Cocoa Flavanols: Natural Agents with Attenuating Effects on Metabolic Syndrome Risk Factors
Abstract
:1. Introduction
2. Cacao Flavanols
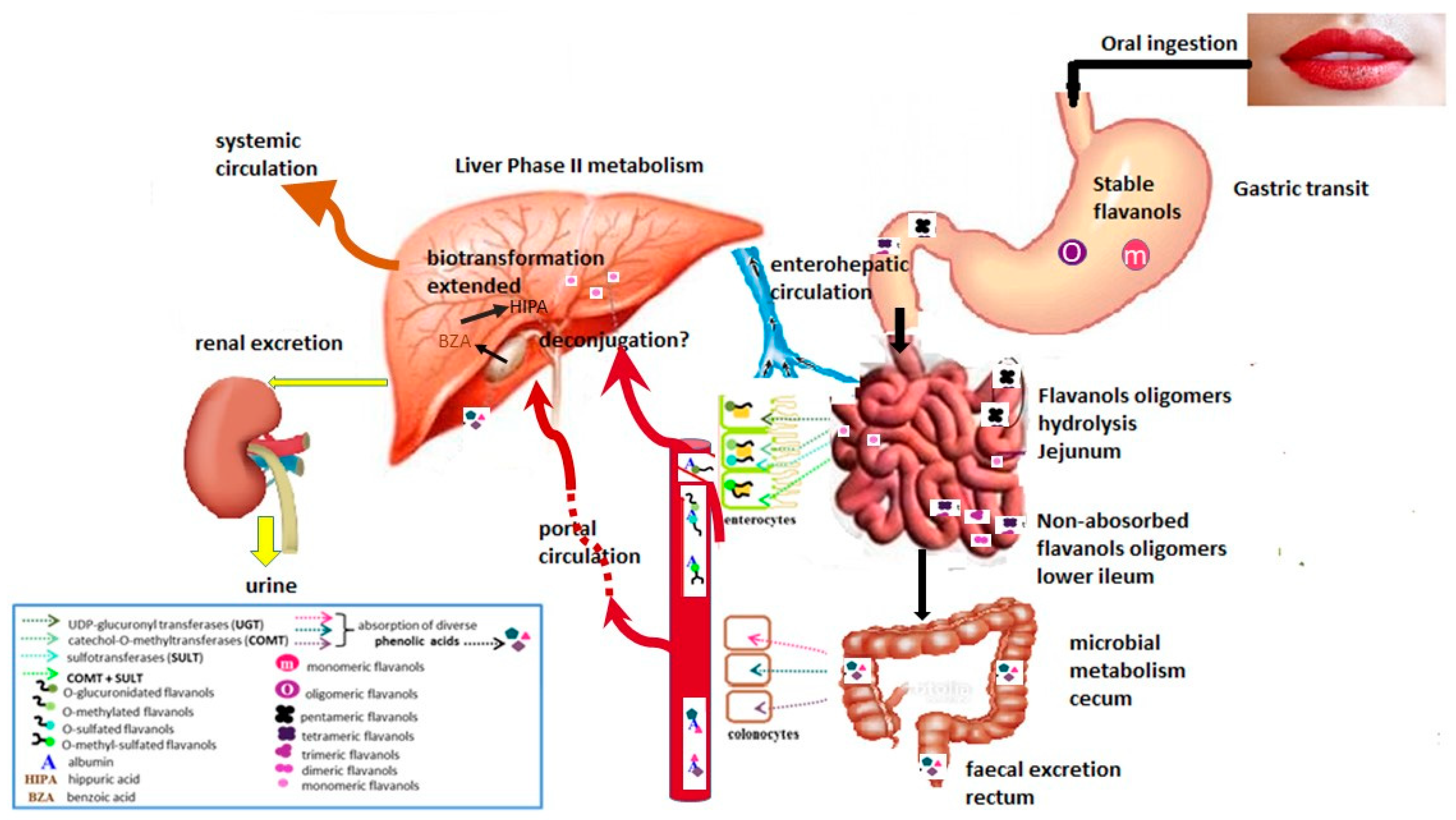
3. Flavanol Bioavailability
4. Cacao Flavanols and Their Health Effects
4.1. Direct Mechanisms
4.2. Indirect Mechanisms
4.2.1. Effects on Inflammation and Oxidation
NADPH Oxidase and Endotheline 1
Endothelial Nitric Oxide Synthase (eNOS) and Arginase
Nuclear Factor κB (NF-κB) and Tumoral Necrosis Factor (TNF-α)
Oxidized Low-Density Lipoproteins (LDLox)
4.2.2. Effects of Flavanols on Lipid Metabolism Disorders
4.2.3. Effects on Hyperglycemia and Insulin Resistance
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Rangel-Fajardo, M.A.; Zavaleta-Mancera, H.A.; Córdova-Téllez, L.; López-Andrade, A.P.; Delgado-Alvarado, A.; Vidales-Fernández, I.; Villegas-Monter, Á. Anatomía e histoquímica de la semilla del cacao (Theobroma cacao L.) criollo mexicano. Anatomy and histochemistry of the mexican cacao (Theobroma cacao L.) seed. Rev. Fitotecnia Mex 2012, 35, 189–197. [Google Scholar]
- Colombo, M.L.; Pinorini-Godly, M.T.; Conti, A. Botany and Pharmacognosy of the Cacao Tree. In Chocolate and Health; Conti, A., Paoletti, R., Poli, A., Visioli, F., Eds.; Springer: Berlin/Heidelberg, Garmany, 2012; pp. 41–62. [Google Scholar]
- Martín, M.A.; Ramos, S. Cocoa polyphenols in oxidative stress: Potential health implications. J. Funct. Foods 2016, 27, 570–588. [Google Scholar] [CrossRef]
- Redovniković, I.R.; Delonga, K.; Mazor, S.; Dragović-Uzelac, V.; Carić, M.; Vorkapić-Furač, J. Polyphenolic content and composition and antioxidative activity of different Cocoa liquors. Czech J. Food Sci. 2009, 27, 330–337. [Google Scholar] [CrossRef]
- Bauer, S.R.; Ding, E.L.; Smit, L.A. Cocoa Consumption, Cocoa Flavonoids, and Effects on Cardiovascular Risk Factors: An Evidence-Based Review. Curr. Cardiovasc. Risk. Rep. 2011, 5, 120–127. [Google Scholar] [CrossRef]
- Sudano, I.; Flammer, A.J.; Roas, S.; Enseleit, F.; Ruschitzka, F.; Corti, R. Cocoa, blood pressure, and vascular function. Curr. Hypertens. Rep. 2012, 14. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Violi, F. Polyphenolic antioxidants and health. Choc. Heal. 2013, 77–85. [Google Scholar] [CrossRef]
- Wang, Y.; Feltham, B.A.; Suh, M.; Jones, P.J.H. Cocoa flavanols and blood pressure reduction: Is there enough evidence to support a health claim in the United States? Trends Food Sci. Technol. 2019, 83, 203–210. [Google Scholar] [CrossRef]
- Corella, D.; Ordovas, J.M. Metabolic syndrome pathophysiology: The role of adipose tissue. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 125–139. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Connell, J.M.C. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr. Metab Cardiovasc. Dis. 2007, 17, 319–326. [Google Scholar] [CrossRef]
- Srikanthan, K.; Feyh, A.; Visweshwar, H.; Shapiro, J.I.; Sodhi, K. Systematic review of metabolic syndrome biomarkers: A panel for early detection, management, and risk stratification in the West Virginian population. Int. J. Med. Sci. 2016, 13, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 2010, 469–480. [Google Scholar]
- Trivedi, R.; Patel, P.N.; Jani, H.J.; Trivedi, B.A. Nutrigenomics: From molecular nutrition to prevention of disease. Biotechnol. Indian J. 2011, 5, 112–116. [Google Scholar]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef] [PubMed]
- Strat, K.M.; Rowley, T.J.; Smithson, A.T.; Tessem, J.S.; Hulver, M.W.; Liu, D.; Davy, B.M.; Davy, K.P.; Neilsonc, A.P. ScienceDirect Mechanisms by which cocoa flavanols improve metabolic syndrome and related disorders ☆. J. Nutr. Biochem. 2016, 35, 1–21. [Google Scholar] [CrossRef]
- Rusconi, M.; Conti, A. Theobroma cacao L., the Food of the Gods: A scientific approach beyond myths and claims. Pharmacol. Res. 2010, 61, 5–13. [Google Scholar] [CrossRef]
- Braicu, C.; Irimie, A.; Berindan; Pilecki, V.; Balacescu, O.; Neagoe, I. The Relationships Between Biological Activities and Structure of Flavan-3-Ols. Int. J. Mol. Sci. 2011, 12, 9342–9353. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Aspects Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E.; Chaudry, F.; Pannala, A.S.; Srai, S.K.; Debnam, E.; Rice-Evans, C. Decomposition of cocoa procyanidins in the gastric milieu. Biochem. Biophys. Res. Commun. 2000, 272, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Scalbert, A.; Rémésy, C.; Rios, L.Y.; Williamson, G.; Lazarus, S.A. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2018, 76, 1106–1110. [Google Scholar]
