Growth Benefits of Own Mother’s Milk in Preterm Infants Fed Daily Individualized Fortified Human Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Nutritional Practices
2.3. Individualized HM Fortification (IHMF)
2.4. Data Collection and Growth Assessment
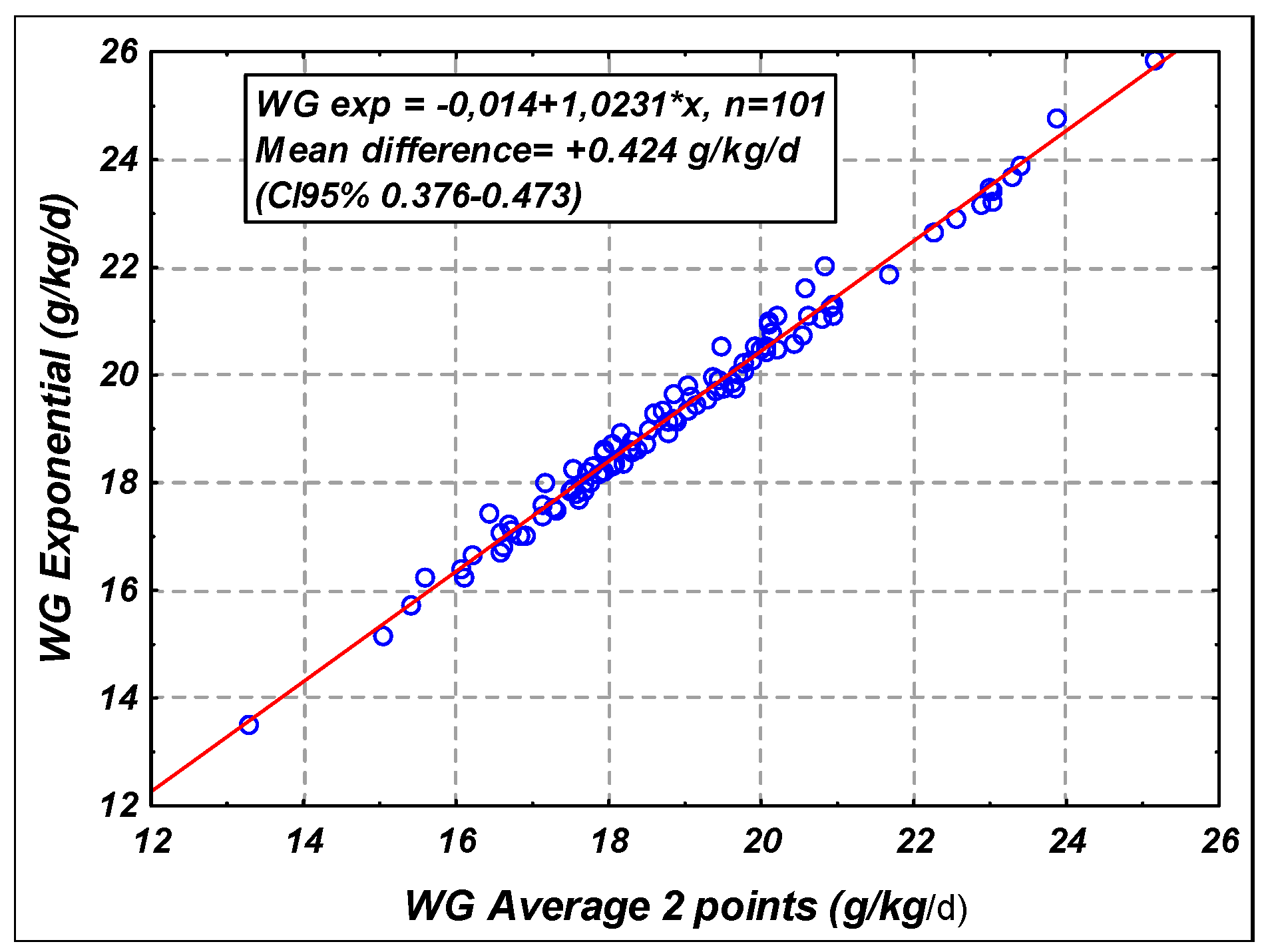
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Clinical Variables
3.3. Influence of OMM Versus DM
3.3.1. Human Milk Composition and Nutritional Intakes
3.3.2. Growth
3.4. Effects of Type of Human Milk (Raw OMM, Pasteurized OMM, and Pasteurized DM)
3.4.1. Human Milk Composition and Nutritional Intakes
3.4.2. Growth
3.5. Univariate and Multivariate Analysis on the Whole Population
3.5.1. Univariate Analysis
3.5.2. Multivariate Analysis
Weight Gain and Weight for Age Z-score Difference
Length Gain and Length for Age Z-score Difference
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Academy of Pediatrics. Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. Available online: https://pediatrics.aappublications.org/content/115/2/496 (accessed on 1 April 2019). [CrossRef] [PubMed]
- Arslanoglu, S.; Corpeleijn, W.; Moro, G.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellöf, M.; Fewtrell, M.; Hojsak, I.; et al. Donor Human Milk for Preterm Infants: Current Evidence and Research Directions. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Committee on Nutrition; Section on Breastfeeding; Committee on Fetus and Newborn. Donor Human Milk for the High-Risk Infant: Preparation, Safety, and Usage Options in the United States. Pediatrics 2017, 139. [Google Scholar] [CrossRef]
- Agostoni, C.; Buonocore, G.; Carnielli, V.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellof, M.; Embleton, N.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.C.; Uauy, R.; Koletzko, B.; Zlotkin, S.H. Summary of Reasonable Nutrient Intakes (mass units) for Preterm infants. In Nutrition of the Preterm Infant, Scientific Basis and Practical Guidelines, 2nd ed.; Tsang, R., Uauy, R., Koletzko, B., Zlotkin, S., Eds.; Digital Educational Publishing: Cincinnati, OH, USA, 2005; 415p. [Google Scholar]
- Koletzko, B.; Poindexter, B.; Uauy, R. Recommended nutrient intake levels for stable, fully enterally fed very low birth weight infants. World Rev. Nutr. Diet. 2014, 110, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.E.; Arslanoglu, S.; Bertino, E.; Corvaglia, L.; Montirosso, R.; Picaud, J.C.; Polberger, S.; Schanler, R.J.; Steel, C.; van Goudoever, J.; et al. XII. Human Milk in Feeding Premature Infants: Consensus Statement. J. Pediatr. Gastroenterol. Nutr. 2015, 61, S16–19. [Google Scholar] [CrossRef]
