High-Fat Breakfast Meal Replacement in Overweight and Obesity: Implications on Body Composition, Metabolic Markers, and Satiety
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. Body Composition
2.4. Resting Metabolic Test
2.5. Steady-State Cardiorespiratory Test
2.6. Fasted Blood Sample
2.7. Questionnaires
2.8. Dietary Analysis
2.9. Supplementation
2.10. Statistical Analysis
3. Results
3.1. Body Composition
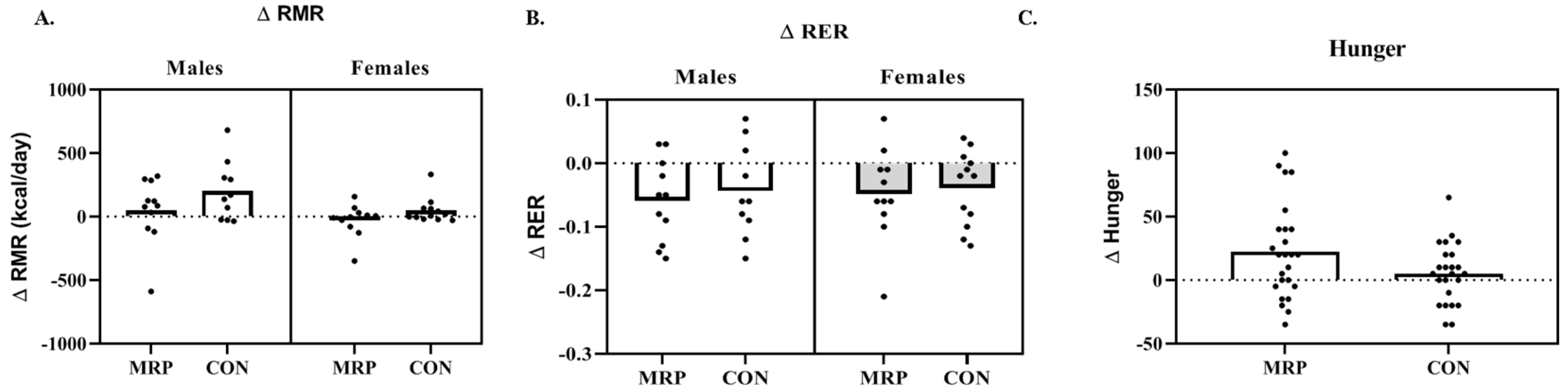
3.2. Metabolism
3.3. Metabolic Blood Markers
3.4. Nutrition
3.5. Questionnaires
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dhurandhar, E.J.; Dawson, J.; Alcorn, A.; Larsen, L.H.; Thomas, E.A.; Cardel, M.; Bourland, A.C.; Astrup, A.; St-Onge, M.P.; Hill, J.O.; et al. The effectiveness of breakfast recommendations on weight loss: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 100, 507–513. [Google Scholar] [CrossRef]
- Geliebter, A.; Astbury, N.M.; Aviram-Friedman, R.; Yahav, E.; Hashim, S. Skipping breakfast leads to weight loss but also elevated cholesterol compared with consuming daily breakfasts of oat porridge or frosted cornflakes in overweight individuals: A randomised controlled trial. J. Nutr. Sci. 2014, 3, e56. [Google Scholar] [CrossRef]
- O’Neil, C.E.; Nicklas, T.A.; Fulgoni, V.L., 3rd. Nutrient intake, diet quality, and weight/adiposity parameters in breakfast patterns compared with no breakfast in adults: National Health and Nutrition Examination Survey 2001–2008. J. Acad. Nutr. Diet. 2014, 114, S27–S43. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S. Fat intake and energy-balance effects. Physiol. Behav. 2004, 83, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Mumme, K.; Stonehouse, W. Effects of medium-chain triglycerides on weight loss and body composition: A meta-analysis of randomized controlled trials. J. Acad. Nutr. Diet. 2015, 115, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Frape, D.L.; Williams, N.R.; Rajput-Williams, J.; Maitland, B.W.; Scriven, A.J.; Palmer, C.R.; Fletcher, R.J. Effect of breakfast fat content on glucose tolerance and risk factors of atherosclerosis and thrombosis. Br. J. Nutr. 1998, 80, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Phillips-Eakley, A.K.; Smith, K.N. The Effects of Breakfast Consumption and Composition on Metabolic Wellness with a Focus on Carbohydrate Metabolism. Adv. Nutr. 2016, 7, 613S–621S. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.E.; Ribeiro, D.N.; Costa, N.M.; Bressan, J.; Alfenas, R.C.; Mattes, R.D. Acute and second-meal effects of peanuts on glycaemic response and appetite in obese women with high type 2 diabetes risk: A randomised cross-over clinical trial. Br. J. Nutr. 2013, 109, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Jones, P.J. Greater rise in fat oxidation with medium-chain triglyceride consumption relative to long-chain triglyceride is associated with lower initial body weight and greater loss of subcutaneous adipose tissue. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Ross, R.; Parsons, W.D.; Jones, P.J. Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes. Res. 2003, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Bourque, C.; Jones, P.J.; Ross, R.; Parsons, W.E. Medium- versus long-chain triglycerides for 27 days increases fat oxidation and energy expenditure without resulting in changes in body composition in overweight women. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Casazza, K.; Fontaine, K.R.; Astrup, A.; Birch, L.L.; Brown, A.W.; Bohan Brown, M.M.; Durant, N.; Dutton, G.; Foster, E.M.; Heymsfield, S.B.; et al. Myths, presumptions, and facts about obesity. N. Engl. J. Med. 2013, 368, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Flechtner-Mors, M.; Ditschuneit, H.H.; Johnson, T.D.; Suchard, M.A.; Adler, G. Metabolic and weight loss effects of long-term dietary intervention in obese patients: Four-year results. Obes. Res. 2000, 8, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.M.; Moon, J.R.; Tobkin, S.E.; Walter, A.A.; Smith, A.E.; Dalbo, V.J.; Cramer, J.T.; Stout, J.R. Minimal nutrition intervention with high-protein/low-carbohydrate and low-fat, nutrient-dense food supplement improves body composition and exercise benefits in overweight adults: A randomized controlled trial. Nutr. Metab. 