- Hackman, R.M.; Polagruto, J.A.; Zhu, Q.Y.; Sun, B.; Fujii, H.; Keen, C.L. Flavanols: Digestion, absorption and bioactivity. Phytochem. Rev. 2008, 7, 195–208. [Google Scholar] [CrossRef]
- Kwik-Uribe, C.; Bektash, R.M. Cocoa flavanols: Measurement, bioavailability and bioactivity. Asia. Pac. J. Clin. Nutr. 2008, 17, 280–283. [Google Scholar]
- Stoupi, S.; Williamson, G.; Drynan, J.W.; Barron, D.; Clifford, M.N. A comparison of the in vitro biotransformation of (-)-epicatechin and procyanidin B2 by human faecal microbiota. Mol. Nutr. Food Res. 2010, 54, 747–759. [Google Scholar] [CrossRef]
- Camps-Bossacoma, M.; Pérez-Cano, F.J.; Franch, À.; Untersmayr, E.; Castell, M. Effect of a cocoa diet on the small intestine and gut-associated lymphoid tissue composition in an oral sensitization model in rats. J. Nutr. Biochem. 2017, 42, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Arola-Arnal, A.; Muguerza, B.; Margalef, M.; Bravo, F.I.; Iglesias-Carres, L.; Pons, Z. Flavanol plasma bioavailability is affected by metabolic syndrome in rats. Food Chem. 2017, 231, 287–294. [Google Scholar]
- Holt, R.R.; Lazarus , S.A.; Sullards, M.C.; Zhu, Q.Y.; Schramm, D.D.; Hammerstone, J.F.; Fraga, C.G.; Schmitz, H.H.; Keen, C.L. Procyanidin dimer B2 [epicatechin-(4β-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am. J. Clin. Nutr. 2018, 76, 798–804. [Google Scholar]
- Baba, S.; Osakabe, N.; Natsume, M.; Muto, Y.; Takizawa, T.; Terao, J. Absorption and urinary excretion of (-)-epicatechin after administration of different levels of cocoa powder or (-)-epicatechin in rats. J. Agric. Food Chem. 2001, 49, 6050–6056. [Google Scholar] [CrossRef]
- Takizawa, T.; Baba, S.; Terao, J.; Osakabe, N.; Muto, Y.; Natsume, M. In Vivo Comparison of the Bioavailability of (+)-Catechin, (−)-Epicatechin and Their Mixture in Orally Administered Rats. J. Nutr. 2018, 131, 2885–2891. [Google Scholar]
- Ottaviani, J.I.; Heiss, C.; Spencer, J.P.E.; Kelm, M.; Schroeter, H. Recommending flavanols and procyanidins for cardiovascular health: Revisited. Mol. Aspects Med. 2018, 61, 63–75. [Google Scholar] [CrossRef]
- Actis-Goretta, L.; Lévèques, A.; Giuffrida, F.; Romanov-Michailidis, F.; Viton, F.; Barron, D.; Duenas-Paton, M.; Gonzalez-Manzano, S.; Santos-Buelga, C.; Williamson, G.; et al. Elucidation of (-)-epicatechin metabolites after ingestion of chocolate by healthy humans. Free Radic Biol. Med. 2012, 53, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef]
- Ohtake, Y.; Moriichi, N.; Shoji, T.; Masumoto, S.; Akiyama, H.; Kanda, T.; Goda, Y. Apple Procyanidin Oligomers Absorption in Rats after Oral Administration: Analysis of Procyanidins in Plasma Using the Porter Method and High-Performance Liquid Chromatography/Tandem Mass Spectrometry. J. Agric Food Chem. 2006, 54, 884–892. [Google Scholar]
- Appeldoorn, M.M.; Vincken, J.-P.; Gruppen, H.; Hollman, P.C.H. Procyanidin Dimers A1, A2, and B2 Are Absorbed without Conjugation or Methylation from the Small Intestine of Rats. J. Nutr. 2009, 139, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Llorente-Cortés, V.; Urpi-Sarda, M.; Sacanella, E.; Camino-López, S.; Vázquez-Agell, M.; Chiva-Blanch, G.; Tobias, E.; Rourae, E.; Andres-Lacueva, C.; Lamuela-Raventos, R.M.; et al. Cocoa consumption reduces NF-κB activation in peripheral blood mononuclear cells in humans. Nutr. Metab. Cardiovasc. Dis. 2011, 23, 257–263. [Google Scholar]
- Aherne, S.A.; O ’brien, N.M. Dietary Flavonols: Chemistry, Food Content, and Metabolism chemistry and structure of the flavonoids. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef]
- Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Membrane effects of Cocoa procyanidins in liposomes and Jurkat T cells. Biol. Res. 2004, 37, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.V.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G.; Oteiza, P.I. Flavan-3-ols and procyanidins protect liposomes against lipid oxidation and disruption of the bilayer structure. Free Radic Biol. Med. 2003, 34, 84–92. [Google Scholar] [CrossRef]
- Repetto, M.; Semprine, J.; Boveris, A. Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination. InTechOpen 2012. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In vitro antioxidant versus metal ion chelating properties of flavonoids: A structure-activity investigation. PLoS ONE 2016, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Loke, W.M.; Proudfoot, J.M.; Hodgson, J.M.; McKinley, A.J.; Hime, N.; Magat, M.; Stocker, R.; Croft, K.D. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein e-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 749–757. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Sheen, J.-M.; Hu, W.L.; Hung, Y.-C. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxid. Med. Cell Longev. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Ali, F.; Ismail, A.; Kersten, S. Molecular mechanisms underlying the potential antiobesity-related diseases effect of cocoa polyphenols. Mol. Nutr. Food Res. 2014, 58, 33–48. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martín, M.A.; Goya, L.; Ramos, S. Cocoa flavonoids protect hepatic cells against high-glucose-induced oxidative stress: Relevance of MAPKs. Mol. Nutr. Food Res. 2015, 59, 597–609. [Google Scholar] [CrossRef]
