Fluctuation of Water Intake and of Hydration Indices during the Day in a Sample of Healthy Greek Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Intake
2.2. Urine Hydration Indices
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
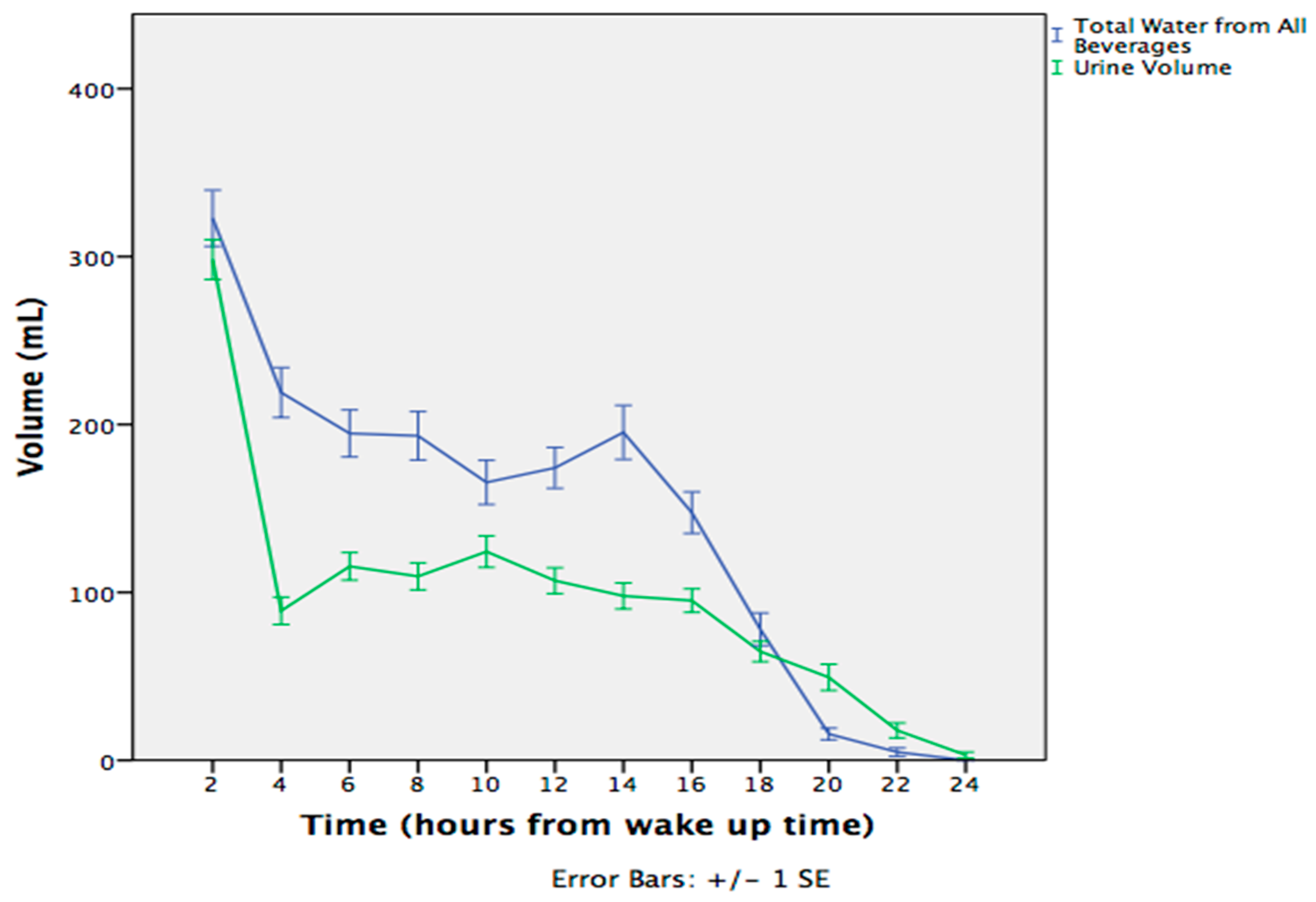
3.1. Fluctuations During the Day
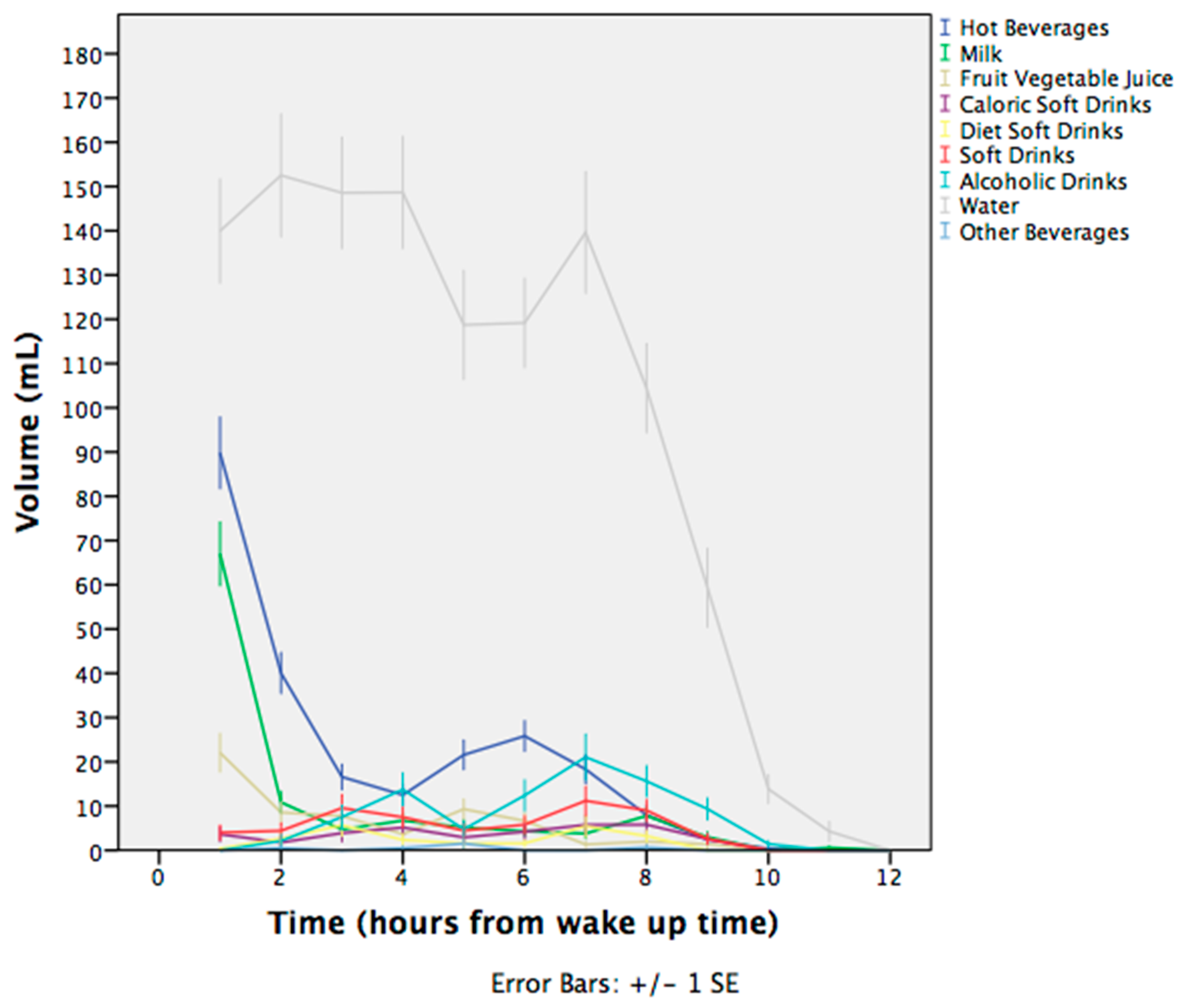
3.2. Water Intake from Beverages
3.3. Urine Hydration Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EFSA. Scientific opinion on the substantiation of health claims related to water and maintenance of normal physical and cognitive functions (ID 1102, 1209, 1294, 1331), maintenance of normal thermoregulation (ID 1208) and “basic requirement of all living things” (ID 1207) pursuant to article 13(1) of regulation (EC) no 1924/2006. EFSA J. 2011, 9, 2075–2091. [Google Scholar]
- WHO. A Manual for Physicians and Other Senior Health Workers—Fact Sheet 107; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, hydration, and health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Pei, L.; Gao, Y.; Zhao, L.; Fang, H.; Bunda, B.; Fisher, L.; Wang, Y.; Li, S.; Li, Y.; et al. Dehydration status predicts short-term and long-term outcomes in patients with cerebral venous thrombosis. Neurocrit. Care 2019, 30, 478–483. [Google Scholar] [CrossRef]