2008, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; van Mierlo, C.A.; van der Knaap, H.C.; Heo, M.; Frier, H.I. Weight management using a meal replacement strategy: Meta and pooling analysis from six studies. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 537–549. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Schouten, L.J.; Jurgens, J.; Endert, E.; Kalsbeek, A.; Fliers, E.; Bisschop, P.H. Breakfast replacement with a low-glycaemic response liquid formula in patients with type 2 diabetes: A randomised clinical trial. Br. J. Nutr. 2014, 112, 504–512. [Google Scholar] [CrossRef]
- Hirsch, K.R.; Smith-Ryan, A.E.; Blue, M.N.; Mock, M.G.; Trexler, E.T.; Ondrak, K.S. Metabolic characterization of overweight and obese adults. Phys. Sportsmed. 2016, 44, 362–372. [Google Scholar] [CrossRef]
- Dennis, B.H. Well-Controlled Diet Studies in Humans: A Practical Guide to Design and Management; American Dietetic Association: Chicago, IL, USA, 1999; p. xiv. 418p. [Google Scholar]
- Wang, Z.; Pi-Sunyer, F.X.; Kotler, D.P.; Wielopolski, L.; Withers, R.T.; Pierson, R.N., Jr.; Heymsfield, S.B. Multicomponent methods: Evaluation of new and traditional soft tissue mineral models by in vivo neutron activation analysis. Am. J. Clin. Nutr. 2002, 76, 968–974. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Ebbeling, C.B.; Zheng, J.; Pietrobelli, A.; Strauss, B.J.; Silva, A.M.; Ludwig, D.S. Multi-component molecular-level body composition reference methods: Evolving concepts and future directions. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2015, 16, 282–294. [Google Scholar] [CrossRef]
- Leite, C.C.; Wajchenberg, B.L.; Radominski, R.; Matsuda, D.; Cerri, G.G.; Halpern, A. Intra-abdominal thickness by ultrasonography to predict risk factors for cardiovascular disease and its correlation with anthropometric measurements. Metabolism 2002, 51, 1034–1040. [Google Scholar] [CrossRef]
- Stoner, L.; Chinn, V.; Cornwall, J.; Meikle, G.; Page, R.; Lambrick, D.; Faulkner, J. Reliability tests and guidelines for B-mode ultrasound assessment of central adiposity. Eur. J. Clin. Investig. 2015, 45, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Gore, C.J.; Withers, R.T. The effect of exercise intensity and duration on the oxygen deficit and excess post-exercise oxygen consumption. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 60, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, H.L.; Smith-Ryan, A.E.; Melvin, M.N.; Roelofs, E.J.; Trexler, E.T.; Hackney, A.C.; Weaver, M.A.; Ryan, E.D. The acute effect of exercise modality and nutrition manipulations on post-exercise resting energy expenditure and respiratory exchange ratio in women: A randomized trial. Sports Med. Open 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, J.; Vuorimaa, T. Heart rate and exercise intensity during sports activities. Practical application. Sports Med. 1988, 5, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Cardello, A.V.; Schutz, H.G.; Lesher, L.L.; Merrill, E. Development and testing of a labeled magnitude scale of perceived satiety. Appetite 2005, 44, 1–13. [Google Scholar] [CrossRef]
- Krotkiewski, M. Value of VLCD supplementation with medium chain triglycerides. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1393–1400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seaton, T.B.; Welle, S.L.; Warenko, M.K.; Campbell, R.G. Thermic effect of medium-chain and long-chain triglycerides in man. Am. J. Clin. Nutr. 1986, 44, 630–634. [Google Scholar] [CrossRef]