- Brown, J.V.; Embleton, N.D.; Harding, J.E.; McGuire, W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst. Rev. 2016, 5, CD000343. [Google Scholar] [CrossRef] [PubMed]
- Pieltain, C.; De Curtis, M.; Gerard, P.; Rigo, J. Weight gain composition in preterm infants with dual energy X-ray absorptiometry. Pediatr. Res. 2001, 49, 120–124. [Google Scholar] [CrossRef]
- Colaizy, T.T.; Carlson, S.; Saftlas, A.F.; Morriss, F.H. Growth in VLBW infants fed predominantly fortified maternal and donor human milk diets: A retrospective cohort study. BMC Pediatr. 2012, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.; Embleton, N.D.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2018, 6, CD002971. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar] [CrossRef]
- Stephens, B.E.; Walden, R.V.; Gargus, R.A.; Tucker, R.; McKinley, L.; Mance, M.; Nye, J.; Vohr, B.R. First-Week Protein and Energy Intakes Are Associated With 18-Month Developmental Outcomes in Extremely Low Birth Weight Infants. Pediatrics 2009, 123, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.; Johnson, M.J.; Leaf, A.A.; Vollmer, B. Nutrition and neurodevelopmental outcomes in preterm infants: A systematic review. Acta Paediatr 2016, 105, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Gibertoni, D.; Corvaglia, L.; Vandini, S.; Rucci, P.; Savini, S.; Alessandroni, R.; Sansavini, A.; Fantini, M.P.; Faldella, G. Positive effect of human milk feeding during NICU hospitalization on 24 month neurodevelopment of very low birth weight infants: An Italian cohort study. PLoS ONE 2015, 10, e0116552. [Google Scholar] [CrossRef]
- Roze, J.C.; Darmaun, D.; Boquien, C.Y.; Flamant, C.; Picaud, J.C.; Savagner, C.; Claris, O.; Lapillonne, A.; Mitanchez, D.; Branger, B.; et al. The apparent breastfeeding paradox in very preterm infants: Relationship between breast feeding, early weight gain and neurodevelopment based on results from two cohorts, epipage and lift. BMJ Open 2012, 2, e000834. [Google Scholar] [CrossRef]
- Vohr, B.R.; Poindexter, B.B.; Dusick, A.M.; McKinley, L.T.; Higgins, R.D.; Langer, J.C.; Poole, W.K. Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics 2007, 120, e953–e959. [Google Scholar] [CrossRef]
- De Halleux, V.; Pieltain, C.; Senterre, T.; Rigo, J. Use of donor milk in the neonatal intensive care unit. Semin. Fetal. Neonatal Med. 2017, 22, 23–29. [Google Scholar] [CrossRef]