- Goya, L.; Martin, M.Á.; Ramos, S. Antidiabetic actions of cocoa flavanols. Mol. Nutr. Food Res. 2016, 60, 1756–1769. [Google Scholar]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (-)-epicatechin and related procyanidins in cell signaling. Free Radic Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef]
- Van Guilder, G.P.; Hoetzer, G.L.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity 2006, 14, 2127–2231. [Google Scholar] [CrossRef] [PubMed]
- Catrysse, L.; van Loo, G. Inflammation and the Metabolic Syndrome: The Tissue-Specific Functions of NF-κB. Trends. Cell Biol. 2017, 27, 417–429. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, S.; Lambert, J.D. Dietary cocoa ameliorates obesity-related inflammation in high fat-fed mice. Eur. J. Nutr. 2014, 53, 149–158. [Google Scholar] [CrossRef]
- Telle-Hansen, V.H.; Christensen, J.J.; Ulven, S.M.; Holven, K.B. Does dietary fat affect inflammatory markers in overweight and obese individuals?—A review of randomized controlled trials from 2010 to 2016. Genes Nutr. 2017, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Schewe, T.; Steffen, Y.; Sies, H. How do dietary flavanols improve vascular function? A position paper. Arch. Biochem. Biophys. 2008, 476, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Steffen, Y.; Gruber, C.; Schewe, T.; Sies, H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch. Biochem. Biophys. 2008, 469, 209–219. [Google Scholar] [CrossRef]
- Rassaf, T.; Kelm, M. Cocoa flavanols and the nitric oxide-pathway: targeting endothelial dysfunction by dietary intervention. Drug Discov. Today Dis. Mech. 2008, 5, 273–278. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Jiménez, R.; Sánchez, M.; Zarzuelo, M.J.; Galindo, P.; Quintela, A.M.; López-Sepulveda, R.; Romero, M.; Tamargo, J.; Vargas, F.; et al. Epicatechin lowers blood pressure, restores endothelial function, and decreases oxidative stress and endothelin-1 and NADPH oxidase activity in DOCA-salt hypertension. Free Radic. Biol. Med. 2011, 52, 70–79. [Google Scholar]
- Reiter, C.E.N.; Kim, J.A.; Quon, M.J. Green tea polyphenol epigallocatechin gallate reduces endothelin-1 expression and secretion in vascular endothelial cells: Roles for AMP-activated protein kinase, Akt, and FOXO1. Endocrinology 2010, 151, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Lauer, T.; Dejam, A.; Kleinbongard, P.; Hamada, S.; Rassaf, T.; Matern, S.; Feelisch, M.; Kelm, M. Plasma nitroso compounds are decreased in patients with endothelial dysfunction. J. Am. Coll. Cardiol. 2006, 47, 573–579. [Google Scholar] [CrossRef]
- Schnorr, O.; Brossette, T.; Momma, T.Y.; Kleinbongard, P.; Keen, C.L.; Schroeter, H.; Sies, H. Cocoa flavanols lower vascular arginase activity in human endothelial cells in vitro and in erythrocytes in vivo. Arch. Biochem. Biophys. 2008, 476, 211–215. [Google Scholar] [CrossRef]
- Hajer, G.R.; Van Haeften, T.W.; Visseren, F.L.J. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 2008, 29, 2959–2971. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, G.G.; Delfino, J.M.; Keen, C.L.; Fraga, C.G.; Oteiza, P.I. Dimeric procyanidins are inhibitors of NF-κB-DNA binding. Biochem. Pharmacol. 2009, 78, 1252–1262. [Google Scholar] [CrossRef]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.M.; Orlando, R.A. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr. Metab. 2008, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Grattagliano, I.; Palmieri, V.O.; Portincasa, P.; Moschetta, A.; Palasciano, G. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J. Nutr. Biochem. 2008, 19, 491–504. [Google Scholar] [CrossRef]
- Steffen, Y.; Schewe, T.; Sies, H. Epicatechin protects endothelial cells against oxidized LDL and maintains NO synthase. Biochem. Biophys. Res. Commun. 2005, 331, 1277–1283. [Google Scholar] [CrossRef]