- Scalfi, L.; Coltorti, A.; Contaldo, F. Postprandial thermogenesis in lean and obese subjects after meals supplemented with medium-chain and long-chain triglycerides. Am. J. Clin. Nutr. 1991, 53, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.; Fathi, M.; Mensi, N.; Girardier, L. Twenty-four-hour energy expenditure and urinary catecholamines of humans consuming low-to-moderate amounts of medium-chain triglycerides: A dose-response study in a human respiratory chamber. Eur. J. Clin. Nutr. 1996, 50, 152–158. [Google Scholar]
- White, M.D.; Papamandjaris, A.A.; Jones, P.J. Enhanced postprandial energy expenditure with medium-chain fatty acid feeding is attenuated after 14 d in premenopausal women. Am. J. Clin. Nutr. 1999, 69, 883–889. [Google Scholar] [CrossRef]
- Poppitt, S.D.; Strik, C.M.; MacGibbon, A.K.; McArdle, B.H.; Budgett, S.C.; McGill, A.T. Fatty acid chain length, postprandial satiety and food intake in lean men. Physiol. Behav. 2010, 101, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Kasai, M.; Takeuchi, H.; Nakamura, M.; Okazaki, M.; Kondo, K. Dietary medium-chain triacylglycerols suppress accumulation of body fat in a double-blind, controlled trial in healthy men and women. J. Nutr. 2001, 131, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Tieken, S.M.; Leidy, H.J.; Stull, A.J.; Mattes, R.D.; Schuster, R.A.; Campbell, W.W. Effects of solid versus liquid meal-replacement products of similar energy content on hunger, satiety, and appetite-regulating hormones in older adults. Horm. Metab. Res. Horm. Und Stoffwechs. Horm. Et Metab. 2007, 39, 389–394. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Age (yrs) | Height (cm) | BMI (kg∙m−2) | %fat |
|---|---|---|---|---|
| MRP (n = 22) | 34.4 ± 9.5 | 170.7 ± 9.2 | 33.3 ± 4.9 | 37.2 ± 7.0 |
| CON (n = 21) | 36.2 ± 8.9 | 169.7 ± 10.7 | 33.8 ± 5.7 | 36.2 ± 8.9 |
| TC (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | TRG (mg/dL) | GLUC (mg/dL) | ||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Male | ||||||||||
| MRP | 197.4 ± 40.1 | 213.9 ± 43.2 | 41.0 ± 7.7 | 40.9 ± 8.6 | 123.5 ± 32.5 | 141.0 ± 30.9 | 165.2 ± 117.9 | 160.6 ± 92.2 | 88.2 ± 11.1 | 90.1 ± 13.7 |
| CON | 182.2 ± 29.7 | 177.1 ± 50.2 | 36.8 ± 8.5 | 36.5 ± 10.4 | 121.9 ± 27.8 | 115.6 ± 42.8 | 117.4 ± 43.4 | 124.2 ± 54.7 | 99.5 ± 43.7 | 109.5 ± 3.5 |
| Female | ||||||||||
| MRP | 167.6 ± 46.7 | 165.1 ± 42.9 | 49.0 ± 13.0 | 46.4 ± 11.6* | 102.5 ± 38.9 | 103.5 ± 35.0 | 81.8 ± 46.5 | 76.5 ± 34.9 | 81.6 ± 5.6 | 85.5 ± 3.9 |
| CON | 180.7 ± 46.6 | 176.0 ± 45.9 | 47.4 ± 12.5 | 47.9 ± 16.0 | 116.7 ± 45.3 | 113.9 ± 37.0 | 82.0 ± 31.5 | 107.1 ± 51.6 | 82.0 ± 5.1 | 87.0 ± 9.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith-Ryan, A.E.; Hirsch, K.R.; Blue, M.N.M.; Mock, M.G.; Trexler, E.T. High-Fat Breakfast Meal Replacement in Overweight and Obesity: Implications on Body Composition, Metabolic Markers, and Satiety. Nutrients 2019, 11, 865. https://doi.org/10.3390/nu11040865
Smith-Ryan AE, Hirsch KR, Blue MNM, Mock MG, Trexler ET. High-Fat Breakfast Meal Replacement in Overweight and Obesity: Implications on Body Composition, Metabolic Markers, and Satiety. Nutrients. 2019; 11(4):865. https://doi.org/10.3390/nu11040865
Chicago/Turabian StyleSmith-Ryan, Abbie E., Katie R. Hirsch, Malia N. M. Blue, Meredith G. Mock, and Eric T. Trexler. 2019. "High-Fat Breakfast Meal Replacement in Overweight and Obesity: Implications on Body Composition, Metabolic Markers, and Satiety" Nutrients 11, no. 4: 865. https://doi.org/10.3390/nu11040865
APA StyleSmith-Ryan, A. E., Hirsch, K. R., Blue, M. N. M., Mock, M. G., & Trexler, E. T. (2019). High-Fat Breakfast Meal Replacement in Overweight and Obesity: Implications on Body Composition, Metabolic Markers, and Satiety. Nutrients, 11(4), 865. https://doi.org/10.3390/nu11040865