- De Halleux, V.; Rigo, J. Variability in human milk composition: Benefit of individualized fortification in very-low-birth-weight infants. Am. J. Clin. Nutr. 2013, 98, 529S–535S. [Google Scholar] [CrossRef]
- De Halleux, V.; Close, A.; Stalport, S.; Studzinski, F.; Habibi, F.; Rigo, J. Intérêt de la supplémentation du lait maternel à la carte. Pediatr. Arch. 2007, 14, S5–S10. (In French) [Google Scholar] [CrossRef]
- Rochow, N.; Fusch, G.; Choi, A.; Chessell, L.; Elliott, L.; McDonald, K.; Kuiper, E.; Purcha, M.; Turner, S.; Chan, E.; et al. Target fortification of breast milk with fat, protein, and carbohydrates for preterm infants. J. Pediatr. 2013, 163, 1001–1007. [Google Scholar] [CrossRef]
- McLeod, G.; Sherriff, J.; Hartmann, P.E.; Nathan, E.; Geddes, D.; Simmer, K. Comparing different methods of human breast milk fortification using measured v. assumed macronutrient composition to target reference growth: A randomised controlled trial. Br. J. Nutr. 2016, 115, 431–439. [Google Scholar] [CrossRef]
- Morlacchi, L.; Mallardi, D.; Giannì, M.L.; Roggero, P.; Amato, O.; Piemontese, P.; Consonni, D.; Mosca, F. Is targeted fortification of human breast milk an optimal nutrition strategy for preterm infants? An interventional study. J. Transl. Med. 2016, 14, 195. [Google Scholar] [CrossRef]
- Andersson, Y.; Savman, K.; Blackberg, L.; Hernell, O. Pasteurization of mother’s own milk reduces fat absorption and growth in preterm infants. Acta Paediatr. 2007, 96, 1445–1449. [Google Scholar] [CrossRef]
- Montjaux-Regis, N.; Cristini, C.; Arnaud, C.; Glorieux, I.; Vanpee, M.; Casper, C. Improved growth of preterm infants receiving mother’s own raw milk compared with pasteurized donor milk. Acta Paediatr. 2011, 100, 1548–1554. [Google Scholar] [CrossRef]
- Madore, L.S.; Bora, S.; Erdei, C.; Jumani, T.; Dengos, A.R.; Sen, S. Effects of Donor Breastmilk Feeding on Growth and Early Neurodevelopmental Outcomes in Preterm Infants: An Observational Study. Clin. Ther. 2017, 39, 1210–1220. [Google Scholar] [CrossRef]
- O’Connor, D.L.; Ewaschuk, J.B.; Unger, S. Human milk pasteurization: Benefits and risks. Curr. Opin. Clin. Nutr. Metab. Care. 2015, 18, 269–275. [Google Scholar] [CrossRef]