- Steffen, Y.; Jung, T.; Klotz, L.O.; Schewe, T.; Grune, T.; Sies, H. Protein modification elicited by oxidized low-density lipoprotein (LDL) in endothelial cells: Protection by (-)-epicatechin. Free Radic Biol. Med. 2007, 42, 955–970. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; Maya, L.; Ceballos, G.; Villarreal, F. (-)-Epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension 2010, 55, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Lorenzo, P.; Ceballos, G.; Villarreal, F.; Varela, C.E.; Rodriguez, A.; Romero-Valdovinos, M.; Mendoza-Loren, P.; Ramirez-Sanchez, I. Browning effects of (-)-epicatechin on adipocytes and white adipose tissue. Eur. J. Pharmacol. 2017, 811, 48–59. [Google Scholar]
- Jia, L.; Liu, X.; Bai, Y.Y.; Li, S.H.; Sun, K.; He, C. Short-term effect of cocoa product consumption on lipid profile: A meta-analysis of randomized controlled trials 1–3. Am. J. Clin. Nutr. 2010, 2, 218–225. [Google Scholar] [CrossRef]
- Tokede, O.A.; Ellison, C.R.; Pankow, J.S.; North, K.E.; Hunt, S.C.; Kraja, A.T.; Arnett, D.K.; Djoussé, L. Chocolate consumption and prevalence of metabolic syndrome in the NHLBI Family Heart Study. e-SPEN J. 2012, 7, e139–e143. [Google Scholar] [CrossRef]
- Bladé, C.; Arola, L.; Salvadó, M.J. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol. Nutr. Food Res. 2010, 54, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Rabadan-Chávez, G.; Reyes-Maldonado, E.; Quevedo-Corona, L.; Jaramillo-Flores, M.E.; Miliar, G.A. Cocoa powder, cocoa extract and epicatechin attenuate hypercaloric diet-induced obesity through enhanced β-oxidation and energy expenditure in white adipose tissue. J. Funct. Foods 2015, 20, 54–67. [Google Scholar] [CrossRef]
- Natsume, M.; Kanegae, M.; Yasuda, A.; Sasaki, K.; Nagaoka, S.; Baba, S.; Natsume, M. Cacao procyanidins reduce plasma cholesterol and increase fecal steroid excretion in rats fed a high-cholesterol diet. BioFactors 2009, 33, 211–223. [Google Scholar]
- Mellor, D.D.; Sathyapalan, T.; Kilpatrick, E.S.; Beckett, S.; Atkin, S.L. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in Type 2 diabetes patients. Diabet. Med. 2010, 27, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Yamagishi, M. Procyanidins in Theobroma cacao Reduce Plasma Cholesterol Levels in High Cholesterol-Fed Rats. J. Clin. Biochem. Nutr. 2009, 45, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Janevski, M.; Antonas, K.N.; Sullivan-Gunn, M.J.; McGlynn, M.A.; Lewandowski, P.A. The effect of cocoa supplementation on hepatic steatosis, reactive oxygen species and LFABP in a rat model of NASH. Comp. Hepatol. 2011, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Tomaru, M.; Takano, H.; Osakabe, N.; Yasuda, A.; Inoue, K.; Yanagisawa, R.; Ohwatari, T.; Uematsu, H. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice. Nutrition 2007, 23, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Faizul, H.A.; Nawalyah, A.G.; Hamid, M.; Ruzaidi, A.; Amin, I. The effect of Malaysian cocoa extract on glucose levels and lipid profiles in diabetic rats. J. Ethnopharmacol. 2005, 98, 55–60. [Google Scholar]
- León, L.; De la Rosa, D.; Gracia, A.; Barranco, D.; Rallo, L. Fatty acid composition of advanced olive. J. Food Sci. Agric. 2008, 1926, 1921–1926. [Google Scholar] [CrossRef]
- Grassi, D.; Lippi, C.; Desideri, G.; Necozione, S.; Ferri, C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2018, 81, 611–614. [Google Scholar] [CrossRef]
- Yamashita, Y.; Okabe, M.; Natsume, M.; Ashida, H. Prevention mechanisms of glucose intolerance and obesity by cacao liquor procyanidin extract in high-fat diet-fed C57BL/6 mice. Arch. Biochem. Biophys. 2012, 527, 95–104. [Google Scholar] [CrossRef]
- Gondoin, A.; Grussu, D.; Stewart, D.; McDougall, G.J. White and green tea polyphenols inhibit pancreatic lipase in vitro. Food Res. Int. [Internet]. 2010, 43, 1537–1544. [Google Scholar] [CrossRef]
- Osada, K.; Suzuki, T.; Kawakami, Y.; Senda, M.; Kasai, A.; Sami, M.; Ohta, Y.; Kanda, T.; Ikeda, M. Dose-dependent hypocholesterolemic actions of dietary apple polyphenol in rats fed cholesterol. Lipids 2006, 41, 133–139. [Google Scholar] [CrossRef]
- Rabadán-Chávez, G.M.; Miliar Garcia, A.; Paniagua Castro, N.; Escalona Cardoso, G.; Quevedo-Corona, L.; Reyes-Maldonado, E.; Jaramillo Flores, M.E. Modulating the expression of genes associated with hepatic lipid metabolism, lipoperoxidation and inflammation by cocoa, cocoa extract and cocoa flavanols related to hepatic steatosis induced by a hypercaloric diet. Food Res. Int. 2016, 89, 937–945. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Nakata, R.; Tamura, E.; Kosuge, Y.; Kariya, A.; Katsukawa, M.; Mishima, S.; Ito, T.; Iinuma, M.; Akao, Y.; et al. Vaticanol C, a resveratrol tetramer, activates PPARalpha and PPARbeta/delta in vitro and in vivo. Nutr. Metab. 2010, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhou, X.-Y.; Gao, D.-M.; Zhou, N.-J.; Xie, J.; Chen, J. Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Arch. Biochem. Biophys. 2011, 518, 61–70. [Google Scholar]