- Krabak, B.J.; Lipman, G.S.; Waite, B.L.; Rundell, S.D. Exercise-associated hyponatremia, hypernatremia, and hydration status in multistage ultramarathons. Wilderness Environ. Med. 2017, 28, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Riebl, S.K.; Davy, B.M. The hydration equation: Update on water balance and cognitive performance. ACSMs Health Fit. J. 2013, 17, 21–28. [Google Scholar]
- Pross, N.; Demazieres, A.; Girard, N.; Barnouin, R.; Santoro, F.; Chevillotte, E.; Klein, A.; Le Bellego, L. Influence of progressive fluid restriction on mood and physiological markers of dehydration in women. Br. J. Nutr. 2013, 109, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Perrier, E.; Rondeau, P.; Poupin, M.; Le Bellego, L.; Armstrong, L.E.; Lang, F.; Stookey, J.; Tack, I.; Vergne, S.; Klein, A. Relation between urinary hydration biomarkers and total fluid intake in healthy adults. Eur. J. Clin. Nutr. 2013, 67, 939–943. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Johnson, E.C.; Munoz, C.X.; Swokla, B.; Le Bellego, L.; Jimenez, L.; Casa, D.J.; Maresh, C.M. Hydration biomarkers and dietary fluid consumption of women. J. Acad. Nutr. Diet. 2012, 112, 1056–1061. [Google Scholar] [CrossRef]