- Brownell, E.A.; Matson, A.P.; Smith, K.C.; Moore, J.E.; Esposito, P.A.; Lussier, M.M.; Lerer, T.J.; Hagadorn, J.I. Dose-response Relationship Between Donor Human Milk, Mother’s Own Milk, Preterm Formula, and Neonatal Growth Outcomes. J. Pediatr. Gastroenterol. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gidrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Picaud, J.C.; Buffin, R.; Gremmo-Feger, G.; et al. Review concludes that specific recommendations are needed to harmonise the provision of fresh mother’s milk to their preterm infants. Acta Paediatr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Senterre, T.; Rigo, J. Reduction in postnatal cumulative nutritional deficit and improvement of growth in extremely preterm infants. Acta Paediatr. 2012, 101, e64–70. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, A.; Delsat, L.; Pieltain, C.; De Halleux, V.; Rigo, J. Evaluation de la qualité bactériologique du lait maternel dans un service de néonatologie (NIC). Revue médicale de Liège 2007, 62, 159–165. (In France) [Google Scholar]
- Simon, L.; Kessen, C.; Rigo, J.; De Halleux, V. Bacteriologic Quality of Colostrum, Comparison with Mature Milk. Thesis for Graduation in Medicine and Pediatrics, University of Nantes, Nantes, France, 2012. [Google Scholar]
- Fenton, T.R.; Anderson, D.; Groh-Wargo, S.; Hoyos, A.; Ehrenkranz, R.A.; Senterre, T. An Attempt to Standardize the Calculation of Growth Velocity of Preterm Infants-Evaluation of Practical Bedside Methods. J. Pediatr. 2018, 196, 77–83. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Rigo, J. Protein, amino acid and other nitrogen compounds. In Nutrition of the Preterm Infants, Scientific Basis and Practical Guidelines, 2nd ed.; Tsang, R.C., Uauy, R., Koletzko, B., Zlotkin, S.H., Eds.; Digital Educational Publishing, Inc.: Cincinnati, OH, USA, 2005; pp. 45–80. [Google Scholar]
- Cormack, B.E.; Embleton, N.D.; Van Goudoever, J.B.; Hay, W.W.; Bloomfield, F.H. Comparing apples with apples: It is time for standardized reporting of neonatal nutrition and growth studies. Pediatr. Res. 2016, 79, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Giuliani, F.; Fenton, T.R.; Ohuma, E.O.; Ismail, L.C.; Kennedy, S.H.; Consortium, I.-s. INTERGROWTH-21st very preterm size at birth reference charts. Lancet 2016, 387, 844–845. [Google Scholar] [CrossRef]
- Villar, J.; Giuliani, F.; Barros, F.; Roggero, P.; Coronado Zarco, I.A.; Rego, M.A.S.; Ochieng, R.; Gianni, M.L.; Rao, S.; Lambert, A.; et al. Monitoring the Postnatal Growth of Preterm Infants: A Paradigm Change. Pediatrics 2018, 141. [Google Scholar] [CrossRef] [PubMed]
- Pearson, F.; Johnson, M.J. How should we chart the growth of very preterm babies? Arch. Dis. Child Fetal. Neonatal. Ed. 2019, 104, F120–F121. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, F.; Prandi, G.; Coscia, A.; Cresi, F.; Di Nicola, P.; Raia, M.; Sabatino, G.; Occhi, L.; Bertino, E. Donor human milk versus mother’s own milk in preterm VLBWIs: A case control study. J. Biol. Regul. Homeost Agents 2012, 26, 19–24. [Google Scholar]
