1.6) 7.9–12.9 |
| Weight at Study Start (lbs.) | 190.4 (46.8) 134–294 | 182.2 (40.5) 145–294 | 197.9 (52.3) 134–292 |
| Body Mass Index | 31.6 (7.0) 24.1–48.9 | 30.7 (6.7) 24.1–48.9 | 32.4 (7.5) 24.5–42.8 |
| % | % | % | |
| % BMI = 24.1–29.9 | 59.3 | 69.2 | 50 |
| % Obese (BMI ≥ 30) | 40.7 | 30.8 | 50 |
| Parity | |||
| Primiparous (no prior live birth) | 66.7 | 53.9 | 78.6 |
| Multiparous: 1 previous live birth | 33.3 | 46.2 | 21.4 |
| Education | |||
| Graduate/professional | 48.2 | 38.5 | 57.1 |
| College | 48.2 | 61.5 | 35.7 |
| High school | 3.7 | 0 | 7.1 |
| Marital Status | |||
| Married | 92.6 | 92.3 | 92.9 |
| Single | 3.7 | 0 | 7.1 |
| Divorced | 3.7 | 7.7 | 0 |
| Race | |||
| Non-Hispanic White | 96.3 | 92.3 | 100 |
| Asian | 3.7 | 7.7 | 0 |
| Family Income | |||
| >$100,000 | 33.3 | 30.8 | 35.7 |
| $40–$100,000 | 44.4 | 61.5 | 28.6 |
| $20–$40,000 | 18.5 | 0 | 35.7 |
| $10–$20,000 | 3.7 | 7.7 | 0 |
| Employment | |||
| Full-time | 81.5 | 84.6 | 78.6 |
| Part-time | 11.1 | 15.4 | 7.1 |
| Self-employed | 3.7 | 0 | 7.1 |
| Other | 3.7 | 0 | 7.1 |
| Model 1: Study Group | |||
|---|---|---|---|
| Term | Est | SE | p |
| Intercept | 1232.8 | 151.2 | <0.0001 |
| Gestational week | −161.9 | 10.34 | <0.0001 |
| Gestational week2 | 5.80 | 0.21 | <0.0001 |
| Prepregnancy BMI | 0.30 | 2.74 | 0.91 |
| Study group: Intervention * | 89.46 | 61.56 | 0.14 |
| Study group * × gestational week | −4.94 | 2.93 | 0.095 |
| Model 2: Study group + Baseline Uncontrolled Eating | |||
|---|---|---|---|
| Term | Est | SE | p |
| Intercept | 1195.7 | 154.5 | <0.0001 |
| Gestational week | −160.9 | 10.34 | <0.0001 |
| Gestational week2 | 5.80 | 0.21 | <0.0001 |
| Prepregnancy BMI | 0.89 | 2.86 | 0.76 |
| Study group: Intervention * | 122.0 | 63.02 | 0.06 |
| Study group * × gestational week | −6.59 | 3.03 | 0.03 |
| Baseline uncontrolled eating | 475.3 | 330.5 | 0.15 |
| Baseline uncontrolled eating × gestational week | −49.04 | 28.21 | 0.08 |
| Baseline uncontrolled eating × gestational week2 | 1.21 | 0.57 | 0.03 |
| Model 3: Study group + Baseline Uncontrolled Eating + Change in Uncontrolled Eating | |||
|---|---|---|---|
| Term | Est | SE | p |
| Intercept | 1270.8 | 154.3 | <0.0001 |
| Gestational week | −159.6 | 10.63 | <0.0001 |
| Gestational week2 | 5.74 | 0.21 | <0.0001 |
| Prepregnancy BMI | −1.45 | 2.71 | 0.60 |
| Study group: Intervention * | 110.3 | 64.65 | 0.09 |
| Study group * × gestational week | −6.56 | 2.96 | 0.03 |
| Baseline uncontrolled eating | 532.6 | 330.8 | 0.11 |
| Baseline uncontrolled eating × gestational week | −53.72 | 28.26 | 0.06 |
| Baseline uncontrolled eating × gestational week2 | 1.32 | 0.57 | 0.02 |
| Change in uncontrolled eating | 252.1 | 181.0 | 0.17 |
| Change in uncontrolled eating × gestational week | −13.94 | 6.74 | 0.04 |
| Model 4: Study Group + Uncontrolled Eating + Cognitive Restraint | |||
|---|---|---|---|
| Term | Est | SE | p |
| Intercept | 1283.4 | 156.4 | <0.0001 |