- Perrier, E.; Vergne, S.; Klein, A.; Poupin, M.; Rondeau, P.; Le Bellego, L.; Armstrong, L.E.; Lang, F.; Stookey, J.; Tack, I. Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br. J. Nutr. 2013, 109, 1678–1687. [Google Scholar] [CrossRef]
- Lemaire, J.B.; Wallace, J.E.; Dinsmore, K.; Lewin, A.M.; Ghali, W.A.; Roberts, D. Physician nutrition and cognition during work hours: Effect of a nutrition based intervention. BMC Health Serv. Res. 2010, 10, 241. [Google Scholar] [CrossRef]
- Malisova, O.; Athanasatou, A.; Pepa, A.; Husemann, M.; Domnik, K.; Braun, H.; Mora-Rodriguez, R.; Ortega, J.F.; Fernandez-Elias, V.E.; Kapsokefalou, M. Water intake and hydration indices in healthy European adults: The European hydration research study (EHRS). Nutrients 2016, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Ix, J.H.; Wassel, C.L.; Stevens, L.A.; Beck, G.J.; Froissart, M.; Navis, G.; Rodby, R.; Torres, V.E.; Zhang, Y.L.; Greene, T.; et al. Equations to estimate creatinine excretion rate: The CKD epidemiology collaboration. Clin. J. Am. Soc. Nephrol. 2011, 6, 184–191. [Google Scholar] [CrossRef]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Trichopoulou, A. Available online: http://www.hhf-greece.gr/tables/Dishes.aspx?l=el (accessed on 5 May 18).