- Sisk, P.M.; Lambeth, T.M.; Rojas, M.A.; Lightbourne, T.; Barahona, M.; Anthony, E.; Auringer, S.T. Necrotizing Enterocolitis and Growth in Preterm Infants Fed Predominantly Maternal Milk, Pasteurized Donor Milk, or Preterm Formula: A Retrospective Study. Am. J. Perinatol. 2017, 34, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Cossey, V.; Vanhole, C.; Eerdekens, A.; Rayyan, M.; Fieuws, S.; Schuermans, A. Pasteurization of mother’s own milk for preterm infants does not reduce the incidence of late-onset sepsis. Neonatology 2013, 103, 170–176. [Google Scholar] [CrossRef]
- Maas, C.; Wiechers, C.; Bernhard, W.; Poets, C.F.; Franz, A.R. Early feeding of fortified breast milk and in-hospital-growth in very premature infants: A retrospective cohort analysis. BMC Pediatr. 2013, 13, 178. [Google Scholar] [CrossRef]
- De Curtis, M.; Senterre, J.; Rigo, J.; Putet, G. Carbohydrate derived energy and gross energy absorption in preterm infants fed human milk or formula. Arch. Dis. Child 1986, 61, 867–870. [Google Scholar] [CrossRef]
- Rigo, J.; Hascoët, J.M.; Billeaud, C.; Picaud, J.C.; Mosca, F.; Rubio, A.; Saliba, E.; Radkë, M.; Simeoni, U.; Guillois, B.; et al. Growth and Nutritional Biomarkers of Preterm Infants Fed a New Powdered Human Milk Fortifier: A Randomized Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 65, e83–e93. [Google Scholar] [CrossRef]
- Schanler, R.J.; Atkinson, S. Human milk. In Nutrition of the Preterm Infant: Scientific Basis and Practice, 2nd ed.; Tsang, R., Uauy, R., Koletzko, B., Zlotkin, S., Eds.; Digital Educational Publishing, Inc.: Cincinnati, OH, USA, 2005; pp. 333–356. [Google Scholar]
- Lapillonne, A.; O’Connor, D.L.; Wang, D.; Rigo, J. Nutritional Recommendations for the Late-Preterm Infant and the Preterm Infant after Hospital Discharge. J. Pediatr. 2013, 162, S90–S100. [Google Scholar] [CrossRef]
- Rochow, N.; Fusch, G.; Ali, A.; Bhatia, A.; Ahmad, S.; Nguyen, A.; Chessell, L.; el Helou, S.; Fusch, C. Target fortification of breast milk with protein, carbohydrate and fat for preterm infants improves growth outcomes: A double-bilnd randomised controlled trial. J. Pediatr. Neonatal Individ. Med. 2017, 6, e060238. [Google Scholar] [CrossRef]
- Fusch, G.; Kwan, C.; Kotrri, G.; Fusch, C. “Bed Side” Human Milk Analysis in the Neonatal Intensive Care Unit: A Systematic Review. Clin. Perinatol. 2017, 44, 209–267. [Google Scholar] [CrossRef]