| Gestational week | −161.4 | 10.49 | <0.0001 |
| Gestational week2 | 5.80 | 0.21 | <0.0001 |
| Prepregnancy BMI | −1.28 | 2.92 | 0.66 |
| Study group: Intervention * | 113.2 | 64.17 | 0.08 |
| Study group * × gestational week | −7.45 | 3.05 | 0.02 |
| Baseline uncontrolled eating | 501.5 | 328.3 | 0.13 |
| Baseline uncontrolled eating × gestational week | −49.78 | 28.03 | 0.08 |
| Baseline uncontrolled eating × gestational week2 | 1.25 | 0.56 | 0.03 |
| Change in uncontrolled eating | −116.4 | 52.68 | 0.03 |
| Baseline cognitive restraint | 11.18 | 42.88 | 0.80 |
| Baseline cognitive restraint × change in uncontrolled eating | −272.3 | 116.9 | 0.02 |
| Model 5: Study Group × Uncontrolled Eating + Cognitive Restraint | |||
|---|---|---|---|
| Term | Est | SE | p |
| Intercept | 1267.4 | 155.7 | <0.0001 |
| Gestational week | −160.6 | 10.40 | <0.0001 |
| Gestational week2 | 5.80 | 0.21 | <0.0001 |
| Prepregnancy BMI | −1.21 | 2.93 | 0.68 |
| Study group: Intervention * | 117.0 | 63.58 | 0.07 |
| Study group * × gestational week | −7.67 | 3.03 | 0.01 |
| Baseline uncontrolled eating | 256.8 | 352.8 | 0.47 |
| Baseline uncontrolled eating × gestational week | −35.22 | 28.64 | 0.22 |
| Baseline uncontrolled eating × gestational week2 | 1.18 | 0.56 | 0.04 |
| Change in uncontrolled eating | −111.3 | 52.60 | 0.04 |
| Baseline cognitive restraint | 10.36 | 42.70 | 0.81 |
| Baseline cognitive restraint × change in uncontrolled eating | −259.0 | 116.1 | 0.03 |
| Study group × baseline uncontrolled eating | 310.6 | 175.3 | 0.08 |
| Study group × baseline uncontrolled eating × gestational week | −17.00 | 8.25 | 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savage, J.S.; Hohman, E.E.; McNitt, K.M.; Pauley, A.M.; Leonard, K.S.; Turner, T.; Pauli, J.M.; Gernand, A.D.; Rivera, D.E.; Symons Downs, D. Uncontrolled Eating during Pregnancy Predicts Fetal Growth: The Healthy Mom Zone Trial. Nutrients 2019, 11, 899. https://doi.org/10.3390/nu11040899
Savage JS, Hohman EE, McNitt KM, Pauley AM, Leonard KS, Turner T, Pauli JM, Gernand AD, Rivera DE, Symons Downs D. Uncontrolled Eating during Pregnancy Predicts Fetal Growth: The Healthy Mom Zone Trial. Nutrients. 2019; 11(4):899. https://doi.org/10.3390/nu11040899
Chicago/Turabian StyleSavage, Jennifer S., Emily E. Hohman, Katherine M. McNitt, Abigail M. Pauley, Krista S. Leonard, Tricia Turner, Jaimey M. Pauli, Alison D. Gernand, Daniel E. Rivera, and Danielle Symons Downs. 2019. "Uncontrolled Eating during Pregnancy Predicts Fetal Growth: The Healthy Mom Zone Trial" Nutrients 11, no. 4: 899. https://doi.org/10.3390/nu11040899
APA StyleSavage, J. S., Hohman, E. E., McNitt, K. M., Pauley, A. M., Leonard, K. S., Turner, T., Pauli, J. M., Gernand, A. D., Rivera, D. E., & Symons Downs, D. (2019). Uncontrolled Eating during Pregnancy Predicts Fetal Growth: The Healthy Mom Zone Trial. Nutrients, 11(4), 899. https://doi.org/10.3390/nu11040899