- Armstrong, L.E.; Pumerantz, A.C.; Fiala, K.A.; Roti, M.W.; Kavouras, S.A.; Casa, D.J.; Maresh, C.M. Human hydration indices: Acute and longitudinal reference values. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 145–153. [Google Scholar] [CrossRef]
- Holbrook, J.T.; Patterson, K.Y.; Bodner, J.E.; Douglas, L.W.; Veillon, C.; Kelsay, J.L.; Mertz, W.; Smith, J.C., Jr. Sodium and potassium intake and balance in adults consuming self-selected diets. Am. J. Clin. Nutr. 1984, 40, 786–793. [Google Scholar] [CrossRef]
- Baron, S.; Courbebaisse, M.; Lepicard, E.M.; Friedlander, G. Assessment of hydration status in a large population. Br. J. Nutr. 2015, 113, 147–158. [Google Scholar] [CrossRef]
- McLean, R.M. Measuring population sodium intake: A review of methods. Nutrients 2014, 6, 4651–4662. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Maalouf, J.; Elliott, P.; Loria, C.M.; Patel, S.; Bowman, B.A. Use of urine biomarkers to assess sodium intake: Challenges and opportunities. Annu. Rev. Nutr. 2015, 35, 349–387. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.C.; Munoz, C.X.; Le Bellego, L.; Klein, A.; Casa, D.J.; Maresh, C.M.; Armstrong, L.E. Markers of the hydration process during fluid volume modification in women with habitual high or low daily fluid intakes. Eur. J. Appl. Physiol. 2015, 115, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Perrier, E.; Demazieres, A.; Girard, N.; Pross, N.; Osbild, D.; Metzger, D.; Guelinckx, I.; Klein, A. Circadian variation and responsiveness of hydration biomarkers to changes in daily water intake. Eur. J. Appl. Physiol. 2013, 113, 2143–2151. [Google Scholar] [CrossRef]
- Bottin, J.H.; Lemetais, G.; Poupin, M.; Jimenez, L.; Perrier, E.T. Equivalence of afternoon spot and 24-h urinary hydration biomarkers in free-living healthy adults. Eur. J. Clin. Nutr. 2016, 70, 904–907. [Google Scholar] [CrossRef][Green Version]
- Jequier, E.; Constant, F. Water as an essential nutrient: The physiological basis of hydration. Eur. J. Clin. Nutr. 2010, 64, 115–123. [Google Scholar] [CrossRef]
- Stookey, J.D.; Hamer, J.; Killilea, D.W. Change in hydration indices associated with an increase in total water intake of more than 0.5 l/day, sustained over 4 weeks, in healthy young men with initial total water intake below 2 l/day. Physiol. Rep. 2017, 5, e13356. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on dietary reference values for water. EFSA J. 2010, 8, 1459–1507. [Google Scholar]
- Zhang, N.; Du, S.; Tang, Z.; Zheng, M.; Yan, R.; Zhu, Y.; Ma, G. Hydration, fluid intake, and related urine biomarkers among male college students in Cangzhou, China: A cross-sectional study-applications for assessing fluid intake and adequate water intake. Int. J. Environ. Res. Public Health 2017, 14, 513. [Google Scholar] [CrossRef] [PubMed]