- Buffin, R.; Decullier, E.; De Halleux, V.; Loys, C.M.; Hays, S.; Studzinsky, F.; Jourdes, E.; Rigo, J.; Picaud, J.C. Assessment of human milk composition using mid-infrared analyzers requires calibration adjustment. J. Perinatol. 2017, 37, 552–557. [Google Scholar] [CrossRef]
| m ± SD | ≥75% OMM n = 37 | 26%–74% OMM n = 31 | ≥75% DM n = 33 | All Subjects n = 101 | p |
|---|---|---|---|---|---|
| Male sex, n (%) | 18 (49) | 15 (48) | 17 (52) | 50 (50) | 0.96 |
| Gestational age, weeks, | 27.7 ± 2.1 | 28.2 ± 1.9 | 27.5 ± 1.8 | 27.8 ±1.9 | 0.26 |
| Birth Weight, g, | 983 ± 244 | 1042 ± 312 | 901 ± 185 | 975 ± 255 | 0.08 |
| Birth Weight < 1000 g, n (%) | 20 (54) | 16 (52) | 24 (73) | 60 (59) | 0.16 |
| Mean Weight z score, | −0.19 ± 0.99 | −0.37 ± 0.89 | −0.48 ± 0.82 | −0.34 ± 0.91 | 0.47 |
| Birth Length, cm, | 35.0 ± 3.3 | 35.8 ± 3.9 | 34.6 ± 2.9 | 35.1 ± 3.4 | 0.34 |
| Birth HC, cm, | 24.9 ± 1.9 | 25.8 ± 2.3 | 24.5 ± 1.7 | 25.0 ± 2.0 | 0.02 |
| Vaginal Delivery, n (%) | 16 (43) | 9 (29) | 7 (21) | 32 (32) | 0.13 |
| Twin, n (%) | 8 (22) | 12 (39) | 6 (18) | 26 (26) | 0.13 |
| Apgar Score 1 min, | 6.5 ± 2.2 | 6.1 ± 2.2 | 6.1 ± 2.0 | 6.2 ± 2.1 | 0.60 |
| Apgar Score 5 min, | 7.9 ± 1.5 | 7.8 ± 1.5 | 7.9 ± 1.1 | 7.9 ± 1.4 | 0.92 |
| Antenatal steroids, n (%) | 35 (95) | 27 (87) | 29 (88) | 91 (90) | 0.30 |
| Study duration, | 27 ± 8 | 27 ± 8 | 24 ± 6 | 26 ± 8 | 0.14 |
| GA age at study day 1, weeks, | 30.5 ± 1,5 | 30.8 ± 1,6 | 30.5 ± 1,5 | 30,6 ± 1,5 | 0.64 |
| GA age at study end, weeks, | 34.2 ± 1.4 | 34.7 ± 1.8 | 33.9 ± 1.5 | 34.3 ± 1.6 | 0.12 |
| ≥75% OMM n = 37 | ≥75% DM n = 33 | p-Value | |
|---|---|---|---|
| Human Milk Category (%) | |||
| Raw OMM | 31.3 ± 33.6 | 0.5 ± 3.0 | <0.001 |
| Pasteurized OMM | 64.1 ± 33.1 | 1.7 ± 4.7 | <0.001 |
| Pasteurized DM | 4.6 ± 7.8 | 97.8 ± 5.4 | <0.001 |
| Human Milk Composition (Infrared) | |||
| Protein, g/dL | 1.44 ± 0.22 | 1.35 ± 0.14 | 0.056 |
| Lipid, g/dL | 3.87 ± 0.59 | 3.61 ± 0.23 | 0.022 |
| Carbohydrates, g/dL | 6.84 ± 0.22 | 6.86 ± 0.19 | 0.695 |
| Nutritional Intakes (Units/kg/day) | |||
| Volume, mL | 167 ± 10 | 166 ± 8 | 0.536 |
| Energy, kcal | 143 ± 8 | 141 ± 6 | 0.148 |
| Protein, g | 4.17 ± 0.15 | 4.15 ± 0.14 | 0.512 |
| OMM ≥ 75% n = 37 | DM ≥ 75% n = 33 | Delta OMM vs. DM | p | |
|---|---|---|---|---|
| Weight gain, g/kg/day | 19.8 ± 2.0 | 18.2 ± 2.2 | +1.6 | 0.002 |
| Length gain, cm/week | 1.17 ± 0.26 | 0.99 ± 0.36 | +0.18 | 0.020 |
| Head circumference, cm/week | 1.13 ± 0.22 | 1.04 ± 0.27 | +0.09 | 0.120 |