- Najim, N.I.; Shah, S.A.; Kazi, A.N.; Dharani, A.M.; Shah, S.R.; Jangda, M.A. Use of dark chocolate for diabetic patients: a review of the literature and current evidence. J. Commu. Hosp. Intern. Med. Perspect. 2017, 7, 218–221. [Google Scholar]
- Reygaert, W. An Update on the Health Benefits of Green Tea. Beverages 2017, 3, 6. [Google Scholar] [CrossRef]

| Type of Study | Product/Compound | Dose | Plasma Metabolites | Plasma Cmax (μmol/L) | Plasma Tmax (H) | Area Under Curve AUC | Urinary Excretion | T ½ | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| In vivo Sprague–Dawley male rats (n = 30) | (-)-epicatechin | 1, 5 and 10 mg/kg | Total 3′-O-methylated forms (conjugated + no conjugated) | 1 ± 0.02; 3.05 ± 0.15; 4.5 ± 0.22 | 1 | - | Total (-)-epicatechin nonmethylated and 3′-O-methylated metabolites (nM/18 h): 397 ± 35 nM; 1870 ± 101 nM; 3003 ± 212nM | - | [27] | |
| Total nonmethylated forms | 0.97 ± 0.14; 3.21 ± 0.29; 4.41 ± 0.50 | |||||||||
| cocoa poder | 150, 750 and 1500 mg/kg | Total 3′-O-methylated forms (conjugated + no conjugated) | 0.12 ± 0.04; 1.05 ± 0.05; 2.49 ± 0.16 | 1 | - | Total (-)-epicatechin metabolites (non-methylated and 3′-O-methylated): 415±18 nM; 1523±120 nM; 3074±218 nM/18 h | - | |||
| Total nonmethylated forms | 0.35 ± 0.04; 2.12 ± 0.05; 5.08 ± 0.43 | |||||||||
| In vivo Sprague–Dawley male rats (n = 20) | (-)-epicatechin | 172 μmol/kg | Total 3′-O-methylated forms | - | - | 78.3 ± 4.9 μmol.h/L | 9.45 ± 0.56 μmol/24 h | - | [28] | |
| Total non-methylated forms | 88.3 ± 12.4 Μm ol.h/L | 16.6 ± 2.3 μmol/24 h | ||||||||
| (+)-catechin | 172 μmol/kg | Total 3′-O-methylated forms | - | - | 23 ± 1.1 μmol.h/L | 3.60 ± 0.07 μmol/24 h | - | |||
| Total non- methylated forms | 66.4 ± 2.8 μmol.h/L | 8.85 ± 0.76 μmol/24 h | ||||||||
| Mix | 345 μmol/kg | Total epicatechin 3′-O-methylated forms | - | - | 76.5 ± 6.8 μmol.h/L | 4.51 ± 0.45 μmol/24 h | - | |||
| Total epicatechin nonmethylated forms | 78.7 ± 4 μmol.h/L | 9.43 ± 0.58 μmol/24 h | ||||||||
| Total catechin 3′-O-methylated forms | 18.9 ± 0.4 μmol.h/L | 2.53 ± 0.34 μmol/24 h | ||||||||
| Total catechin nonmethylated forms | 56.5 ± 3.5 μmol.h/L | 7.21 ± 0.51 μmol/24 h | ||||||||
| In vivo Wistar albino male rats | [14 C] procyanidin B2 | 21 mg/kg IV | - | - | AUC(0−24): 149 ± 21μg.h/min | 75.6 ± 5.4 % of total dose/24 h | 6.67 ± 0.95 | [23] | ||
| 21 mg/kg IG | 2.60 ± 0.93 μg/Ml | 6.11 ± 0.43 | AUC(0−24): 17 ± 2.7μg.h/min | 62.9 ± 5.48 % of dose | 7.3 ± 2.07 | |||||
| 10.5 mg/kg IG | 1.38 ± 0.28 μg/mL | 5.56 ± 0.98 | AUC(0−24): 5.18 ± 1.35μg.h/min | 62.2 ± 7.6 % of dose | 4.57 ± 1.46 | |||||
| (−)-epicatechin-3′-sulfate; (−)-epicatechin-5-sulfate; (−)-epicatechin-7-sulfate | 331 ± 26 nM; 37 ± 3 nM; 12 ± 1 nM assessed using authentic standards | 2 | ||||||||
| Unmetabolized (−)-epicatechin | 4 ± 1 nM | 1 | ||||||||
| Healthy volunteers (n = 5; 23.47 ± 3.3 years) | 100g of Nestle’ Noir 70% chocolate | Content: 79mg (-)-epicatechin 26mg (+)-catechin 49mg procyanidin B2 | (-)-epicatechin-3′-β-D-glucuronide; (-)-epicatechin-4′-β-D-glucuronide; (-)-epicatechin-7-β-D-glucuronide | 290 ± 49 nM; 44 ± 11 nM; 22 ± 6 nM | 3.2 ± 0.2; 3.4 ± 0.3; 12.8 ± 4.8 | 1276 ± 182 nM/h; 164 ± 38 nM/h; 360 ± 50 nM/h | 13.3 ± 3.85 μmol/24 h; 1.03 ± 0.06 μmol/24 h; 7.27 ± 1.35 μmol/24 h | 3.8 ± 1.0; 1.8 ± 0.3; 5.6 ± 1.1 | [30] | |
| (-)-epicatechin 3′-sulfate; (-)-epicatechin 4′-sulfate | 233 ± 60 nM; 11 ± 3nM | 3.2 ± 0.2; 3.5 ± 0.3 | 954 ± 207 nM/h; 66 ± 8 nM/h | 8.53 ± 2.71 μmol/24 h; 0.56 ± 0.13μmol/24 h; (-)-epicatechin 5-sulfate: 1.15 ± 0.20μmol/24 h | 2.3 ± 0.8; 4.1 ± 0.9; | |||||
| 3′-O-methyl-(-)-epicatechin 4′-sulfate; 3′-O-methyl-(-)-epicatechin 5-sulfate; 3′-O-methyl-(-)-epicatechin 7-sulfate; 4′-O-methyl- (-)-epicatechin 5-sulfate 4′-O-methyl-(-)-epicatechin 7-sulfate | 49 ± 14 nM; 153 ± 43 nM; 40 ± 10 nM; 18 ± 6 nM; 13 ± 4 nM | 3.6 ± 0.3; 3.8 ± 0.2; 3.8 ± 0.2; 3.8 ± 0.3; 3.8 ± 0.2 | 269 ± 74 nM/h; 679 ± 160 nM/h; 222 ± 59 nM/h; 94 ± 19 nM/h; 70 ± 22 nM/h | 1.67 ± 0.62 μmol/24 h; 14.1 ± 3.88 μmol/24 h; 2.33 ± 0.68 μmol/24 h; 1.37 ± 0.34 μmol/24 h; 0.73 ± 0.23 μmol/24 h | 2.5 ± 0.6, 2.1 ± 0.6; 2.1 ± 0.6; 2.3 ± 0.5; 2.0 ± 0.8 | |||||
| Type of Study | Product/Compound | Dose/Duration | Intervention | Target | Outcome (S) | Reference |
|---|---|---|---|---|---|---|