- Medicine, I.O. Panel on Dietary Reference Intakes for Electrolytes and Water: Dietary Reference Intakes for Water, Potassium, Sodium, Chloride and Sulfate; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Wolfram, G. New reference values for nutrient intake in Germany, Austria and Switzerland (dach-reference values). Forum. Nutr. 2003, 56, 95–97. [Google Scholar]
- Armstrong, L.E.; Johnson, E.C. Water intake, water balance, and the elusive daily water requirement. Nutrients 2018, 10, 1928. [Google Scholar] [CrossRef]
- Gibson, S.; Shirreffs, S.M. Beverage consumption habits “24/7” among British adults: Association with total water intake and energy intake. Nutr. J. 2013, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Pego, C.; Guelinckx, I.; Moreno, L.A.; Kavouras, S.A.; Gandy, J.; Martinez, H.; Bardosono, S.; Abdollahi, M.; Nasseri, E.; Jarosz, A.; et al. Total fluid intake and its determinants: Cross-sectional surveys among adults in 13 countries worldwide. Eur. J. Nutr. 2015, 54 (Suppl. 2), 35–43. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, Q.; Liu, A.; Zuo, J.; Zhang, W.; Zou, S.; Li, X.; Lu, L.; Pan, H.; Hu, X. Fluid intake of adults in four chinese cities. Nutr. Rev. 2012, 70 (Suppl. 2), S105–S110. [Google Scholar] [CrossRef]
- Bougatsas, D.; Arnaoutis, G.; Panagiotakos, D.B.; Seal, A.D.; Johnson, E.C.; Bottin, J.H.; Tsipouridi, S.; Kavouras, S.A. Fluid consumption pattern and hydration among 8–14 years-old children. Eur. J. Clin. Nutr. 2018, 72, 420–427. [Google Scholar] [CrossRef]
- Vuckovic, N.; Ritenbaugh, C.; Taren, D.L.; Tobar, M. A qualitative study of participants’ experiences with dietary assessment. J. Am. Diet. Assoc. 2000, 100, 1023–1028. [Google Scholar] [CrossRef]
| Category of Beverage | Morning (A) | Afternoon (B) | Evening (C) | Night (D) | 24 h |
|---|---|---|---|---|---|
| Hot beverages (mL) | |||||
| Total | 211 (120) | 123 (78) | 115 (75) | 91 (49) | 376 (245) |
| LOW | 197 (101) | 112 (79) | 83 (78, 129) | 88 (42) | 360 (232) |
| HIGH | 225 (137) | 141 (74) | 83 (80, 117) | 94 (56) | 394 (260) |
| Winter | 200 (124) | 123 (85) | 83 (82, 142) | 95 (55) | 379 (269) |
| Summer | 223 (114) | 124 (64) | 80 (67, 110) | 86 (41) | 373 (215) |
| Milk (mL) | |||||
| Total | 133 (104) | 57 (57) | 70 (48) | - | 160 (123) |
| LOW | 127 (97) | 47 (52) | 80 (17, 92) | - | 146 (109) |
| HIGH | 140 (112) | 80 (12, 127) | 80 (35, 97) | - | 178 (138) |
| Winter | 117 (92) | 45 (11, 83) | 83 (16, 83) | - | 146 (108) |
| Summer | 151 (115) | 33 (4, 83) | 80 (12, 105) | - | 177 (138) |
| Fruit & Vegetable Juice (mL) | |||||
| Total | 134 (85) | 104 (78) | 75 (33, 103) | - | 147 (101) |
| LOW | 83 (67, 165) | 83 (77, 112) | 75 (46, 83) | - | 115 (75) * |
| HIGH | 110 (83, 220) | 83 (75, 98) | 75 (9, 162) | - | 176 (115) |
| Winter | 83 (67, 167) | 93 (79, 93) | - | - | 126 (79) |