| Weight Z-score gain, g/kg/d | 0.13 ± 0.35 | −0.26 ± 0.41 | +0.39 | <0.001 |
| Length Z-score gain, cm/week | −0.25 ± 0.41 | −0.59 ± 0.52 | +0.33 | 0.004 |
| HC Z-score gain, cm/week | 0.59 ± 0.50 | −0.24 ± 0.65 | +0.35 | 0.013 |
| Human Milk Type Volume Intake (%) | DM 88.5 ± 16.9 | POMM 70.3 ± 22.6 | Delta vs. DM | p vs. DM | ROMM 69.1 ± 19.9 | Delta vs. DM | p vs. DM | Delta vs. POMM | p vs. POMM |
|---|---|---|---|---|---|---|---|---|---|
| n | 45 | 41 | 15 | ||||||
| Energy, kcal/kg/day | 141.3 ± 6.3 | 142.4 ± 7.3 | - | 0.432 | 143.7 ± 6.2 | 0.210 | - | 0.552 | |
| Protein, g/kg/d | 4.15 ± 0.14 | 4.19 ± 0.13 | - | 0.211 | 4.18 ± 0.15 | 0.494 | - | 0.855 | |
| Weight gain, g/kg/d | 18.2 ± 1.9 | 19.1 ± 1.8 | +0.87 | 0.035 | 21.1 ± 1.6 | +2.83 | <0.001 | +1.96 | <0.001 |
| Length gain, cm/week | 1.04 ± 0.36 | 1.13 ± 0.33 | +0.10 | 0.193 | 1.17 ± 0.28 | +0.14 | 0.194 | +0.04 | 0.697 |
| HC gain, cm/week | 1.04 ± 0.24 | 1.10 ± 0.20 | +0.05 | 0.258 | 1.10 ± 0.24 | +0.06 | 0.409 | +0.01 | 0.937 |
| Weight Z-score gain | –0.23 ± 0.39 | 0.09 ± 0.31 | +0.31 | <0.001 | 0.15 ± 0.44 | +0.38 | 0.003 | +0.06 | 0.546 |
| Length Z-score gain | –0.53 ± 0.52 | –0.36 ± 0.45 | +0.17 | 0.116 | –0.14 ± 0.50 | +0.39 | 0.013 | +0.22 | 0.114 |
| HC Z-score gain | 0.28 ± 0.59 | 0.51 ± 0.56 | +0.23 | 0.068 | 0.70 ± 0.41 | +0.41 | 0.016 | +0.18 | 0.252 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Halleux, V.; Pieltain, C.; Senterre, T.; Studzinski, F.; Kessen, C.; Rigo, V.; Rigo, J. Growth Benefits of Own Mother’s Milk in Preterm Infants Fed Daily Individualized Fortified Human Milk. Nutrients 2019, 11, 772. https://doi.org/10.3390/nu11040772
de Halleux V, Pieltain C, Senterre T, Studzinski F, Kessen C, Rigo V, Rigo J. Growth Benefits of Own Mother’s Milk in Preterm Infants Fed Daily Individualized Fortified Human Milk. Nutrients. 2019; 11(4):772. https://doi.org/10.3390/nu11040772
Chicago/Turabian Stylede Halleux, Virginie, Catherine Pieltain, Thibault Senterre, Frédéric Studzinski, Catheline Kessen, Vincent Rigo, and Jacques Rigo. 2019. "Growth Benefits of Own Mother’s Milk in Preterm Infants Fed Daily Individualized Fortified Human Milk" Nutrients 11, no. 4: 772. https://doi.org/10.3390/nu11040772
APA Stylede Halleux, V., Pieltain, C., Senterre, T., Studzinski, F., Kessen, C., Rigo, V., & Rigo, J. (2019). Growth Benefits of Own Mother’s Milk in Preterm Infants Fed Daily Individualized Fortified Human Milk. Nutrients, 11(4), 772. https://doi.org/10.3390/nu11040772