| 2-year-old male Wistar rats (n = 48) | (-)-epicatechin | 2 and 10 mg/kg bw intragastric administration, during 5 weeks | DOCA-salt induced hypertension vs. DOCA-salt EPI2 and DOCA-salt EPI10 | Vascular Nox activity Protein expression of Nox p47phox and p22phox subunits | DOCA-salt–EPI10 ↓ Nox activity in aortic rings by suppression of protein over-expression of p47phox and p22phox subunits and ↓ in ET-1 plasma levels Both DOCA-salt–EPI2 and EPI10 restored impaired endothelial function due to an ↑ in eNOS phosphorylation and a ↓ in O2− vascular content | [56] |
| Double blind study with crossover-design in healthy volunteers (n = 10) | High-flavanol cocoa beverage (98 mg total flavanols: 183 mg epicatechin and 215 mg dimers) Low-flavanol cocoa beverage (80.4 mg total flavanols: 19.8 mg epicatechin and 23.1 mg dimers) | 54 g/200 mL of high or low-flavanol cocoa beverage | High-flavol cocoa (HFC) vs. low-flavanol cocoa (LFC) | Erythrocyte arginase activity | Ingestion of a high-flavanol cocoa beverage resulted in the highest decrease in erythrocyte arginase activity after 24 h (HFC: 3.0± 0.4; p < 0.05 vs. LFC: 3.5 ± 0.5 μmol urea mg protein-1 h-1) | [60] |
| Jurkat T cells culture HCAEC (human coronary artery endothelial cells) culture Obesity mice 2 months old C57BL | Procyanidin A1, procyanidin A2, procyanidin B1 and procyanidin B2 (-)-epicatechin | Cells (1×106 cells/ml) were pre-incubated with 2.5–50 μM A1, A2, B1 or B2 for 24 h Incubation of 0.1 nM-100 μmol/L during 10 minutes 1 mg epicatechin/kG Body weight 15 days | Effect of preincubation of Jurkat T cells (further incubation with or without the addition of either TNF-α or PMA) Identification of epicatechin intracellular signaling pathways on eNOS-NO production Inflammatory status: TNF αand IL-6 | NF-κB-DNA binding eNOS activation | Pre-incubation (24 h) with B1 or B2 procyanidins (50 μM) ↓ NF-κB-Luc activity (34–52%) and ↓ by 80 and 85% IL-2 release in Jurkat cells subsequently treated with TNF-α or PMA A concentration-dependent (5-50μM) inhibition of NF-κB-DNA binding was observed in cells pre-incubated with B1 or B2 procyanidins At 100 nM, B1 and B2 caused a 29–38% and 38–47% inhibition of either p50 or RelA binding to its DNA consensus sequence Epicatechin (1 μM/L) induced eNOS activation via Ser1177 and Ser633 phosphorylation and Thr495 de-phosphorylation Epicatechin (1 μM/L) activated eNOS via Akt phosphorylation (induction of Ser1177 phosphorylation) Epicatechin stimulated dissociation of eNOS from Cav-1 and therefore stimulated its activation TNF α level decreased by 50 % while IL-6 decreased by 30% | [62] [68] [69] |
| RAEC, BAEC and human umbilical endothelial cells (HUVEC) cultures | (-)-epicatechin | 20 μM incubation for 24 h | Protective effects of (-)-epicatechin against oxLDL protein damage | NADPH oxidase (NOX) activity and oxLDL protein damage | Pretreatment of BAEC and RAEC with epicate-chin prevented oxLDL-elicited downregulation of eNOS protein and par-tially the upregulation of iNOS protein In BAEC and HUVEC incubated with oxLDL, (-)-epicatechin showed a potent O2− scavenging activity and a strong inhibition of its production (10 μM) Pretreatment of HUVEC with (-)-epicatechin su-ppressed the formation of all 3 types of modified proteins (protein carbo-nyls and tyrosine-nitrated proteins) in a dose-dependent manner (com-plete inhibition at 10 μM) | [67] |
| HUVEC culture | (-)-epicatechin, its metabolites (3′-O-methyl epicatechin, 4′-O- methyl epicatechin) and pB2 | 0.1-100 μM incubation for 24 h | Effect of pB2, epicatechin and its metabolites on NADPH oxidase activity | NADPH oxidase (NOX) activity and O2− generation | All 4 compouds (10 μM) inhibited O2− re-lease in Angiotensin-II estimulated HUVEC, after 24 h preincubation Methylated epicatechin metabolites proved to be Nox inhibitors (100 μM) without O2− scavenging activity Epicatechin showed O2− scavenging activity (100 μM) dependent on the duration of preincuba-tion, but did not affect NOX oxidation pB2 showed both inhibitory Nox and O2− scavenging activities | [54] |
| Sprague–Dawley male rats (n = 10) | Cocoa powder (11 mg epicatechin/g and 43 mg procyanidins/g) | Purified egg white protein-based diet containing 40 g cocoa/kg diet, during 28 days | Diet 0% cocoa vs. Diet 4% cocoa | Renal arginase activity | 4% cocoa supplementation ↓ renal arginase activity, compared with control group (0.13 ± 0.02 vs. 0.18 ± 0.02 U/mg protein) | [60] |
| HUVEC culture | (-)-epicatechin flavanol metabolite mixture (2.6 μM total flavanols: 0.1 μM epicatechin and 2.15 μM epi-catechin metaboli-tes found in human plasma 2 h after high-flavanol cocoa beverage consumption) | mix: 0.4, 2.6 and 7.8 μM epicatechin: 1, 3 and 10 μM 48 hour incubation | Comparison between different concentrations of flavanol mix and epicatechin | Arginase-2 (Arg-2) mRNA expression and activity | Flavanol mix and epicatechin signifi-cantly ↓ Arg-2 mRNA expression in HUVEC, at 24 h in a dose-dependent manner Cells incubated with flavanol mix and epica-techin exhibited ↓ Arg-2 activity, at 48 h in a dose-dependent manner | [60] |