| Summer | 137 (82, 217) | 83 (75, 113) | - | - | 165 (116) |
| Caloric Soft Drinks (mL) | |||||
| Total | 110 (50, 158) | 110 (83, 167) | 133 (83, 167) | - | 188 (120) |
| LOW | 110 (33, 167) | 110 (83, 167) | 117 (50, 187) | - | 167 (83, 250) |
| HIGH | 105 (58, 202) | 97 (77, 175) | 147 (96, 167) | - | 167 (83, 267) |
| Winter | 110 (108, 167) | 97 (65, 188) | 83 (37, 167) | 143 (83, 243) | |
| Summer | 67 (33, 150) | 110 (83, 167) | 160 (100, 180) | 210 (110, 300) | |
| Diet Soft Drinks (mL) | |||||
| Total | 167 (75, 230) | 110 (88, 153) | 110 (77, 167) | - | 165 (108, 257) |
| LOW | 138 (75, 167) | 167 (167, 167) * | 97 (76, 143) | - | 167 (100, 235) |
| HIGH | 227 (93, 371) | 105 (75, 110) | 110 (72, 250) | - | 110 (105, 407) |
| Winter | 167 (87,200) | 133 (88, 167) | 110 (80, 142) | 165 (92, 235) | |
| Summer | - | 110 (65, 110) | - | 167 (110, 540) | |
| Alcoholic Drinks (mL) | |||||
| Total | 88 (42, 167) | 123 (103) | 154 (138) | 58 (25, 94) | 198 (237) |
| LOW | 83 (42, 156) | 83 (41, 130) | 88 (72, 167) | 75 (75, 75) | 145 (134) * |
| HIGH | 130 (26, 479) | 100 (70, 167) | 109 (81, 216) | - | 261 (311) |
| Winter | 63 (20, 104) | 75 (42, 113) | 83 (42, 167) | - | 120 (90) * |
| Summer | 145 (80, 271) | 110 (67, 193) | 122 (83, 220) | 58 (25, 94) | 266 (298) |
| Drinking Water (mL) | |||||
| Total | 465 (290) | 414 (247) | 345 (272) | 83 (83, 162) | 1168 (666) |
| LOW | 321 (224) ** | 326 (198) ** | 254 (179) ** | 83 (83, 133) | 831 (455) ** |
| HIGH | 627 (270) | 511 (261) | 444 (319) | 100(80,167) | 1574 (655) |
| Winter | 355 (227) ** | 337 (205) ** | 271 (198) ** | 83 (83, 83) | 902 (448) ** |
| Summer | 578 (305) | 499 (263) | 428 (317) | 133 (80, 167) | 1473 (742) |
| Other Beverages (mL) | |||||
| Total | 83 (83, 83) | 83 (83, 83) | 110 | - | 110 (83, 167) |
| LOW | 83 (83, 83) | 83 (83, 83) | 110 (110,110) | - | 138 (110, 138) |
| HIGH | 83 (83, 83) | - | - | - | 83 (83, 83) |
| Winter | 83 (83, 83) | 83 (83, 83) | - | - | 138 (110, 138) |
| Summer | 83 (83, 83) | - | - | 83 (83, 83) | |
| Total Water Intake (mL) | |||||
| Total | 863 (425) | 750 (357) | 533 (333) | 22 (64) | 2266 (781) |
| LOW | 640 (271) ** | 602 (278) ** | 419 (240) ** | 19 (61) | 1564(333) ** |
| HIGH | 1147 (416) | 938 (359) | 679 (377) | 26 (67) | 2741 (622) |
| Winter | 718 (330) ** | 642 (325) ** | 462 (280) ** | 22 (72) | 1942 (527) ** |
| Summer | 1019 (461) | 865 (355) | 610 (367) | 23 (53) | 2611 (859) |
| Variety Score | |||||
| Total | 2.7 (1.1) | 2.3 (1.2) | 1.8 (1.1) | 0.7(0.7) | 3.6 (1.4) |
| LOW | 2.6 (1.0) * | 2.2 (1.0) | 1.8 (1.0) | 0.6 (0.6) | 3.5 (1.4) |
| HIGH | 2.9 (1.1) | 2.3 (1.2) | 2.0 (1.0) | 0.8 (0.7) | 3.7 (1.4) |
| Winter | 2.7 (1.0) | 3.7 (1.1) | 1.9 (1.1) | 0.7 (0.6) | 3.7 (1.1) |
| Summer | 2.6 (1.3) | 3.5 (1.6) | 1.8 (1.1) | 0.7 (0.7) | 3.5 (1.6) |
| Hydration Indices | Morning | Afternoon | Evening | Night | First Morning Urine | 24 h |