| Randomized, crossover clinical trial in healthy volunteers (n = 18) | Cocoa powder | 40 g cocoa powder (28.2mg epicatechin and 25.5 mg pB2/40 g) with 250 mL whole milk or water, during 3 weeks | Cocoa powder with milk (CM) vs. cocoa powder with water (CW) | NF-κB activation and protein expression of adhesion molecules (sICAM-1, sVCAM-1 and sE-selectin) in PBMC (periphe-ral blood mono-nuclear cells) | CW significantly ↓NF-κB activation (determined by protein expression) after 6 h of ingestion, compared with CM Both CM and CW ↓ serum [sICAM-1] after intervention but only CW ↓ [sE-selectin] | [34] |
| Human hepatoma HepG2, | (-)-Epicatechin (EC) and cocoa phenolic extract (CPE) | 10 µM EC or 1 µg//mL CPE were added to the cells for 24 h; | Comparison between epicatechin and polyphenol extract | Nrf2; GPx, GX and CAT | Antioxidant exnzymes were regulating and Nfr2 has been stimulated. | [46] |
| Type OF Study | Product/Compound | Dose/Duration | Intervention | Target | Outcome (s) | Reference |
|---|---|---|---|---|---|---|
| Comparative, double-blind study in normo and mild hyper cholesterolemic japanese subjects (n = 160) | Low PFT cocoa powder (64.5 mg epicatechin and 36.3 mg pB2/g) middle PFT cocoa powder (96.7 mg epicatechin and 54.4 mg pB2/g) high PFT cocoa powder (129 mg epicatechin and 72.5 mg pB2/g) | Consumption of 13 g low PFT cocoa; or 19.5 g middle PFT cocoa; or 26 g high PFT cocoa, during 4 weeks | Intake of low PFT cocoa powder vs. middle PFT cocoa vs. high PFT cocoa in normo and mild hyper cholesterolemic subjects | Serum LDL, HDL and oxLDL | Consumption of 3 cocoa doses in subjects with LDL ≥3.23 mmol/L, resulted in significantly ↓ serum [LDL], after 4 wk Consumption of 3 cocoa doses (normo and mild hypercholesterolemic subjects) resulted in ↑ serum [HDL], compared with baseline after 4 wk Plasma oxLDL levels were significantly ↓ after 4 wk consumption of 3 cocoa doses (normo and mild hyper cholesterole-mic subjects) | [72] |
| Randomized, placebo-controlled, double blind, crossover study in DM 2 subjects (n = 12) | High (16.6 mg epicatechin) and low (<2 mg epicatechin) polyphenol content chocolate | 45 g high or low polyphenol chocolate, during 8 week | High polyphenol chococolate (HPC) intake vs. low polyphenol chococolate (LPC) intake | Serum c-HDL, c-LDL, TG, HbA1c, fasting glucose and insulin and C- reactive protein | Consumption of HPC and LPC improved lipid profile through ↑ HDL/↓ LDL No changes were observed in fasting glucose or HbA1c levels in none of the 2 treat-ment groups Insulin levels showed an ↑ after LPC intake No changes were observed in C-reactive protein levels in none of the 2 treatment groups | [75] |
| Cross-sectional study in 4098 patients from NHLBI | Chocolate | - | Association between self-reported chococlate consumption and prevalence of metabolic syndrome (MS) in adult population | ATP-III criteria for clinical diagnosis of metabolic syndrome | Higher intake of chocolate was associated with ↓ prevalence of coronary heart disease and ↓ glycemia From the lowest to the highest levels of choco-late consumption, the prevalence of MS odd ratios were: + women: 1.0 (0/wk); 1.26 (<1/wk); 1.15 (1–4/wk) and 0.9 (+5/wk) + men: 1.0 (0/wk); 1.13 (<1/wk); 1.02 (1-4/wk) and 1.21 (+5/wk) the highest odd ratios of obesity prevalence were observed with higher chocolate consumption | [71] |
| 9-week-old male Sprague–Dawley rats (n = 40) 12-week-old female Sprague–Dawley rats (n = 56) | Cacao procya-nidins (CP) extracted from cacao liquor (CLPr: 79.3% total polyphenols; 5.9% epicatechin; and 4% PB2) Cocoa powder | High-cholesterol diet (HCD: 1% cholesterol and 15% fat) supplemented with 0.5 or 1.0% of CLPr C1: methionine-choline deficient diet (MCD) + 28 d of 12.5% cocoa supplementation C2: MCD diet + 56 d of cocoa supplementation C3: 80 d of MCD + cocoa supp. C4: 108 d of MCD + cocoa supplementation | HCD with 0.5% CP vs. HCD with 1.0% CP C1 and C2 were selected to test NASH treatmet effects of cocoa supplementation C3 and C4 were used to test if cocoa supple- mentation could prevent NASH development | Plasma and liver cholesterol liver and feces TG mRNA and protein expression of LFABP serum TG, glucose and superoxide levels | Both CP groups (0.5 and 1%) inhibited drastic elevation of plasma TC levels Liver cholesterol and TG levels were significantly ↓ in HCD groups supplemented with both CP doses (more marked effects in 1% CP) All cocoa supplemented groups showed ↓ serum TG and glucose levels C3 group had the ↓ superoxide levels, compared to C1, janeC2 and C4 groups C1 had the ↑ mRNA and protein expression levels of LFABP | [76] [77] |
| 3-week-old female diabetic obese mice (n = 44) Streptozotocin-diabetic male Wistar rats (n = 80: 200–300 g) | Cacao liquor procyanidins (72.32% total polyphenols; 5.89% epi-catechin and 3.93% PB2) Cocoa beans extract (CE) | Supplementation with 0.5 or 1 % CLPr, during 3 weeks Supplementation with 1, 2 or 3% CE (1 g CE/100 g diet) | Dietary supplementation with 0% CLPr vs. supplementation with 0.5 or 1.0% CLPr Streptozotocin-diabetic rats + normal diet vs. diabetic induced rats + 1, 2 or 3% CE | Plasma glucose (hyperglycemia) and renal function Serum CT, HDL, LDL, TG and glucose | Levels of blood glucose were significantly ↓ in mice fed 1% CLPr At the end of the study, group supplemented with 1% CLPr had ↓ levels of BUN and creatinine and suppressed membrane lipoxidation in kidney (↓ 4-hidroxy-2-nonenal antibody levels) All 3 diabetic groups treated with CE showed significant ↓ in body weight gain and serum TG levels Diabetic groups treated with 1 and 3% CE exhibited significant ↓ in plasma glucose levels Diabetic group treated with 1% CE had the ↓ serum levels of CT and LDL | [78] [79] |
| Male Sprague–Dawley rats (n= 90) | Polyphenol-rich cocoa extract (CE) | Intragastric administration of 1, 2 or 3% CE (1 mL/100 g bw) during 4 weeks | Assessment of CE effectiveness in reducing hyperglyce-mia in diabetic-induced rats | Plasma glucose levels and body weight gain | A significant body weight reduction was observed (p < 0.05) in diabetic-induced rats treated with 1 and 2% CE Diabetic-induced rats treated with 3% CE showed the most significant ↓ in glucose levels | [80] |
| Glucose-responsive pancreatic cell lines (BRIN-BD11) Randomized, crossover trial in patients with hypertension (HTA-I) and impaired glucose tolerance (IGT) (n = 19) | Polyphenol-rich cocoa extract (CE) Flavanol-rich dark chocolate bar (FRDC: 110.9 mg epicatechin/bar) | Incubation with CE at 2, 1, 0.5, 0.1 and 0.05 mg/mL 100 g of dark chocolate bar, during 15 days | Evaluation of different concentrations of CE on insulin secretion Flavanol rich dark chocolate bar vs. flavanol free chocolate bar | Insulin-release from rat pancreatic β-cells Fasting glucose and insulin sensitivity Serum C-reactive protein (CRP) Serum lipid profile (HDL, CT, LDL and TG) | Pancreatic cell lines treated with 0.1 mg/mL of CE showed the ↑ insulin secretion FRDC intake enhanced insulin sensitivity and β-cell function in HTA-I patients with IGT (measured by ↑ QUICKI; ↓ HOMA-IR; ↑ ISI0 and ICI20) FRDC ingestion significantly ↑ FMD FRDC intake ↓ serum CT and LDL, but did not affect TG and HDL neither FRDC nor FFWC ingestion affected serum CRP | [81] |
| Male C57BL/6 4-week-old mice (n = 36) | cacao liquor procyanidin extract (CLPr: 6.12% epicatechin and 3.60% PB2) | Supplementation with 0.5 or 2% CLPr, during 13 weeks | High fat diet (HFD) vs. HFD + 0.5% (HF-0.5) or 2% (HF-2) CLPr | Glucose parameters mRNA and protein expression of UCP-1, UCP-2, GLUT-4 and AMPKα | At week 7, fasting glucose levels in HF-2 group were significantly lower At week 11, OGTTe showed ↓ glucose levels in HF-2 group (0 and 15 minutes after glucose load) At the end of the study, HF-0.5 and HF-2 completely suppressed HF diet-induced hyper-glycemia, hyper-insulinemia (↓ HOMA-IR) and hypercholestero-lemia, compared to control group CLPr supplementation promoted AMPKα phosphorilation (BAT, WAT, liver and skeletal muscle), which enhanced GLUT-4 translocation to plasma membrane in BAT and skeletal muscle in a dose-dependent manner CLPr ↑ UCP-1 and UCP-2 gene and protein expression in BAT and WAT, respectively | [82] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo Flores, M.E. Cocoa Flavanols: Natural Agents with Attenuating Effects on Metabolic Syndrome Risk Factors. Nutrients 2019, 11, 751. https://doi.org/10.3390/nu11040751
Jaramillo Flores ME. Cocoa Flavanols: Natural Agents with Attenuating Effects on Metabolic Syndrome Risk Factors. Nutrients. 2019; 11(4):751. https://doi.org/10.3390/nu11040751
Chicago/Turabian StyleJaramillo Flores, Maria Eugenia. 2019. "Cocoa Flavanols: Natural Agents with Attenuating Effects on Metabolic Syndrome Risk Factors" Nutrients 11, no. 4: 751. https://doi.org/10.3390/nu11040751
APA StyleJaramillo Flores, M. E. (2019). Cocoa Flavanols: Natural Agents with Attenuating Effects on Metabolic Syndrome Risk Factors. Nutrients, 11(4), 751. https://doi.org/10.3390/nu11040751