|---|---|---|---|---|---|---|
| Volume (mL) | ||||||
| Total | 557 (231) | 378 (205) | 290 (158) | 177 (149) | 339 (123) | 1331 (44) |
| LOW | 524 (203) * | 353 (200) | 272 (146) | 174 (160) | 327 (109) | 1239 (474) * |
| HIGH | 601 (258) | 410 (209) | 312 (171) | 182 (138) | 354 (138) | 1454 (589) |
| Winter | 578 (212) | 413 (206) * | 294 (146) | 228 (163) * | 340 (115) | 1397 (472) |
| Summer | 531 (251) | 334 (197) | 284 (172) | 126 (112) | 338 (132) | 1253 (597) |
| Osmolality (mOsmol/kg H2O) | ||||||
| Total | 620 (240) | 627 (258) | 580 (254) | 271 (157) | 691 (224) | 665 (223) |
| LOW | 645 (206) | 679 (257) * | 610 (220) | 310 (171) * | 719 (225) | 703 (209) * |
| HIGH | 588 (278) | 558 (243) | 540 (291) | 221 (123) | 655 (219) | 620 (233) |
| Winter | 624 (242) | 605 (243) | 575 (210) | 290 (189) | 681 (208) | 664 (209) |
| Summer | 616 (240) | 655 (274) | 586 (303) | 266 (137) | 701 (240) | 667 (237) |
| USG | ||||||
| Total | 1.017 (0.006) | 1.017 (0.006) | 1.019 (0.007) | 1.016 (0.007) | 1.018 (0.008) | 1.017 (0.006) |
| LOW | 1.018 (0.006) | 1.018 (0.006) * | 1.020 (0.006) * | 1.017 (0.007) | 1.019 (0.007) | 1.018 (0.006) * |
| HIGH | 1.017 (0.007) | 1.016 (0.006) | 1.017 (0.007) | 1.016 (0.008) | 1.017 (0.008) | 1.016 (0.006) |
| Winter | 1.017 (0.006) | 1.017 (0.006) | 1.019 (0.006) | 1.014 (0.007) ** | 1.019 (0.006) | 1.017 (0.006) |
| Summer | 1.017 (0.007) | 1.018 (0.007) | 1.019 (0.008) | 1.020 (0.007) | 1.017 (0.009) | 1.017 (0.006) |
| Color | ||||||
| Total | 3 (2) | 3 (1) | 3 (2) | 1 (2) | 3 (1) | 4 (2) |
| LOW | 3 (1) | 3 (1) | 3 (1) | 1 (1) | 3 (1) | 4 (2) |
| HIGH | 3 (2) | 3 (1) | 3 (1) | 2 (2) | 3 (2) | 3 (2) |
| Winter | 3 (1) | 3 (1) ** | 3 (1) * | 1 (1) | 3 (1) | 4 (2) |
| Summer | 3 (1) | 3 (1) | 3 (1) | 1 (1) | 3 (2) | 4 (2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasatou, A.; Kandyliari, A.; Malisova, O.; Kapsokefalou, M. Fluctuation of Water Intake and of Hydration Indices during the Day in a Sample of Healthy Greek Adults. Nutrients 2019, 11, 793. https://doi.org/10.3390/nu11040793
Athanasatou A, Kandyliari A, Malisova O, Kapsokefalou M. Fluctuation of Water Intake and of Hydration Indices during the Day in a Sample of Healthy Greek Adults. Nutrients. 2019; 11(4):793. https://doi.org/10.3390/nu11040793
Chicago/Turabian StyleAthanasatou, Adelais, Aikaterini Kandyliari, Olga Malisova, and Maria Kapsokefalou. 2019. "Fluctuation of Water Intake and of Hydration Indices during the Day in a Sample of Healthy Greek Adults" Nutrients 11, no. 4: 793. https://doi.org/10.3390/nu11040793
APA StyleAthanasatou, A., Kandyliari, A., Malisova, O., & Kapsokefalou, M. (2019). Fluctuation of Water Intake and of Hydration Indices during the Day in a Sample of Healthy Greek Adults. Nutrients, 11(4), 793. https://doi.org/10.3390/nu11040793





